Introduction
Colorectal carcinoma (CRC) remains one of the most
common malignancies worldwide and represents a global health
problem (1). An increasing number
of Asian countries, including China, Japan, South Korea and
Singapore, have experienced a 2- to 4-fold increase in the
incidence of CRC over the last few decades (2). The pathogenesis of CRC ordinarily
occurs in a staged progression from normal colonic mucosa to
adenoma and finally to carcinoma over a period of ~7–10 years
(3–5). This sequenced progression over time
provides an opportunity for early diagnosis and treatment.
It has previously been indicated that delayed
diagnosis is the main reason for a poor prognosis (6). The traditional non-invasive and
invasive methods of screening modalities include fecal occult blood
testing, fecal immunochemical test, double-contrast barium enema,
flexible sigmoidoscopy and colonoscopy (7–9).
Although some of these screening modalities have been demonstrated
to reduce the rates of malignancy or mortality, there remains the
issue of reducing cancer-related mortality by removing premalignant
adenomas and early localized cancer prior to the onset of more
advanced stages. Therefore, an effective approach to early
screening, diagnosis and follow-up monitoring of CRC is
required.
Over the last few years, extensive investigations
have focused on serum tumor markers (STMs). In patients with CRC,
single STMs exhibit low sensitivity and specificity, whereas the
simultaneous measurement of several STMs may increase their
diagnostic accuracy (10). It was
demonstrated that the combined use of carcionembryonic antigen
(CEA) and carbohydrate antigen (CA) 19-9 was effective in the
screening and diagnosis of CRC (11). CA724 was previously identified as a
type of STM specific for gastric cancer (12) and the correlation between CA724 and
CRC has been attracting increasing attention (13).
Furthermore, due to the numerous characteristics
shared by colon carcinoma (CC) and rectal carcinoma (RC), the two
are discussed as a single entity. Whether CC and RC should be
considered as a single or two distinct entities remains
controversial (14). The aim of
the present study was to investigate serum CA724 levels in patients
with different clinical stages of CC and RC and analyze the
correlation between serum CA724 levels and the clinical stages of
CRC.
Patients and methods
Patients
In this study, a total of 100 patients (63 male and
37 female) with histologically confirmed CRC were investigated. The
patient sample was comprised of 51 patients with CC (32 male and 19
female) and 49 patients with RC (31 male and 18 female). The CC and
RC patients were classified into four stages according to the 2003
TNM classification. Stages I and II were considered as early-stage,
whereas stages III and IV were considered as advanced-stage
disease. The patients in the advanced-stage group had either lymph
node (stage III) or distant metastases (stage IV). Patient
information, such as gender, age and pathological TNM staging, is
summarized in Table I. The ethics
committee for the Second Affiliated Hospital of Dalian Medical
University provided approval for this study (Dalian, China)
 | Table I.Clinicopathological characteristics of
100 CRC patients. |
Table I.
Clinicopathological characteristics of
100 CRC patients.
| Variables | No. of CC | No. of RC |
|---|
| Gender | | |
| Male | 32 | 31 |
| Female | 19 | 18 |
| Age (years) | | |
| ≤50 | 9 | 11 |
| >50 | 42 | 38 |
| Lymph node
metastasis | | |
| No | 18 | 22 |
| Yes | 33 | 27 |
| Distant
metastasis | | |
| No | 35 | 39 |
| Yes | 16 | 10 |
| Stage | | |
| I | 6 | 14 |
| II | 12 | 8 |
| III | 17 | 17 |
| IV | 16 | 10 |
Serum collection and CA724 assay
The values of CA724 were measured prior to the
patients receiving radiation treatment or chemotherapy. Blood
samples were collected, separated by centrifugation and the serum
samples were stored at −20°C until assays were performed. The CA724
kit was provided by Diagnostic Products Corporation (DPC, Tianjin,
China). The serum CA724 levels were determined with an
immunoradiometric gamma counter (DPC-GAMMA-C12; DPC). The patient
details were furnished by the First and Second Hospitals affiliated
to Dalian Medical University, between 2010 and 2012.
Statistical analysis
The CA724 levels were assessed in different groups
according to different site, TNM classification, gender, age and
metastatic status of the patients. Since the serum CA724 levels in
each group did not follow a normal distribution, the statistical
significance of the differences between the groups was calculated
by non-parametric statistics (Mann-Whitney and Kruskall-Wallis
tests). The centralized tendency of each group was described as
means plus standard error of the mean (SEM). All statistical tests
were performed using SPSS software, version 11.5 (SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Differences among CRC patients in
different groups
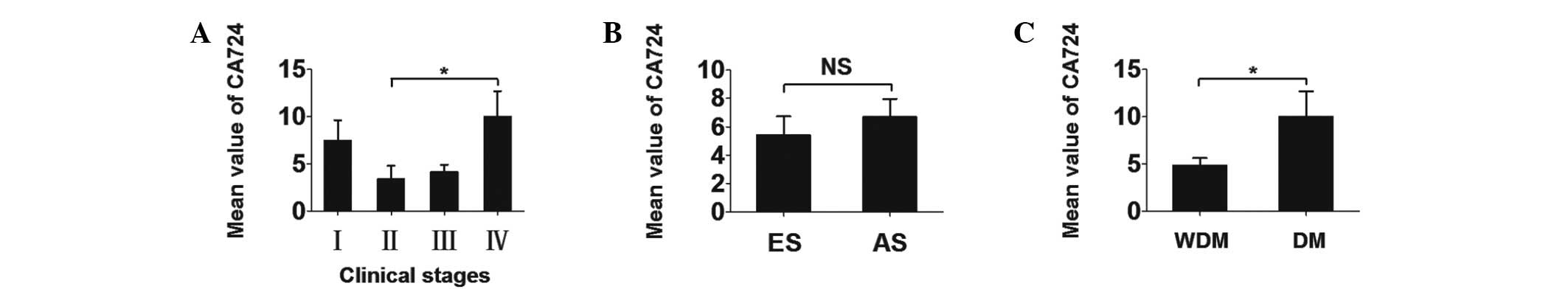
The mean serum CA724 values ± SEM were 6.808±1.462
U/ml in CC patients and 5.524±1.151 U/ml in RC patients, with no
statistically significant difference. Therefore, we first analyzed
the two types of cancer as a single entity. The different CA724
levels among CRC patients in different groups are presented in
Table II. The differences in the
CA724 values between CRC clinical stages were compared (Fig. 1). Further investigations indicated
that the mean serum CA724 concentrations ± SEM in patients with
early- and advanced-stage disease were 5.414±1.317 and 6.689±1.281
U/ml, respectively. The differences between the two groups were not
statistically significant. However, the differences between the CRC
patient group with and that without distant metastasis was
statistically significant (P=0.043).
 | Table II.Differences in CA724 levels among
patients with colorectal carcinoma in different groups. |
Table II.
Differences in CA724 levels among
patients with colorectal carcinoma in different groups.
| Variables | Pt no. (CA724
levelsa) | P-value |
|---|
| Site | | NS |
| Colon | 51 (6.808±1.462) | |
| Rectum | 49 (5.524±1.151) | |
| Lymph node
metastasis | | NS |
| No | 40 (5.414±1.317) | |
| Yes | 60 (6.689±1.281) | |
| Distant
metastasis | | 0.043 |
| No | 74 (4.835±0.791) | |
| Yes | 26
(10.005±2.677) | |
| Stage | | NS |
| I | 20 (7.464±2.156) | |
| II | 20 (3.364±1.426) | |
| III | 34 (4.153±0.760) | |
| IV | 26
(10.005±2.677) | |
| Gender | | NS |
| Male | 63 (5.771±1.039) | |
| Female | 37 (7.148±1.798) | |
| Age | | NS |
| 30–50 | 20 (5.026±1.560) | |
| >50 | 80 (6.467±1.096) | |
To determine whether gender and age affected the
results in CRC patients mentioned above, the differences in the
serum CA724 values between genders was also assessed. Table III shows that there were no
significant differences between male and female CRC patients.
Furthermore, there were no significant differences between CRC
patients aged 30–50 years and those aged >50 years. Therefore,
the variables of gender and age were eliminated (Table II). Since there were distinct
differences among CRC patients, in order to interpret the
differences described above when assessing the two groups as a
single entity, we proceeded to analyze CC and RC separately.
 | Table III.Differences in CA724 levels among CC
patients. |
Table III.
Differences in CA724 levels among CC
patients.
| Variables | Pt no. (CA724
levelsa) | P-value |
|---|
| Lymph node
metastasis | | <0.001 |
| No | 18 (1.663±0.237) | |
| Yes | 33 (9.614±2.109) | |
| Distant
metastasis | | <0.001 |
| No | 35
(3.143±0.493) | |
| Yes | 16
(14.985±3.875) | |
| Stage | | <0.001 |
| I | 6
(1.437±0.535) | |
| II | 12
(1.777±0.249) | |
| III | 17
(4.559±0.855) | |
| IV | 16
(14.985±3.875) | |
| Gender | | NS |
| Male | 32
(6.451±1.508) | |
| Female | 19
(7.409±3.051) | |
| Age (years) | | NS |
| 30–50 | 9
(4.664±1.160) | |
| >50 | 42
(7.267±1.755) | |
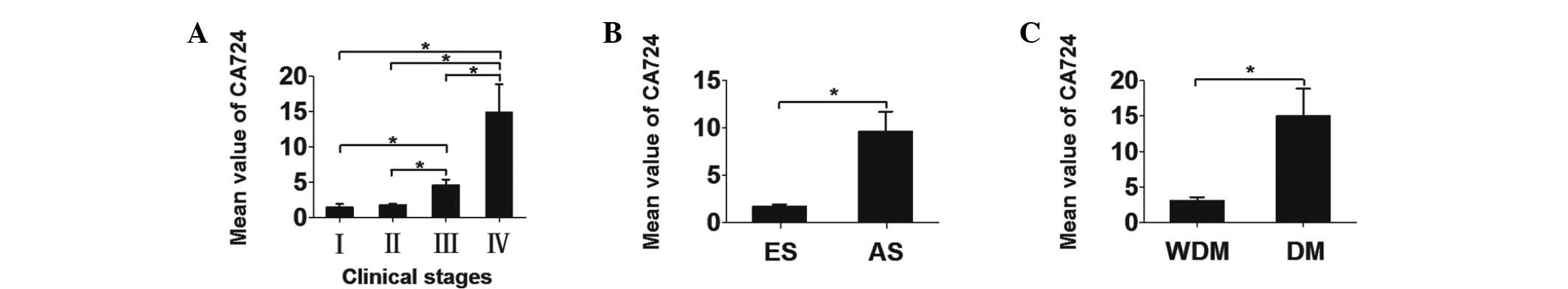
Ascending gradient among CC patients
The different CA724 levels among CC patients in
different groups are presented in Table III. There was an ascending gradient
of serum CA724 values with increasing clinical stage, except for
the difference between stages I and II (Fig. 2). The mean CA724 values ± SEM for
each stage were as follows: stage I, 1.437±0.535 U/ml; stage II,
1.777±0.249 U/ml; stage III, 4.559±0.855 U/ml; and stage IV,
14.985±3.875 U/ml. The differences in the CA724 values between
early- and advanced-stage disease were statistically significant
(P<0.001), as were those between patients with and those without
distant metastasis (P<0.001) (Fig.
2).
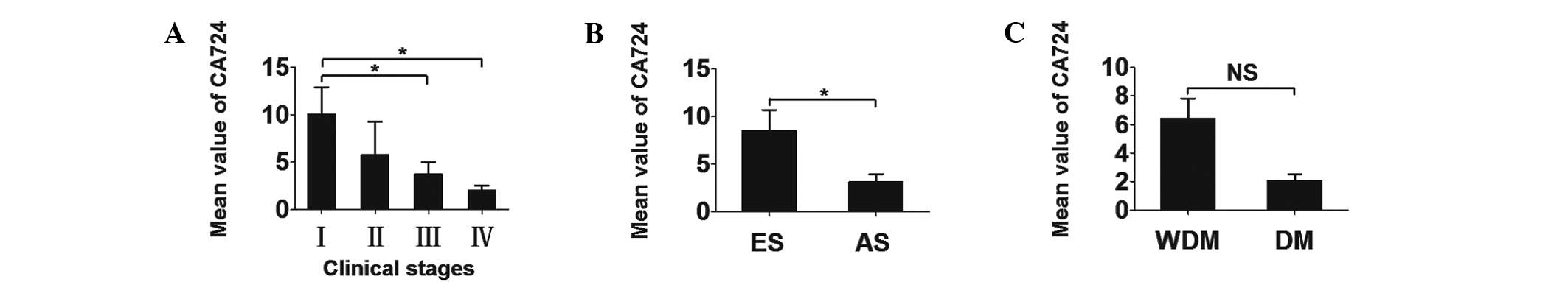
Descending gradient among RC
patients
The different CA724 levels among CC patients in
different groups are presented in Table IV. The mean serum CA724 values ±
SEM for each stage were as follows: stage I, 10.046±2.819 U/ml;
stage II, 5.745±3.506 U/ml; stage III, 3.748±1.277 U/ml; and stage
IV, 2.036±0.491 U/ml, these results are based on the data provided
in Table V. Therefore, a
descending gradient was identified among RC patients when each
clinical stage was separately analyzed (Fig. 3). Similarly, analysis with SPSS
software, version 11.5, revealed a significant correlation between
early- and advanced-stage disease (P=0.010). However, the
differences in the CA724 values between patients with and those
without distant metastasis did not reach a statistical significance
(Fig. 3).
 | Table IV.Differences in CA724 levels among RC
patients. |
Table IV.
Differences in CA724 levels among RC
patients.
| Variables | Pt no. (CA724
levelsa) | P-value |
|---|
| Lymph node
metastasis | | 0.011 |
| No | 22
(8.482±2.196) | |
| Yes | 27
(3.114±0.830) | |
| Distant
metastasis | | NS |
| No | 39
(6.419±1.396) | |
| Yes | 10 (
2.036±0.491) | |
| Stage | | 0.022 |
| I | 14
(10.046±2.819) | |
| II | 8
(5.745±3.506) | |
| III | 17
(3.748±1.277) | |
| IV | 10
(2.036±0.491) | |
| Gender | | NS |
| Male | 31
(5.069±1.442) | |
| Female | 18
(6.308±1.905) | |
| Age (years) | | NS |
| 30–50 | 11
(5.322±2.739) | |
| >50 | 38
(5.583±1.261) | |
 | Table V.Clinicopathological characteristics
of 100 patients with colorectal carcinoma. |
Table V.
Clinicopathological characteristics
of 100 patients with colorectal carcinoma.
| No. | Pathological
diagnosis | Site | Pathological TNM
staging | Lesion | Age/gender | CA724 |
|---|
| 1 | Adenocarcinoma | Colon | T2N0M0 | Primary tumor | 61/M | 4.05 |
| 2 | Adenocarcinoma | Colon | T2N0M0 | Primary tumor | 77/M | 1.19 |
| 3 | Adenocarcinoma | Colon | T2N0M0 | Primary tumor | 61/M | 1.13 |
| 4 | Adenocarcinoma | Colon | T1N0M0 | Primary tumor | 63/F | 1.09 |
| 5 | Adenocarcinoma | Colon | T2N0M0 | Primary tumor | 69/M | 0.59 |
| 6 | Adenocarcinoma | Colon | T2N0M0 | Primary tumor | 58/F | 0.57 |
| 7 | Adenocarcinoma | Colon | T4N0M0 | Primary tumor | 65/F | 3.44 |
| 8 | Adenocarcinoma | Colon | T4N0M0 | Primary tumor | 61/F | 3.04 |
| 9 | Adenocarcinoma | Colon | T4N0M0 | Primary tumor | 56/F | 2.78 |
| 10 | Adenocarcinoma | Colon | T3N0M0 | Primary tumor | 75/F | 1.81 |
| 11 | Adenocarcinoma | Colon | T4N0M0 | Primary tumor | 64/M | 1.71 |
| 12 | Adenocarcinoma | Colon | T3N0M0 | Primary tumor | 48/M | 1.67 |
| 13 | Adenocarcinoma | Colon | T3N0M0 | Primary tumor | 33/M | 1.49 |
| 14 | Adenocarcinoma | Colon | T4N0M0 | Primary tumor | 51/F | 1.38 |
| 15 | Adenocarcinoma | Colon | T3N0M0 | Primary tumor | 55/M | 1.24 |
| 16 | Adenocarcinoma | Colon | T4N0M0 | Primary tumor | 67/M | 1.10 |
| 17 | Adenocarcinoma | Colon | T3N0M0 | Primary tumor | 69/M | 0.85 |
| 18 | Adenocarcinoma | Colon | T4N0M0 | Primary tumor | 48/F | 0.81 |
| 19 | Adenocarcinoma | Colon | T4N1M0 | Primary tumor | 65/M | 11.90 |
| 20 | Adenocarcinoma | Colon | T3N1M0 | Primary tumor | 64/M | 10.22 |
| 21 | Adenocarcinoma | Colon | T3N1M0 | Primary tumor | 45/M | 8.92 |
| 22 | Adenocarcinoma | Colon | T4N1M0 | Primary tumor | 52/F | 8.44 |
| 23 | Adenocarcinoma | Colon | T3N1M0 | Primary tumor | 60/F | 6.44 |
| 24 | Adenocarcinoma | Colon | T4N1M0 | Primary tumor | 45/F | 6.31 |
| 25 | Adenocarcinoma | Colon | T3N1M0 | Primary tumor | 51/M | 4.78 |
| 26 | Adenocarcinoma | Colon | T3N2M0 | Primary tumor | 61/M | 3.86 |
| 27 | Adenocarcinoma | Colon | T3N2M0 | Primary tumor | 62/F | 3.38 |
| 28 | Adenocarcinoma | Colon | T3N1M0 | Primary tumor | 35/M | 2.90 |
| 29 | Adenocarcinoma | Colon | T3N2M0 | Primary tumor | 55/M | 2.05 |
| 30 | Adenocarcinoma | Colon | T3N2M0 | Primary tumor | 54/F | 1.82 |
| 31 | Adenocarcinoma | Colon | T3N1M0 | Primary tumor | 70/F | 1.74 |
| 32 | Adenocarcinoma | Colon | T3N1M0 | Primary tumor | 68/M | 1.67 |
| 33 | Adenocarcinoma | Colon | T3N1M0 | Primary tumor | 67/M | 1.09 |
| 34 | Adenocarcinoma | Colon | T3N1M0 | Primary tumor | 66/M | 1.03 |
| 35 | Adenocarcinoma | Colon | T4N1M0 | Primary tumor | 67/F | 0.95 |
| 36 | Adenocarcinoma | Colon | T2N0M1 | Primary tumor | 66/F | 57.79 |
| 37 | Adenocarcinoma | Colon | T3N0M1 | Primary tumor | 81/M | 43.30 |
| 38 | Adenocarcinoma | Colon | T2N0M1 | Primary tumor | 70/F | 19.99 |
| 39 | Adenocarcinoma | Colon | T4N2M1 | Primary tumor | 61/M | 19.90 |
| 40 | Adenocarcinoma | Colon | TxNxM1 | Primary tumor | 64/F | 16.56 |
| 41 | Adenocarcinoma | Colon | T4N2M1 | Primary tumor | 56/M | 16.17 |
| 42 | Adenocarcinoma | Colon | TxNxM1 | Primary tumor | 59/M | 15.15 |
| 43 | Adenocarcinoma | Colon | T4N1M1 | Primary tumor | 80/M | 12.23 |
| 44 | Adenocarcinoma | Colon | T3N2M1 | Primary tumor | 37/M | 10.76 |
| 45 | Adenocarcinoma | Colon | T4N2M1 | Primary tumor | 75/M | 7.37 |
| 46 | Adenocarcinoma | Colon | TxNxM1 | Primary tumor | 74/M | 7.03 |
| 47 | Adenocarcinoma | Colon | T4N1M1 | Primary tumor | 42/M | 5.54 |
| 48 | Adenocarcinoma | Colon | T4N0M1 | Primary tumor | 42/M | 3.58 |
| 49 | Adenocarcinoma | Colon | T4N0M1 | Primary tumor | 74/F | 2.43 |
| 50 | Adenocarcinoma | Colon | TxNxM1 | Primary tumor | 36/M | 1.32 |
| 51 | Adenocarcinoma | Colon | T3N0M1 | Primary tumor | 61/M | 0.64 |
| 52 | Adenocarcinoma | Rectum | T2N0M0 | Primary tumor | 66/M | 29.60 |
| 53 | Adenocarcinoma | Rectum | T2N0M0 | Primary tumor | 72/M | 26.03 |
| 54 | Adenocarcinoma | Rectum | T2N0M0 | Primary tumor | 79/M | 24.86 |
| 55 | Adenocarcinoma | Rectum | T2N0M0 | Primary tumor | 56/F | 18.30 |
| 56 | Adenocarcinoma | Rectum | T2N0M0 | Primary tumor | 55/M | 16.02 |
| 57 | Adenocarcinoma | Rectum | T2N0M0 | Primary tumor | 67/F | 6.48 |
| 58 | Adenocarcinoma | Rectum | T2N0M0 | Primary tumor | 73/F | 3.93 |
| 59 | Adenocarcinoma | Rectum | T2N0M0 | Primary tumor | 65/M | 3.91 |
| 60 | Adenocarcinoma | Rectum | T2N0M0 | Primary tumor | 57/M | 2.35 |
| 61 | Adenocarcinoma | Rectum | T2N0M0 | Primary tumor | 78/M | 2.14 |
| 62 | Adenocarcinoma | Rectum | T2N0M0 | Primary tumor | 62/F | 2.07 |
| 63 | Adenocarcinoma | Rectum | T2N0M0 | Primary tumor | 59/M | 1.95 |
| 64 | Adenocarcinoma | Rectum | T2N0M0 | Primary tumor | 79/F | 1.88 |
| 65 | Adenocarcinoma | Rectum | T2N0M0 | Primary tumor | 72/F | 1.13 |
| 66 | Adenocarcinoma | Rectum | T3N0M0 | Primary tumor | 55/F | 29.85 |
| 67 | Adenocarcinoma | Rectum | T3N0M0 | Primary tumor | 67/F | 5.92 |
| 68 | Adenocarcinoma | Rectum | T4N0M0 | Primary tumor | 54/M | 4.27 |
| 69 | Adenocarcinoma | Rectum | T3N0M0 | Primary tumor | 55/F | 2.06 |
| 70 | Adenocarcinoma | Rectum | T3N0M0 | Primary tumor | 67/M | 1.40 |
| 71 | Adenocarcinoma | Rectum | T3N0M0 | Primary tumor | 66/F | 0.95 |
| 72 | Adenocarcinoma | Rectum | T3N0M0 | Primary tumor | 67/F | 0.83 |
| 73 | Adenocarcinoma | Rectum | T4N0M0 | Primary tumor | 73/M | 0.68 |
| 74 | Adenocarcinoma | Rectum | T4N2M0 | Primary tumor | 38/F | 15.27 |
| 75 | Adenocarcinoma | Rectum | T3N1M0 | Primary tumor | 79/F | 14.91 |
| 76 | Adenocarcinoma | Rectum | T3N1M0 | Primary tumor | 68/M | 13.70 |
| 77 | Adenocarcinoma | Rectum | T4N2M0 | Primary tumor | 48/M | 3.14 |
| 78 | Adenocarcinoma | Rectum | T3N1M0 | Primary tumor | 72/M | 3.13 |
| 79 | Adenocarcinoma | Rectum | T4N2M0 | Primary tumor | 73/M | 2.93 |
| 80 | Adenocarcinoma | Rectum | T4N1M0 | Primary tumor | 57/M | 1.48 |
| 81 | Adenocarcinoma | Rectum | T3N1M0 | Primary tumor | 55/M | 1.14 |
| 82 | Adenocarcinoma | Rectum | T2N1M0 | Primary tumor | 71/M | 1.13 |
| 83 | Adenocarcinoma | Rectum | T3N1M0 | Primary tumor | 54/M | 1.05 |
| 84 | Adenocarcinoma | Rectum | T3N1M0 | Primary tumor | 47/F | 1.00 |
| 85 | Adenocarcinoma | Rectum | T3N1M0 | Primary tumor | 48/M | 0.96 |
| 86 | Adenocarcinoma | Rectum | T4N1M0 | Primary tumor | 69/M | 0.91 |
| 87 | Adenocarcinoma | Rectum | T4NxM0 | Primary tumor | 76/M | 0.86 |
| 88 | Adenocarcinoma | Rectum | T4N1M0 | Primary tumor | 43/M | 0.80 |
| 89 | Adenocarcinoma | Rectum | T3N2M0 | Primary tumor | 44/M | 0.67 |
| 90 | Adenocarcinoma | Rectum | T3N2M0 | Primary tumor | 72/M | 0.63 |
| 91 | Adenocarcinoma | Rectum | TxNxM1 | Primary tumor | 74/F | 5.80 |
| 92 | Adenocarcinoma | Rectum | TxN1M1 | Primary tumor | 54/M | 2.34 |
| 93 | Adenocarcinoma | Rectum | T4N1M1 | Primary tumor | 67/F | 0.95 |
| 94 | Adenocarcinoma | Rectum | T3N1M1 | Primary tumor | 62/M | 3.33 |
| 95 | Adenocarcinoma | Rectum | T4NxM1 | Primary tumor | 43/F | 1.59 |
| 96 | Adenocarcinoma | Rectum | T4NxM1 | Primary tumor | 61/M | 2.26 |
| 97 | Adenocarcinoma | Rectum | T4N1M1 | Primary tumor | 67/M | 1.32 |
| 98 | Adenocarcinoma | Rectum | TxNxM1 | Primary tumor | 39/M | 1.10 |
| 99 | Adenocarcinoma | Rectum | T4NxM1 | Primary tumor | 62/M | 1.04 |
| 100 | Adenocarcinoma | Rectum | T3N2M4 | Primary tumor | 53/F | 0.63 |
Discussion
This comprehensive integrative analysis of 100 CRC
patients indicated that serum CA724 levels may be of predictive
value in CRC, particularly in the analysis of clinical stage. As
regards CA724, recent studies have mainly focused on the
sensitivity and specificity of its diagnostic and early detection
value for recurrences. The combinations of CA724 with other STMs
were considered to be satisfactory (10,13,15–17).
In our study, we focused on the differences in the CA724 levels
among patients with different disease stages.
The differences in serum CA724 values between
patients with early- and advanced-stage disease suggest that CA724
may be associated with the metastasis of CC and RC. A similar
association was observed between CEA levels and CRC metastasis
(18,19). When comparing and analysing CC and
RC, we may surmise that the differences in the serum levels of
CA724 represent a sign of distant metastasis in CC. However, we did
not observe a statistically significant difference between RC
patients with and those without distant metastasis. We then
compared the clinical stages of CC and RC. Although we were unable
to verify a statistically significant difference for each stage
transition, there was a distinct variation tendency among the
stages. Therefore, we consider that monitoring serum CA724 levels
may be indicative of clinical stage, particularly in patients for
whom the determination of the pathological stage is difficult. The
CA724 value may reflect advanced stage, progression and metastasis
of CRC. Due to the widely variable prognosis of CRC, a previous
study attempted to identify a parameter useful in the selection of
patients who may be candidates for more tailored treatment
(20). Whether CA724 is such a
parameter requires further verification; however, our results
suggest that it has potential as a tumor marker. Considering the
association between CA724 and CRC, we may surmise the presence of
similar associations between other STMs and carcinomas.
Our study demonstrated the presence of two opposite
trends in the levels of CA724 according to the progression of the
clinical CRC stage. As was mentioned previously, there was an
ascending gradient among CC patients and a descending gradient
among RC patients. These two opposite trends possibly reflect
biological, advancing and metastatic differences between CC and RC.
Due to the similarities in morphology and configuration and the
fact that one is considered to be the continuation of the other, CC
and RC are often considered as a single disease entity. However, an
increasing number of studies refer to the differences between these
two types of cancer. Firstly, the colon embryologically originates
from the midgut and hindgut, whereas the rectum originates from the
cloaca. Furthermore, relevant studies have demonstrated that there
were differences regarding blood supply, biological function,
histochemical reactions and the level of mRNA expression (21–26).
Those studies indicated that the normal colon and rectum are
different. A previous study also reported that the prognosis of CC
is better compared to that of RC and that RC exhibits a higher
expression of CEA15. Additionally, a gene-level analysis
demonstrated that methylation and mutations were more common in the
right colon (27). All those
results suggested that there are biological differences between CC
and RC. The exact mechanism underlying the descending gradient in
the CA724 levels among RC patients has not been elucidated.
Although this study included a small number of
patients, the results regarding the association between CA724
levels and CRC patients were considered to be statistically
significant. Due to the small number of stage I CC patients (n=6),
further investigations are required to confirm these findings.
Furthermore, the exact nature of the association and the underlying
pathophysiological mechanisms of the opposite trends of serum CA724
in CC and RC require further investigation by future large
prospective studies.
In conclusion, we demonstrated a close correlation
between serum CA724 levels and tumor migration in CRC. The opposite
variation tendencies of CA724 levels in the different evolution
groups of CC and RC may reflect the differences between these two
types of cancer. The evaluation of serum CA724 levels, particularly
elevation in patients with CC, may be of monitoring and predictive
value and may also assist in the development of treatment
strategies for CRC patients.
Acknowledgements
This study was supported by grants
from NSFC (31270867), the State Key Development Program of Basic
Research of China (2012CB822103) and the Department of Science and
Technology for Liaoning Province (2012225020).
References
|
1.
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2009. CA Cancer J Clin. 59:225–249. 2009. View Article : Google Scholar
|
|
2.
|
Sung JJ, Lau JY, Goh KL, et al: Increasing
incidence of colorectal cancer in Asia: implications for screening.
Lancet Oncol. 6:871–876. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Hofstad B and Vatn M: Growth rate of colon
polyps and cancer. Gastrointest Endosc Clin N Am. 7:345–363.
1997.PubMed/NCBI
|
|
4.
|
Winawer SJ, Fletcher RH, Miller L, et al:
Colorectal cancer screening: clinical guidelines and rationale.
Gastroenterology. 112:594–642. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Wong JJ, Hawkins NJ and Ward RL:
Colorectal cancer: a model for epigenetic tumorigenesis. Gut.
56:140–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Diallo Owono FK, Nguema Mve R, Ibaba J,
Mihindou C and Ondo N’dong F: Epidemiological and diagnostic
features of colorectal cancer in Libreville, Gabon. Med Trop
(Mars). 71:605–607. 2011.(In French).
|
|
7.
|
U.S. Preventive Services Task Force:
Screening for colorectal cancer: U.S. Preventive Services Task
Force recommendation statement. Ann Intern Med. 149:627–637. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Levin B, Lieberman DA, McFarland B, et al
American Cancer Society Colorectal Cancer Advisory Group; US
Multi-Society Task Force; American College of Radiology Colon
Cancer Committee: Screening and surveillance for the early
detection of colorectal cancer and adenomatous polyps, 2008: a
joint guideline from the American Cancer Society, the US
Multi-Society Task Force on Colorectal Cancer, and the American
College of Radiology. CA Cancer J Clin. 58:130–160. 2008.
View Article : Google Scholar
|
|
9.
|
Dominic OG, McGarrity T, Dignan M and
Lengerich EJ: American College of Gastroenterology Guidelines for
Colorectal Cancer Screening 2008. Am J Gastroenterol.
104:2626–2627; author reply 2628–2629. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Lumachi F, Marino F, Orlando R, Chiara GB
and Basso SM: Simultaneous multianalyte immunoassay measurement of
five serum tumor markers in the detection of colorectal cancer.
Anticancer Res. 32:985–988. 2012.PubMed/NCBI
|
|
11.
|
Wang JY, Lu CY, Chu KS, et al: Prognostic
significance of pre- and postoperative serum carcinoembryonic
antigen levels in patients with colorectal cancer. Eur Surg Res.
39:245–250. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Chen XZ, Zhang WK, Yang K, et al:
Correlation between serum CA724 and gastric cancer: multiple
analyses based on Chinese population. Mol Biol Rep. 39:9031–9039.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Nicolini A, Ferrari P, Duffy MJ, et al:
Intensive risk-adjusted follow-up with the CEA, TPA, CA19.9, and
CA72.4 tumor marker panel and abdominal ultrasonography to diagnose
operable colorectal cancer recurrences: effect on survival. Arch
Surg. 145:1177–1183. 2010. View Article : Google Scholar
|
|
14.
|
Li M, Li JY, Zhao AL and Gu J: Colorectal
cancer or colon and rectal cancer? Clinicopathological comparison
between colonic and rectal carcinomas. Oncology. 73:52–57. 2007.
View Article : Google Scholar
|
|
15.
|
Filella X, Molina R, Mengual PJ, et al:
Significance of CA72.4 in patients with colorectal cancer.
Comparison with CEA and CA19.9. J Nucl Biol Med. 35:158–161.
1991.PubMed/NCBI
|
|
16.
|
Yu JK, Yang MQ, Jiang TJ and Zheng S: The
optimal combination of serum tumor markers with bioinformatics in
diagnosis of colorectal carcinoma. J Zhejiang Univ. 33:407–410.
2004.(In Chinese).
|
|
17.
|
Newton KF, Newman W and Hill J: Review of
biomarkers in colorectal cancer. Colorectal Dis. 14:3–17. 2012.
View Article : Google Scholar
|
|
18.
|
Wiggers T, Arends JW, Verstijnen C, et al:
Prognostic significance of CEA immunoreactivity patterns in large
bowel carcinoma tissue. Br J Cancer. 54:409–414. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kitadai Y, Radinsky R, Bucana CD, et al:
Regulation of carcinoembryonic antigen expression in human colon
carcinoma cells by the organ microenvironment. Am J Pathol.
149:1157–1166. 1996.PubMed/NCBI
|
|
20.
|
Seregni E, Ferrari L, Martinetti A and
Bombardieri E: Diagnostic and prognostic tumor markers in the
gastrointestinal tract. Semin Surg Oncol. 20:147–166. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Araki K, Furuya Y, Kobayashi M, et al:
Comparison of mucosal microvasculature between the proximal and
distal human colon. J Electron Microsc (Tokyo). 45:202–206. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Skinner SA and O’Brien PE: The
microvascular structure of the normal colon in rats and humans. J
Surg Res. 61:482–490. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Devesa SS and Chow WH: Variation in
colorectal cancer incidence in the United States by subsite of
origin. Cancer. 71:3819–3826. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Shamsuddin AM, Phelps PC and Trump BF:
Human large intestinal epithelium: light microscopy,
histochemistry, and ultrastructure. Hum Pathol. 13:790–803. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Macfarlane GT, Gibson GR and Cummings JH:
Comparison of fermentation reactions in different regions of the
human colon. J Appl Bacteriol. 72:57–64. 1992.PubMed/NCBI
|
|
26.
|
Mercurio MG, Shiff SJ, Galbraith RA and
Sassa S: Expression of cytochrome P450 mRNAs in the colon and the
rectum in normal human subjects. Biochem Biophys Res Commun.
210:350–355. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Cancer Genome Atlas Network: Comprehensive
molecular characterization of human colon and rectal cancer.
Nature. 487:330–337. 2012. View Article : Google Scholar : PubMed/NCBI
|