Introduction
Lung cancer is a major health problem worldwide. In
China, lung cancer has become the most commonly diagnosed cancer
and the leading cause of cancer-associated mortality (1). Non-small cell lung cancer (NSCLC)
accounts for 85% of the lung cancer cases diagnosed worldwide
(2), and for 85% of these, the
cases are too advanced for surgery to be the primary treatment
option on diagnosis (3). For these
cases, radiotherapy is considered.
Stereotactic body radiation therapy and radiotherapy
combined with chemotherapy have been demonstrated to accurately and
effectively kill tumor cells, thereby prolonging patient survival
(4). However, radiation resistance
has been observed to develop in patients undergoing long-term
radiotherapy and this restricts their treatment (5). Radiation resistance of neoplastic
cells has been reported to be affected by the level of oxygen,
genome stability, and the capacity of these cells to undergo DNA
double-strand break (DSB) repair, proliferation and anti-apoptotic
signaling (6-8). In the microenvironment of a tumor,
tumor cells, fibroblasts, mesenchymal stem cells and cytokines
including interleukin 6, transforming growth factor β, tumor
necrosis factor are present, and all of these components can
potentially contribute to the radioresistance of tumor cells
(9,10).
Early studies demonstrated that autocrine signaling
is associated with radiation resistance (11,12).
In breast cancer, the autocrine cytokine, human growth hormone, may
promote cell regrowth by contributing to ionizing radiation
(IR)-induced resistance (13).
Vascular endothelial growth factor (VEGF) has been documented to
promote malignant astrocytoma growth and radioresistance via VEGF
receptor 2 (also known as kinase insert domain receptor) and
co-activation of c-Raf/mitogen-activated protein kinase,
phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/Akt and
phospholipase C/protein kinase C signaling pathways (14). In another study, the ability of
cytokines to support DNA repair pathways and an ATM
serine/threonine kinase-dependent DNA response were observed
(15). Additionally, cell secreted
exosomes, which represent extracellular microvesicles with
diameters ranging from 30 to 100 nm that contain an abundance of
proteins, messenger RNAs, microRNAs, and long non-coding RNAs, may
contribute to tumor metastasis, angiogenesis and migration via
autocrine/paracrine signaling (16). Recently, the role of exosomes in
promoting tumor radioresistance was confirmed in head and neck
cancer cells (17). Based on the
results of these prior studies, it may be assumed that cells use
different autocrine secretions as inducing factors to enhance their
own radiation resistance and thus survival. The present study
focused on the identification of changes in H460 cells that lead to
radiation resistance in an autocrine environment.
Materials and methods
Cell cultures and γ-irradiation
The human NSCLC cell line, NCI-H460, was obtained
from the Institute of Basic Medical Sciences at the Chinese Academy
of Medical Sciences and Peking Union Medical College (Tianjin,
China), and were cultured in Dulbecco’s modified Eagle’s medium
(DMEM)/F12 (Hyclone; GE Healthcare Life Science, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The NCI-H1299 cell line was
obtained from American Type Culture Collection (Manassas, VA, USA)
and was cultured in RPMI-1640 medium (Hyclone; GE Healthcare Life
Science) supplemented with 10% FBS. The cells were incubated in 5%
CO2 at 37°C. DSBs were induced in H460 cells by applying
a 137Cs Gammacell-40 radiation source (Atomic Energy of
Canada Limited, Chalk River, Ontario, Canada) at a photon dose rate
of 1.02 Gy/min.
Preparation of conditioned medium (CM)
and exosome isolation
To prepare CM, H460 cells were grown to 80–90%
confluence on 100 mm plates (5×106 cells). The cells
were then rinsed with phosphate-buffered saline (PBS) and incubated
with DMEM/F12 containing 10% FBS. After 48 h, the medium was
collected, membrane-filtered (0.22 µm; EMD Millipore, Billerica,
MA, USA) and stored at −80°C.
To isolate exosomes, H460 cells were incubated in
serum-free DMEM/F12 until they reached 80-90% confluency. Following
an additional 48 h of culturing, the medium was collected and then
subjected to serial centrifugation at 4°C (300 × g for 10 min;
2,000 × g for 20 min; and 10,000 × g for 30 min). Following
filtration of the supernatant (0.22 µm), exosomes were pelleted by
ultracentrifugation at 100,000 × g for 2 h at 4°C with a 32Ti rotor
(Optima L-100XP Ultracentrifuge; Beckman Coulter, Inc., Brea, CA,
USA). The pellet was resuspended in PBS and then subjected to a
second ultracentrifugation step (100,000 × g for 2 h at 4°C). The
pelleted exosomes were resuspended in 100 µl PBS and stored at
−80°C. Bicinchoninic acid protein assays (Beyotime Institute of
Biotechnology, Haimen, China) were used to determine total protein
concentration for the exosome preparation.
Morphological identification of
exosomes
The mixed exosomes were added to a copper net for 2
min, and the residual liquid was removed with filter paper, which
was followed by staining with 2% phosphotungstic acid solution (pH
6.4) for 2 min at room temperature. The prepared copper nets were
then observed under a transmission electron microscope (HT77000;
Hitachi, Ltd., Tokyo, Japan). Images of the exosomes were obtained
at ×10 magnification.
Co-culture clonogenic survival assay
To evaluate radiosensitivity, H460 cells were plated
in 6-well plates (500 cells/well). The next day, Transwells (0.4
µm; Corning Incorporated, Corning, NY, USA) containing H460 cells
(2.5×104 cells/Transwell) were transferred into the
6-well plates. The whole plate was then treated with
137Cs γ-radiation (1.02 Gy/min) at variable doses (0, 2
and 4 Gy). In a control group, Transwells containing unirradiated
H460 cells (2.5×104 cells) were placed in a 6 well-plate
alongside previously plated and irradiated H460 cells (500
cells/well). H1299 cells were plated in 6-well plates (1,000
cells/well) and exposed to 0, 2 or 4 Gy of γ-rays. Transwells
containing unirradiated H1299 cells (2.5×104
cells/Transwell) were subsequently added. A second set of
experiments was conducted with H460 cells plated in 12-well plates
(2.5×104 cells/well) and treated with 0, 2 and 4 Gy
irradiation. CM was collected from both irradiated and
non-irradiated H460 cells and these samples were filtered (0.22 µm)
and added to the appropriate 12-well plates every 48 h for 7
days.
To verify the effect of exosomes on the
radiosensitivity of H460 cells, 30 µg exosomes was added to each
well of a 6-well plate containing irradiated H460 cells (18).
At 7 days after seeding, all plates were fixed with
anhydrous methanol for 20 min and stained with 10% Giemsa stain for
30 min, all at room temperature. The number of colonies was counted
under a microscope (Nikon Eclipse TS100; Nikon Corporation, Tokyo,
Japan), which a colony consisting of at least 50 cells.
Cell viability assay
H460 cells were seeded into 96-well plates (1,500
cells/well) and treated with 4 Gy γ-irradiation. After 24 h, the
medium was replaced with varying volumes of conditioned medium (50,
100, 150, 200 and 250 µl) and a corresponding volume of fresh
medium to achieve a total volume of 300 µl. After 72 h, MTT reagent
(Beijing Solarbio Science & Technology, Co., Ltd., Beijing,
China) was added to each well. After 4 h, the medium was removed
and the cells were lysed with dimethyl sulfoxide to dissolve the
formazan present. Absorbance values were measured at 570 nm with a
spectrophotometric plate reader (Synergy™ HT; BioTek Instruments,
Inc., Winooski, VT, USA).
Intracellular reactive oxygen species
(ROS)
To investigate the effect of CM on the level of ROS,
H460 cells (1×105) were seeded into 60-mm plates,
exposed to 4 Gy of γ-rays, and the medium was subsequently replaced
with CM or fresh medium. After 24 h, the cells were stained with 5
µM dichlorodihydrofluorescein diacetate (DCFH-DA) from a Reactive
Oxygen Species Assay kit (Beyotime Institute of Biotechnology) for
30 min at room temperature and then were washed 3 times with
DMEM/F12. Immunofluoresent images were obtained with a fluorescence
microscope (EVOS™; Thermo Fisher Scientific). For flow cytometry
analysis, the cells were stained as above and then digested into
single cell suspensions with 0.25% trypsin and washed 3 times with
PBS at room temperature. Single cell suspensions were resuspended
in 500 µl PBS, and analyzed by flow cytometry (Mindray BriCyte E6;
Mindray, Shenzhen, China).
Western blot assay
H460 cells were treated or untreated for 2, 6, 12 or
24 h with CM following exposure to 0 or 4 Gy irradiation. Cell
extracts were subsequently collected and lysed with
radioimmunoprecipitation assay lysis buffer (CWBio, Beijing, China)
at 4°C. Proteins were isolated by centrifugation at 12,000 × g for
10 min at 4°C, then quantified by the bicinchoninic acid assay
prior to being separated by 12% SDS-PAGE (30 µg protein per lane).
Following transfer of the separated proteins to polyvinylidene
difluoride membranes, they were blocked with a 1% BSA solution
(Solarbio, China). All of the antibodies were diluted in a 1%
bovine serum albumin (BSA) solution (0.1 g BSA in 10 ml
tris-buffered saline with Tween-20). Target proteins were probed
with antibodies recognizing superoxide dismutase (SOD)-1 (1:1,000;
cat. no. ab20926; Abcam, Cambridge, UK), γH2A histone family member
X (γH2AX; 1:1,000; cat. no. 9718s; Cell Signaling Technology, Inc.,
Danvers, MA, USA), Rad51 recombinase (1:1,000; cat. no. sc-398587),
cyclin A (1:500; cat. no. sc-271645; both from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), the Mre11-Rad50-Nbs1 (MRN)
complex (1:1,000; cat. no. 8344T, MRN Complex Antibody Sampler
kit), phosphorylated (p)-ATM (cat. no. 5883T) and ATM (1:1,000;
cat. no. 2873T; all from Cell Signaling Technology, Inc.). An
analysis of the exosome protein lysates was conducted according to
the same method, except that anti-cluster of differentiation 9
(CD9; 1:1,000; cat. no. ab1832-500) and anti-tumor susceptibility
gene 101 (TSG101; 1:1,000; cat. no. ab125011; both from Abcam)
antibodies were used. Detection of β-tubulin (1:5,000; cat. no.
66240-1-Ig) and β-actin (1:5,000; cat. no. 66009-1-Ig; both from
ProteinTech Group, Inc., Chicago, IL, USA) was also performed to
provide internal positive controls for the immunoblots. The primary
antibodies were incubated with the membranes overnight at 4°C. The
secondary antibodies of goat anti-mouse immunoglobulin (Ig)G (H+L),
horseradish peroxidase (HRP) conjugate (1:5,000; cat. no.
SA00001-1) and goat anti-rat IgG (H+L), HRP conjugate (1:5,000;
cat. no. SA00001-15; both from ProteinTech Group, Inc.) were
incubated with the membranes at room temperature for 1 h. Bound
antibodies were detected with electrochemiluminescence reagents
(ProteinTech Group, Inc.) and a ChemiDoc MP Imaging System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Western blot data was
analyzed quantitatively with Image Lab 5.2.1 (Bio-Rad Laboratories,
Inc.).
Cell cycle analysis by flow
cytometry
H460 cells were treated or untreated with CM with
and without irradiation (4 Gy) for 24 h. The cells were then
collected and rinsed with PBS three times. The cells were fixed in
70% ethanol overnight at −20°C. The next day, the cells were washed
three times with cold PBS and labeled with propidium iodide (PI;
Beijing Solarbio Science & Technology, Co., Ltd.) for 20 min at
37°C in the dark. The labeled cells were subsequently detected by
flow cytometry (Mindray BriCyte E6) and analyzed with FlowJo v10
software (FlowJo LLC, Ashland, OR, USA).
Immunocytofluorescence
H460 cells (4.5×104) were seeded into
12-well plates containing coverslips and incubated overnight. The
next day, the H460 cells were exposed to 0 or 4 Gy and cultured in
normal medium or CM. After 2, 6, 12 and 24 h, the cells were washed
3 times with PBS and fixed with 4% paraformaldehyde for 20 min at
room temperature. After three additional rinses in PBS, the cell
membranes were permeabilized with 0.3% Triton X-100 at room
temperature. After 20 min, the cells were rinsed three times with
PBS and then incubated in blocking buffer (1% BSA) at room
temperature. After 1 h, the cells were incubated with anti-γH2AX
antibodies (1:200) at room temperature. After an additional 2 h,
the cells were rinsed three times in PBS and then incubated with
cyanine 3-conjugated anti-goat/mouse antibodies (1:200; cat. no.
SA00009-1; ProteinTech Group, Inc.) at room temperature. After 1 h,
the cells were rinsed again in PBS three times and antifade
mounting medium containing DAPI (Vector Laboratories, Inc.,
Burlingame, CA, USA) was used to mount the specimens. Imaging was
performed via fluorescence microscopy.
Statistical analysis
Data are presented as the mean ± standard deviation
from at least three independent experiments, with each experiment
performed in triplicate. The Student’s t-test and one-way analysis
of variance (ANOVA) followed by Duncan’s multiple range test were
applied with SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) and P<0.05
was considered to indicate a significant difference between the
groups. The intracellular ROS levels were analyzed by Duncan’s
multiple range test and other data were analyzed by t-test.
Results
Autocrine secretions enhance the
radioresistance of H460 cells
To examine whether autocrine signaling improves the
radioresistance of NSCLC cells, H460 colonies were co-cultured with
H460 cells contained in Transwells (Fig. 1A). When the cells were incubated
with irradiated or unirradiated H460 cells, the number of H460
colonies markedly increased following 2 or 4 Gy irradiation in the
co-culture group, compared with in the control group (Fig. 1B and C). These data indicated that
the irradiated H460 cells exhibited increased radioresistance
following exposure to autocrine factors, and these factors were
present independent of whether the added H460 cells had undergone
irradiation. To verify whether other NSCLC cell lines also mediate
this effect, H1299 cells were tested in the Transwell model. The
same effect was observed (Fig.
1D).
Interactions between cells are dynamic and complex.
To facilitate studies of these interactions, CM was used to
simulate the effect of cell secretions on clonal formation. CM was
collected from H460 cells with or without prior irradiation, and
the effects of these CM samples on the formation of H460 colonies
following irradiation was observed (Fig. 1E). Both non-irradiated and
irradiated CM enhanced the radiation resistance of the H460 cells,
and these results were consistent with those obtained from the
Transwell assays. Consequently, only non-irradiated CM was used as
a relevant model in subsequent radioresistance-related studies.
Next, it was postulated that H460-derived CM
promotes cell proliferation. When H460 cell viability was measured
in MTT assays, the H460 cells that were irradiated and then
cultured in CM exhibited greater viability compared with those
cells cultured in normal medium (Fig.
1F). Interestingly, the observed effects were dependent on the
volume of the CM that was applied: As the volume of CM increased,
an initial increase in cell viability was observed, followed by a
decrease after 4 Gy irradiation (Fig.
1G, right panel). At the higher volumes of CM, cytotoxicity
appeared to be partially induced (Fig.
1G, left panel).
Autocrine secretions reduce degradation
of ROS in H460 cells
To detect the level of intracellular ROS, cells were
stained with the fluorescence indicator, DCFH-DA. Fluorescence
intensity (in arbitrary units) measured by flow cytometry
demonstrated that the level of ROS increased ~1.25-fold 24 h after
H460 cells were irradiated (4 Gy) and then cultured in CM
(5,195.87±316.76), compared with in those cells cultured in normal
medium (4,152.57±296.77; Fig. 2A and
B). The level of intracellular ROS was also directly observed
by fluorescence microscopy. In H460 cells cultured in CM after 4 Gy
irradiation, the level of intracellular ROS appeared higher than
that in the normal-culture irradiated group (Fig. 2C).
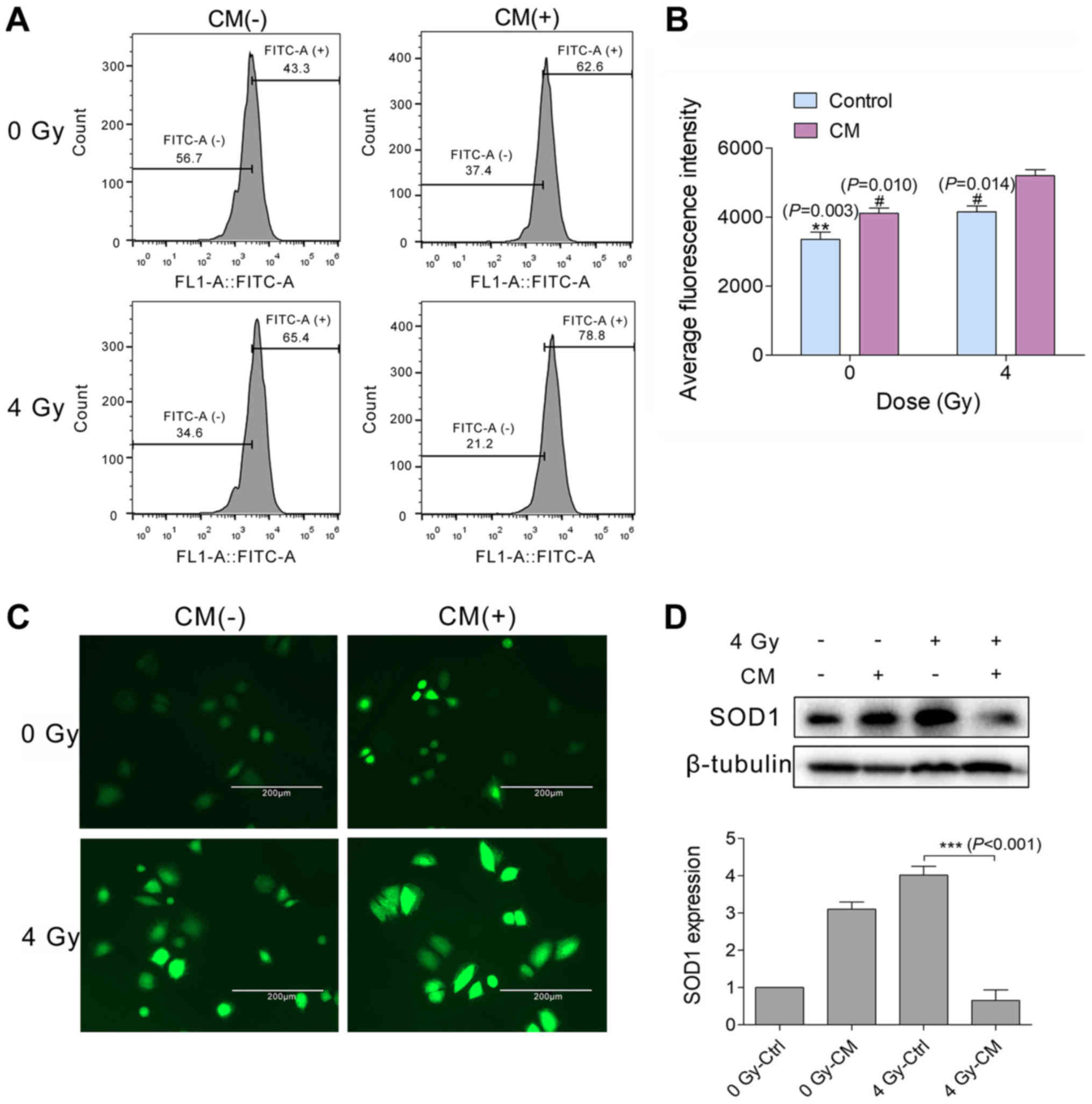 | Figure 2Autocrine secretions reduce the
degradation of ROS in H460 cells. (A-D) H460 cells were incubated
with or without H460 CM for 24 h after receiving 0 or 4 Gy
irradiation. (A and C) ROS were labeled with the fluorescence
indicator, dichlorodihydrofluorescein diacetate, and then detected
by flow cytometry and fluorescence microscopy (magnification,
×400); (B) bar graph of the average ROS levels detected in the H460
samples indicated and analyzed by Duncan’s multiple range test (n=3
independent experiments), the 4Gy-CM group served as control.
**P<0.01, #P<0.05. (D) Relative
expression levels of SOD1 protein in the H460 cell samples
indicated. Detection of β-tubulin served as an internal reference
for normalizing target protein expression,
***P<0.001. ROS, reactive oxygen species; CM,
conditioned medium; Ctrl, control; SOD1, superoxide dismutase
1. |
The balance of intracellular ROS is regulated by
various signaling molecules and signaling pathways (19). Presently, it was hypothesized that
SODs may mediate the level of ROS in irradiated H460 cells. On
western blot analysis, the H460 cells that were irradiated and then
cultured in CM were observed to contain lower levels of SOD1,
compared with in those cells cultured in normal medium (Fig. 2D). These results suggest that
secretions deriving from H460 cells reduce the degradation of ROS
in a SOD1-dependent manner.
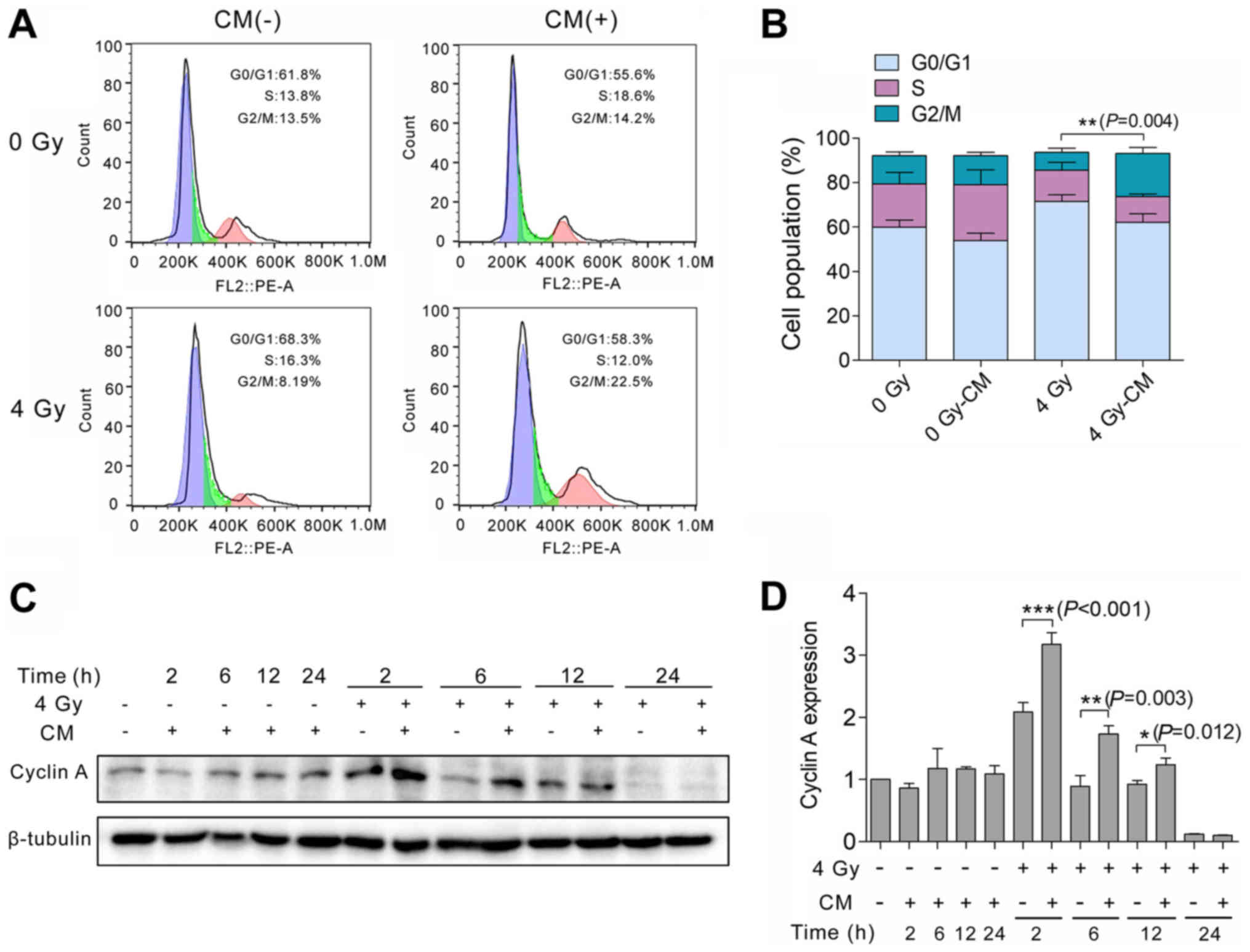
Autocrine secretions alter the cell cycle
of H460 cells
To determine whether IR-treated H460 cells undergo
cell cycle arrest, H460 cells were treated with 0 or 4 Gy
irradiation and then cultured with CM or normal medium. After 24 h,
the cells were stained with PI. Flow cytometry detected a greater
number of cells in the G2/M phase of the cell cycle among the H460
cells that were administered with 4 Gy irradiation followed by CM,
compared with those H460 cells that were irradiated and then
cultured in normal medium (Fig. 3A and
B). To further characterize the observed cell cycle arrest, the
amount of cyclin A in H460 cells with and without irradiation and
subsequent culturing in CM was analyzed at various time points (0,
2, 6, 12 and 24 h) after irradiation. These experiments were
performed based on previous reports that cyclin A is critical for
the S and G2 phases of the cell cycle and is degraded in
prometaphase (20,21). When IR-treated H460 cells were
co-cultured with the secretions of H460 cells for 2 h, cyclin A was
identified to be significantly upregulated compared with in the
IR-treated, normal cultured cells at the same time point.
Degradation of cyclin A was observed at the subsequent time points
assayed (Fig. 3C and D).
Autocrine secretions promote DNA repair
in H460 cells
One of the earliest responses to DSBs is
phosphorylation of histone H2AX at serine 139 (γH2AX) (22). This response has been reported to
persist over a longer period of time in radiosensitive cell lines
than in radioresistant lines (23). Here, the persistence of γH2AX foci
was examined 2, 6, 12 and 24 h after H460 cells were treated with 4
Gy irradiation followed by culture in CM or normal medium. Among
the IR-treated, CM-cultured cells, fewer positive cells were
detected at the 6 h time point compared with in the population of
IR-treated, normal cultured cells (normal medium: 80.13±1.48%; CM:
62.63±2.98; Fig. 4A and B).
Detection of γH2AX protein by western blotting further confirmed
these results (Fig. 4C).
Interestingly, it was also noted that when normal H460 cells were
treated with CM, the content of γH2AX appeared to increase with
time.
 | Figure 4Autocrine secretions promote DNA
repair in H460 cells. (A) Expression of γH2AX in H460 cells was
detected 6 h after irradiation in immunofluo-rescence assays
(magnification, ×400). (B and C) H460 cells received 4 Gy
irradiation and then were immediately incubated with H460 CM for 2,
6, 12 or 24 h. The percentage of γH2AX-positive cells (γH2AX foci
>10) was calculated and the level of γH2AX protein was detected
by western blotting. (D) H460 cells received 4 Gy irradiation and
were immediately incubated with H460 CM for 6, 12 or 24 h. Rad51,
Rad50, Mre11, NBS1 and p-NBS1 in these samples were detected by
western blotting. (E) Relative protein expression of p-Nbs1/Nbs1
was analyzed. (F) Levels of p-ATM and ATM in these samples were
also detected by western blotting; the relative protein expression
of p-ATM/ATM was analyzed. Detection of β-tubulin served as an
internal reference for normalizing protein expression.
***P<0.001, **P<0.01,
*P<0.05. γH2AX, γH2A histone family member X; ATM,
ATM serine/threonine kinase; p-, phosphorylated; CM, conditioned
medium; Ctrl, control. |
Homologous recombination (HR) and non-homologous
end-joining (NHEJ) are the main pathways that mediate the repair of
IR-induced DSBs in DNA, with the HR pathway being dominant in the S
and G2 phases of the cell cycle (24). To examine HR repair in H460 cells,
expression of Rad51 was detected in western blots. In the H460
cells that underwent irradiation and then incubation with CM, a
significant increase in Rad51 expression was observed compared with
in the control cells at the 6 and 12 h time points following
irradiation (Fig. 4D).
Additionally, the Rad50, NBS1 and Mre11 proteins (which constitute
the MRN complex) exhibited marked increases in expression when
cultured in CM for 12 h and/or 24 h after irradiation (Fig. 4D). Meanwhile, it was observed that
not only the protein content of p-ATM was increased, but also the
total amount of ATM following culture in CM for 24 h after
irradiation (Fig. 4E). These data
indicate that radiation resistance in H460 cells is achieved with
cell cycle arrest in the G2/M phase and DNA repair via HR.
Exosomes from H460 cells have little
effect on radioresistance
The above experiments suggested that secretions from
H460 cells reduce the radiosensitivity of IR-treated H460 cells. To
determine whether exosomes contribute to this effect, exosomes were
purified from H460 cell medium by differential ultra-centrifugation
and then were characterized by western blotting (Fig. 5A). Exosome markers are typically
identified by TSG101 and CD9 (25), but the result showed that the
purified exosomes expressed the exosomal marker protein TSG101
without CD9. This may be due to the exosomes secreted by H460 cells
not expressing CD9 protein. When the purified exosomes were
visualized by transmission electron microscopy, round, bi-layer
structures were observed and their diameters ranged from 30 to 100
nm (Fig. 5B).
Clonal formation assays were subsequently conducted
with H460 cells that were treated with 0 or 4 Gy irradiation and
then incubated with or without purified exosomes. The irradiated
H460 cells that were cultured with H460 cell secretions in a
Transwell system exhibited a significant ~1.5-fold increase in
radioresistance (percent survival, 8.47±0.61%) compared with the
control cells (5.60±1.31%). The addition of purified exosomes did
not increase this effect (8.73±0.42%; Fig. 5C). Taken together, these data
suggest that exosomes secreted from H460 cells do not influence the
radiosensitivity of H460 cells.
Discussion
In recent years, it has been demonstrated that
secretions by cells in the tumor microenvironment may enhance the
radiation resistance of tumor cells (26,27),
including recently studied exosomes (28). Regarding the relationship between
NSCLC and radiation resistance, many studies have focused on
identifying internal regulatory factors of these cells (29,30).
In the present study, it was demonstrated that H460 cell-derived
secretions could provide a favorable tumor microenvironment for
protecting the cells against radiation effects, and that the
mediating factors were not exosome-dependent.
Initially, a combination of co-culturing and typical
clonal formation assays were used to verify whether secretions from
H460 cells that enhance the radioresistance of H460 cells post
γ-ray irradiation are independent of prior irradiation of the
secreting cells. In parallel, autocrine secretions from H1299 cells
were also observed to enhance the radiation resistance of H1299
cells. However, the NSCLC cell line, A549, did not exhibit the same
effect (data not shown). Taken together, these results suggest that
not all NSCLCs can enhance the radiation resistance of the tumor
via autocrine signaling. Further experiments indicated that the
observed effect was due to the promotion of cell proliferation by
the secreted factors, although higher volumes of CM appeared to
negatively influence cell survival. It is possible that the large
quantity of cell metabolites present in the higher volumes of CM
are not balanced by sufficient resources in tumor cells, and this
imbalance results in cytotoxic effects. Recent studies have also
demonstrated that exosomes contribute to a radiation-induced
bystander effect (31,32). Thus, it was hypothesized that
exosomes that are produced in response to autocrine signaling could
have some effect on radioresistance. However, the exosomes that
were collected from H460 cells did not affect the IR-treated H460
cells in the present experiments. Therefore, it may be postulated
that the autocrine substances are produced directly by H460 cells,
and that these enhance radioresistance independently of exosomes.
Moreover, this protection may not depend on the microenvironment of
the cells prior to irradiation since the cells received CM
following irradiation.
Previous study has shown that an IR-induced increase
in intracellular ROS levels may cause DSBs in DNA and further
induce cell death (33). The
balance of kinase activity and phosphatase activity involving the
PI3K/Akt pathway has been implicated in mediating the association
between ROS and decreased sensitivity to induced apoptosis
(34). Additionally,
cancer-associated fibroblast-secreted C-X-C motif chemokine ligand
1 has been documented to inhibit the expression of the
ROS-scavenging enzyme, SOD1, thereby leading to increased ROS
accumulation and downstream enhanced DNA repair and radioresistance
(35). In the present study,
irradiated H460 cells that were cultured in CM were identified to
have higher levels of ROS and lower levels of SOD1 than those cells
in normal medium. Meanwhile, it was observed that both the ROS and
SOD1 levels of non-irradiated H460 cells were increased by CM,
compared with normal medium. The reason for the increase of ROS and
SOD1 may be the increase of ROS production, with the increased
level of SOD1 expression serving as a response to eliminate the
excess ROS produced in the cells. However, when the level of ROS
produced by the combination of CM and irradiation was increased, it
may have exceeded the threshold of the level of ROS that can induce
an increase in SOD1, manifesting as a decrease in the expression of
SOD1. Based on these results, it may be hypothesized that a
reduction in SOD1 degradation leads to an increase in ROS (35), thereby enhancing a cellular
adaptive response and promoting cell survival. However, if the ROS
content of a cell exceeds the standard adaptive response, cell
death is induced.
Cell cycle arrest provides an opportunity for cells
to repair their DNA. In the present study, a greater proportion of
irradiated H460 cells incubated with CM were in the G2/M phase of
the cell cycle. In addition, cyclin A was observed to be
significantly upregulated following culture in CM for 2 h after
irradiation. γH2AX is a marker of damage repair and may indicate
the extent of DNA repair that has occurred (36). At 2-12 h after irradiation, the
irradiated H460 cells cultured in CM were found to have
significantly lower expression of γH2AX foci. Interestingly, longer
incubation times appeared to result in increasing expression of
γH2AX in the non-irradiated H460 cells. This phenomenon may be due
to: i) the CM containing metabolites that exert cytotoxic effects
on cells; ii) the CM containing secreted proteins that increase the
expression of γH2AX in H460 cells; or iii) the potential for high
levels of γH2AX expression to promote cell regeneration and
proliferation, as previously demonstrated (37). In general, the results of the
current study suggest an ability of CM from H460 cells to promote
DNA repair in H460 cells treated with 4 Gy irradiation.
In radiation-resistant cells, HR serves an important
role in the repair of damage due to DSBs in late S/G2 phase
(24). Rad51 is an important
indicator of HR repair, and blockade of Rad51 has been demonstrated
to enhance the radiosensitivity of cells (38). The findings of the present study
further confirm that HR repair may promote radioresistance by
enhancing the expression of Rad51 in H460 cells cultured in CM
post-γ-ray irradiation. Moreover, to maintain genome stability and
promote cell growth, the MRN complex participates in HR repair by
activating and recruiting ATM to the DNA cleavage end, while also
contributing to DNA repair and cell cycle control processes
(39-41). In the present study, expression of
the MRN complex and levels of p-AMT/ATM were seemingly enhanced by
autocrine factors secreted by the H460 cells.
Previous study has investigated the effect of
individual cytokines in the tumor microenvironment on tumor
radiation resistance (15). In the
present study, enhanced radioresistance of H460 cells was examined
in the presence of a milieu of autocrine factors, and their effects
were indicated to be independent of the presence of exosomes.
Furthermore, promotion of radiation resistance appeared to be
mainly mediated via enhancement of DNA repair capacity. In future
studies by our group, a proteomic analysis will be conducted to
further investigate the specific mechanism by which H460 cells
exhibit enhanced radioresistance via autocrine signaling, which is
anticipated to provide novel insights in the study of
radioresistance mechanisms.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 31670859
and 81302803), the Concord Youth Fund, special funds of the Central
University Basic Scientific Research Business (grant no.
3332016100), the Concord Small-Scale Characteristics of School
Funding (grant no. 10023201601602) and the Chinese Academy of
Medical Sciences Medical and Health Science and Technology
Innovation Project (grant no. 2017-I2M-1-021).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors’ contributions
SW, LD and QL conceived and designed the study. SW,
PG and NL performed the experiments. PC, JW, NH and KJ contributed
to the analysis and interpretation of the data. SW wrote the paper.
All authors approved the final version of the manuscript to be
published.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Abbreviations:
|
CM
|
conditioned medium
|
|
IR
|
ionizing radiation
|
|
DSBs
|
DNA double-strand breaks
|
|
HR
|
homologous recombination
|
|
NHEJ
|
non-homologous end-joining
|
|
NSCLC
|
non-small cell lung cancer
|
|
ROS
|
reactive oxygen species
|
|
VEGF
|
vascular endothelial growth factor
|
|
PI3K
|
phosphatidylinositol-4,5-bisphosphate
3-kinase
|
|
ATM
|
ATM serine/threonine kinase
|
|
DMEM
|
Dulbecco’s modified Eagle’s medium
|
|
FBS
|
fetal bovine serum
|
|
PBS
|
phosphate-buffered saline
|
|
DCFH-DA
|
dichlorodihydrofluorescein
diacetate
|
|
BSA
|
bovine serum albumin
|
|
SOD
|
superoxide dismutase
|
|
γH2AX
|
γH2A histone family member X
|
|
MRN
|
Mre11-Rad50-Nbs1 complex
|
|
CD9
|
cluster of differentiation 9
|
|
TSG101
|
anti-tumor susceptibility gene 101
|
|
PI
|
propidium iodide
|
|
p-
|
phosphorylated
|
Acknowledgments
This study was deposited in the Dissertation
Management System of Peking Union Medical College (Tianjin, China;
http://dissertation.imicams.ac.cn/index.action) as
part of the Master of Research thesis of Miss Shuang Wang.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Z, Fillmore CM, Hammerman PS, Kim CF
and Wong KK: Non-small-cell lung cancers: A heterogeneous set of
diseases. Nat Rev Cancer. 14:535–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pathak AK, Bhutani M, Mohan A, Guleria R,
Bal S and Kochupillai V: Non small cell lung cancer (NSCLC):
current status and future prospects. Indian J Chest Dis Allied Sci.
46:191–203. 2004.PubMed/NCBI
|
|
4
|
Verma V, McMillan MT, Grover S and Simone
CB II: Stereotactic body radiation therapy and the influence of
chemotherapy on overall survival for large (≥5 centimeter)
non-small cell lung cancer. Int J Radiat Oncol Biol Phys.
97:146–154. 2017. View Article : Google Scholar
|
|
5
|
Arechaga-Ocampo E, Lopez-Camarillo C,
Villegas-Sepulveda N, Gonzalez-De la Rosa CH, Perez-Añorve IX,
Roldan-Perez R, Flores-Perez A, Peña-Curiel O, Angeles-Zaragoza O,
Rangel Corona R, et al: Tumor suppressor miR-29c regulates
radioresistance in lung cancer cells. Tumour Biol.
39:10104283176950102017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hsieh CH, Lee CH, Liang JA, Yu CY and Shyu
WC: Cycling hypoxia increases U87 glioma cell radioresistance via
ROS induced higher and long-term HIF-1 signal transduction
activity. Oncol Rep. 24:1629–1636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Desai A, Webb B and Gerson SL: CD133+
cells contribute to radioresistance via altered regulation of DNA
repair genes in human lung cancer cells. Radiother Oncol.
110:538–545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Condon LT, Ashman JN, Ell SR, Stafford ND,
Greenman J and Cawkwell L: Overexpression of Bcl-2 in squamous cell
carcinoma of the larynx: A marker of radioresistance. Int J Cancer.
100:472–475. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Efimova E, Khodarev N, Darga T, Labay E,
Levina V, Lokshin A and Weichselbaum R: Tumor radioresistance is
linked to resistance to death signaling pathways associated with
tumor microenvironment. Proceedings of the 98th AACR Annual
Meeting. American Association for Cancer Research; Los Angeles, CA.
2007;
|
|
10
|
Chan R, Sethi P, Jyoti A, McGarry R and
Upreti M: Investigating the radioresistant properties of lung
cancer stem cells in the context of the tumor microenvironment.
Radiat Res. 185:169–181. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schlehaider U, Hill HZ, Pashapour A and
Hill GJ: Influence of an autocrine multitherapy resistance factor
on radiation responses of melanoma cells. Melanoma Res. 4:21–27.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Neta R, Perlstein R, Vogel SN, Ledney GD
and Abrams J: Role of interleukin 6 (IL-6) in protection from
lethal irradiation and in endocrine responses to IL-1 and tumor
necrosis factor. J Exp Med. 175:689–694. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bougen NM, Steiner M, Pertziger M,
Banerjee A, Brunet-Dunand SE, Zhu T, Lobie PE and Perry JK:
Autocrine human GH promotes radioresistance in mammary and
endometrial carcinoma cells. Endocr Relat Cancer. 19:625–644. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Knizetova P, Ehrmann J, Hlobilkova A,
Vancova I, Kalita O, Kolar Z and Bartek J: Autocrine regulation of
glioblastoma cell cycle progression, viability and radioresistance
through the VEGF-VEGFR2 (KDR) interplay. Cell Cycle. 7:2553–2561.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Centurione L and Aiello FB: DNA Repair and
Cytokines: TGF-β, IL-6, and Thrombopoietin as Different Biomarkers
of Radioresistance. Front Oncol. 6:1752016. View Article : Google Scholar
|
|
16
|
Keller S, Sanderson MP, Stoeck A and
Altevogt P: Exosomes: From biogenesis and secretion to biological
function. Immunol Lett. 107:102–108. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mutschelknaus L, Peters C, Winkler K,
Yentrapalli R, Heider T 1, Atkinson MJ and Moertl S: Exosomes
derived from squamous head and neck cancer promote cell survival
after ionizing radiation. PLoS One. 11:e01522132016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raimondo S, Saieva L, Corrado C, Fontana
S, Flugy A, Rizzo A, De Leo G and Alessandro R: Chronic myeloid
leukemia-derived exosomes promote tumor growth through an autocrine
mechanism. Cell Commun Signal. 13:82015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prasad S, Gupta SC and Tyagi AK: Reactive
oxygen species (ROS) and cancer: Role of antioxidative
nutraceuticals. Cancer Lett. 387:95–105. 2017. View Article : Google Scholar
|
|
20
|
Pagano M, Pepperkok R, Verde F, Ansorge W
and Draetta G: Cyclin A is required at two points in the human cell
cycle. EMBO J. 11:961–971. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mateo F, Vidal-Laliena M, Canela N, Busino
L, Martinez-Balbas MA, Pagano M, Agell N and Bachs O: Degradation
of cyclin A is regulated by acetylation. Oncogene. 28:2654–2666.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Celeste A, Petersen S, Romanienko PJ,
Fernandez-Capetillo O, Chen HT, Sedelnikova OA, Reina-San-Martin B,
Coppola V, Meffre E, Difilippantonio MJ, et al: Genomic instability
in mice lacking histone H2AX. Science. 296:922–927. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang JA, Liu BH, Shao LM, Guo ZT, Yang Q,
Wu LQ, Ji BW, Zhu XN, Zhang SQ, Li CJ, et al: LRIG1 enhances the
radio-sensitivity of radioresistant human glioblastoma U251 cells
via attenuation of the EGFR/Akt signaling pathway. Int J Clin Exp
Pathol. 8:3580–3590. 2015.
|
|
24
|
Tamulevicius P, Wang M and Iliakis G:
Homology-directed repair is required for the development of
radioresistance during S phase: Interplay between double-strand
break repair and checkpoint response. Radiat Res. 167:1–11. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schey KL, Luther JM and Rose KL:
Proteomics characterization of exosome cargo. Methods. 87:75–82.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ji X, Ji J, Shan F, Zhang Y, Chen Y and Lu
X: Cancer-associated fibroblasts from NSCLC promote the
radioresistance in lung cancer cell lines. Int J Clin Exp Med.
8:7002–7008. 2015.PubMed/NCBI
|
|
27
|
Chen Y, Tsai Y, Hsu JW, Duan SZ, Keng P
and Lee SO: Abstract LB-210: IL-6 promotes stemness of cancer stem
cells and may contribute to radiation resistance of non-small cell
lung cancer. Cancer Res. 74(Suppl 19): LB-2102014. View Article : Google Scholar
|
|
28
|
Ma C, Nguyen H, Paradiso L, Putz U, Luwor
R, Kaye A and Morokoff A: P08.35 Exosomes derived from Glioma Stem
Cells (GSCs) promote cell migration, proliferation and radiation
resistance in brain cancer. Neuro Oncol. 19(Suppl 3): iii612017.
View Article : Google Scholar :
|
|
29
|
Teng K, Zhang Y, Hu X, Ding Y, Gong R and
Liu L: Nimotuzumab enhances radiation sensitivity of NSCLC H292
cells in vitro by blocking epidermal growth factor receptor nuclear
translo-cation and inhibiting radiation-induced DNA damage repair.
OncoTargets Ther. 8:809–818. 2015. View Article : Google Scholar
|
|
30
|
McLaughlin KA, Nemeth Z, Bradley CA,
Humphreys L, Stasik I, Fenning C, Majkut J, Higgins C, Crawford N,
Holohan C, et al: FLIP: A Targetable Mediator of Resistance to
Radiation in Non-Small Cell Lung Cancer. Mol Cancer Ther.
15:2432–2441. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jella KK, Rani S, O’Driscoll L, McClean B,
Byrne HJ and Lyng FM: Exosomes are involved in mediating radiation
induced bystander signaling in human keratinocyte cells. Radiat
Res. 181:138–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu S, Wang J, Ding N, Hu W, Zhang X, Wang
B, Hua J, Wei W and Zhu Q: Exosome-mediated microRNA transfer plays
a role in radiation-induced bystander effect. RNA Biol.
12:1355–1363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie Q, Zhou Y, Lan G, Yang L, Zheng W,
Liang Y and Chen T: Sensitization of cancer cells to radiation by
selenadiazole derivatives by regulation of ROS-mediated DNA damage
and ERK and AKT pathways. Biochem Biophys Res Commun. 449:88–93.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Clerkin JS, Naughton R, Quiney C and
Cotter TG: Mechanisms of ROS modulated cell survival during
carcinogenesis. Cancer Lett. 266:30–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang H, Yue J, Jiang Z, Zhou R, Xie R, Xu
Y and Wu S: CAF-secreted CXCL1 conferred radioresistance by
regulating DNA damage response in a ROS-dependent manner in
esophageal squamous cell carcinoma. Cell Death Dis. 8:e27902017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cleaver JE: γH2Ax: Biomarker of damage or
functional participant in DNA repair ‘all that glitters is not
gold!’. Photochem Photobiol. 87:1230–1239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Turinetto V, Orlando L, Sanchez-Ripoll Y,
Kumpfmueller B, Storm MP, Porcedda P, Minieri V, Saviozzi S,
Accomasso L, Cibrario Rocchietti E, et al: High basal γH2AX levels
sustain self-renewal of mouse embryonic and induced pluripotent
stem cells. Stem Cells. 30:1414–1423. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen X, Wong P, Radany EH, Stark JM,
Laulier C and Wong JY: Suberoylanilide hydroxamic acid as a
radiosen-sitizer through modulation of RAD51 protein and inhibition
of homology-directed repair in multiple myeloma. Mol Cancer Res.
10:1052–1064. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Limbo O, Chahwan C, Yamada Y, de Bruin RA,
Wittenberg C and Russell P: Ctp1 is a cell-cycle-regulated protein
that functions with Mre11 complex to control double-strand break
repair by homologous recombination. Mol Cell. 28:134–146. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Helmink BA, Bredemeyer AL, Lee BS, Huang
CY, Sharma GG, Walker LM, Bednarski JJ, Lee WL, Pandita TK, Bassing
CH, et al: MRN complex function in the repair of chromosomal
Rag-mediated DNA double-strand breaks. J Exp Med. 206:669–679.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lavin MF, Kozlov S, Gatei M and Kijas AW:
ATM-Dependent Phosphorylation of All Three Members of the MRN
Complex: From Sensor to Adaptor. Biomolecules. 5:2877–2902. 2015.
View Article : Google Scholar : PubMed/NCBI
|