Introduction
Haematogenous soft tissue metastasis (STM),
including skeletal muscle metastasis (SMM) and subcutaneous STM, is
clinically uncommon (1,2). However, the detection of STM may
affect cancer staging and prognosis. According to previous studies,
lung cancer, gastrointestinal tumours and colorectal carcinoma are
the primary malignancies that most commonly metastasize to the soft
tissues (3,4). Metastases occur more rarely in
association with other tumours, such as melanoma, breast cancer,
pancreatic carcinoma and sarcoma of the muscle. A recent study by
Surov et al retrospectively analysed the data of 5,170
patients with metastasized solid malignant tumours and concluded
that SMM occurred more frequently in patients with carcinoma of the
cervix and uterus, malignant melanoma and ovarian carcinoma
(5). Statistical data from a study
in Japan suggested that only 0.16% of patients with lung cancer
developed SMM (6). In an autopsy
series of patients with renal cell carcinoma, the prevalence of SMM
ranged from 0.4 to 3.4% (7). In
addition, the prevalence of SMM in patients with colorectal cancer
was reported to be 0.028% (8).
However, the prevalence of SMM did not exceed 5% in any of the
different types of malignancy. In this study, we observed that the
primary malignant tumour most frequently associated with SMM was
lung cancer, in which adenocarcinoma accounted for 88.2% of the
cases.
Metastatic tumours presenting as soft tissue masses
are relatively rare and may be the source of diagnostic confusion
clinically as well as pathologically (2). The differential diagnosis between STM
and primary soft tissue sarcoma is crucial, as their treatment and
prognosis are markedly different. The majority of the currently
available clinical or radiological data on STM consist of isolated
case reports or reviews and do not provide statistical evidence. In
this study, we reviewed 17 cases of patients with STM who underwent
18F-fluorodeoxyglucose (18F-FDG) positron
emission tomography (PET)/computed tomography (CT) scanning. The
imaging and clinical data of these patients were retrospectively
analysed. The aim of this study was to report the manifestations,
origin and distribution of STM detected by 18F-FDG
PET/CT and determine its value in the characterisation of STM. We
also aimed to improve our knowledge of 18F-FDG PET/CT
findings in the diagnosis of STM and determine its value in the
staging of malignant tumours.
Materials and methods
Patients
The medical records of 17 patients with a total of
221 STM lesions were collected. The patients included 10 men and 7
women who underwent 18F-FDG PET/CT scanning at our
hospital between August, 2010 and May, 2012. The cases were 15 of
adenocarcinoma (15/17) and 2 of squamous cell carcinoma (2/17) and
comprised 8 lung cancer cases (7 adenocarcinomas and 1 squamous
cell carcinoma), 2 oesophageal carcinoma cases (1 squamous cell
carcinoma and 1 adenocarcinoma), 5 cases of adenocarcinoma of the
gastrointestinal tract and 2 cases of endometrial carcinoma. The
majority of the patients had no obvious clinical symptoms, although
1 patient presented with a mass in the right lower limb and 1
patient exhibited subcutaneous diffuse small nodules on his
body.
Inclusion and exclusion criteria
The criteria for inclusion were the presence of a
pathologically proven malignancy and the development of metastatic
soft tissue lesions in the skeletal muscles and/or subcutaneous
tissues confirmed by correlations with surgical pathology, other
imaging modalities, or clinical follow-up. The exclusion criteria
included lymph node metastasis, such as to the axillary or inguinal
lymph nodes; bone metastasis invading soft tissues; lymphoma;
malignant melanoma; neurofibromatosis; and primary soft tissue
sarcoma.
PET/CT scanning
All the patients underwent 18F-FDG PET/CT
scanning for diagnostic evaluation using a GE Discovery STE
16PET/CT scanner (Discovery 16STE; GE Healthcare,
Waukesha, WI, USA). All the patients were fasted for >6 h, with
routinely measured blood glucose values of <6.60 mmol/l. A
standard dose (0.1 mCi/kg) of an 18F-FDG tracer with a
radiochemical purity of >95% was intravenously injected. The
patients were then instructed to sit or lie quietly in an injection
room without talking during the subsequent 40–60 min of the FDG
uptake phase and were allowed to breathe normally during image
acquisition without specific instructions. The patients were orally
administered 500–800 ml of water to fill the gastrointestinal tract
prior to scanning. True whole-body (TWB) 18F-FDG PET/CT
scans acquired from the top of the skull through the bottom of the
feet were obtained in 2 cases and limited whole-body (LWB)
18F-FDG PET/CT data acquired from the top of the skull
to the upper thigh were obtained in 15 cases. The imaging
parameters were as follows for CT scanning: 120 kV; 200–250 mA;
thickness, 3.75 mm; pitch, 1.0; and for PET scanning: 3D scanning;
scan 6–7 frames at 2 min per frame. Iterative attenuation
correction reconstruction was performed to obtain axial, sagittal
and coronal sections with a slice thickness of 3.75 mm for the PET,
CT and fused PET/CT images.
Image analysis
All the acquired PET, CT and fused PET/CT images
were separately analysed by 3 radiologists for a comparative
analysis. Disagreements regarding the final conclusions were
resolved by consensus. The maximum standardized uptake value
(SUVmax) was measured in the abnormal radiation uptake
areas with a semi-quantitative analysis. The PET positive standard
was a lesion with a higher FDG uptake compared to the normal uptake
of muscle and subcutaneous soft tissue. The CT evaluation of the
STM included measurement of the greatest diameter and a density
judgment (iso-, hyper-, or hypodense relative to the surrounding
tissues). Given the limited anatomical delimitation of isodense
muscular lesions from the surrounding normal muscular tissue on CT,
the size of the lesions was estimated on the PET images.
Results
In 17 cases of STM, the primary tumour was an
adenocarcinoma, accounting for 88.2% (15/17) of the cases. A total
of 221 soft tissue lesions were present in the 17 patients.
Thirteen patients had SMM, 2 patients had subcutaneous metastases
and 2 patients had both muscle and subcutaneous STM. The incidence
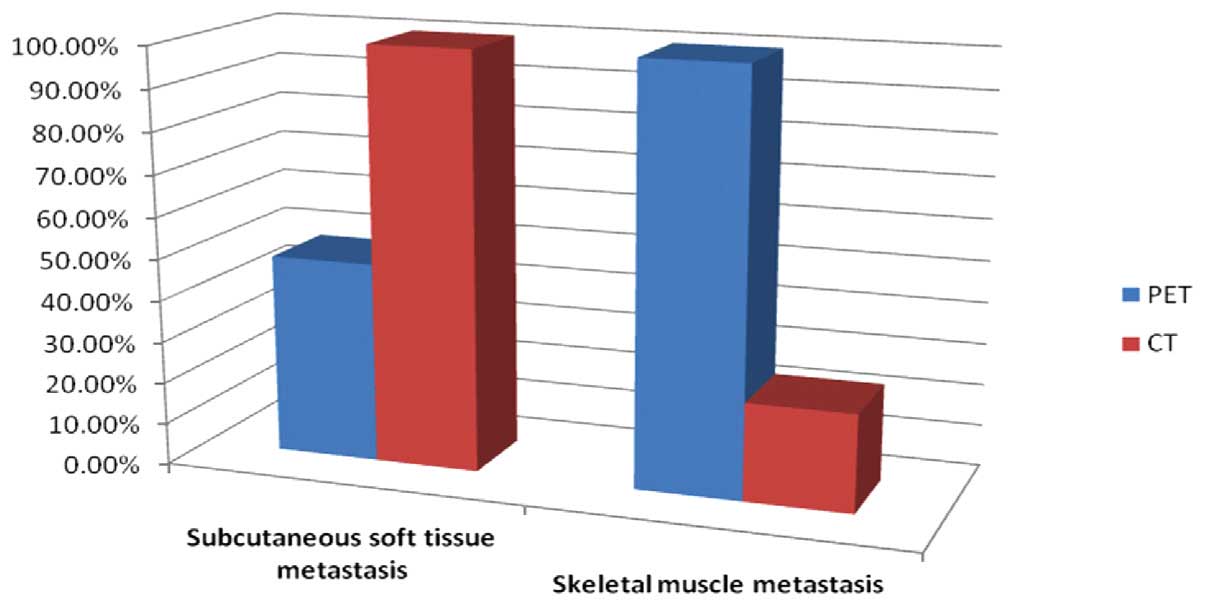
of muscle metastasis was 88.2%, while that of subcutaneous STM was
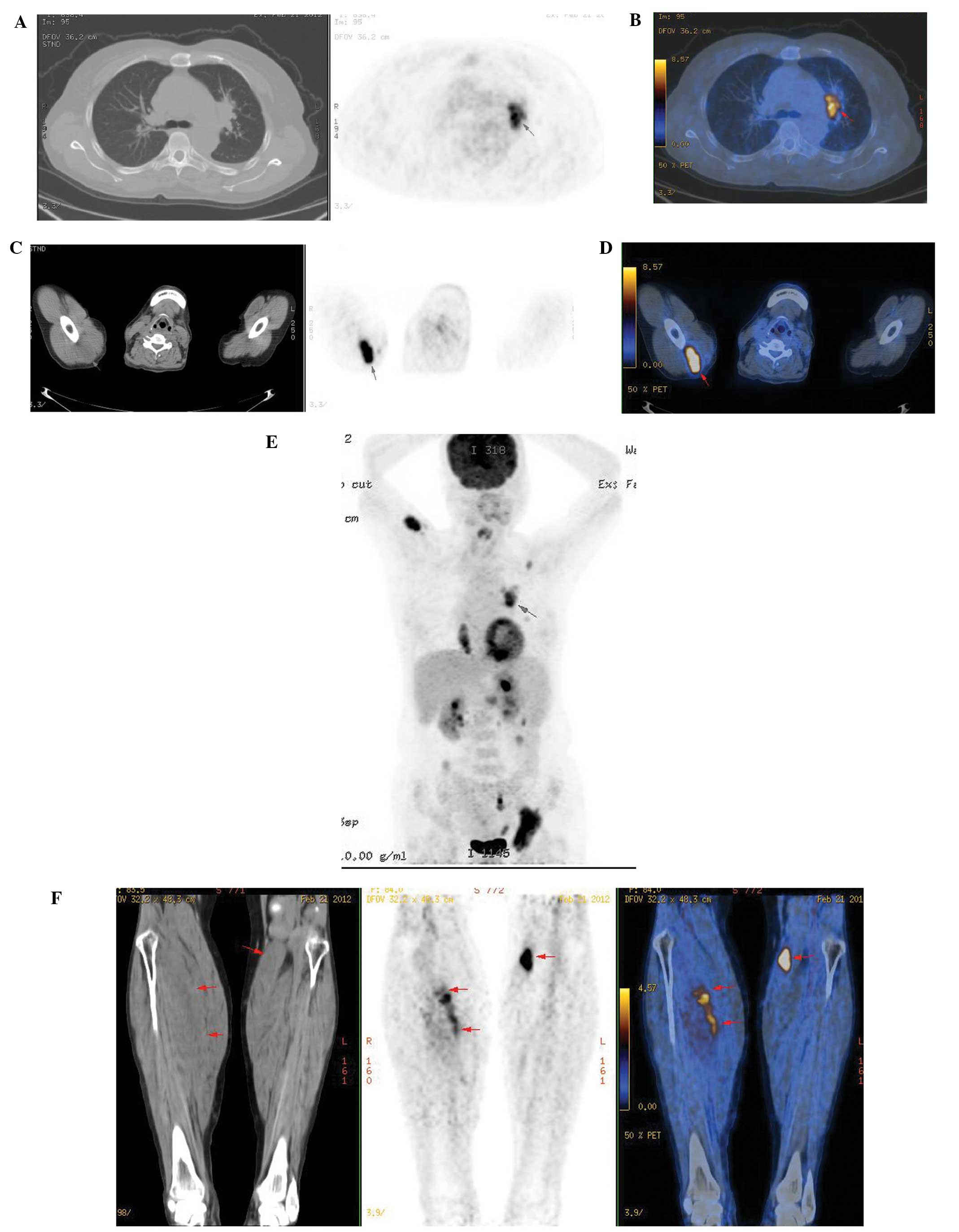
23.5%. PET/CT detected 51 muscle metastases, whereas PET detected
all the muscle lesions with a 100% detection rate (51/51) (Table I and Fig. 1); the skeletal muscle lesions were
hypodense in 12 and isodense in 39 of the 51 lesions (Fig. 2). A CT scan was only able to detect
12 of the 51 muscle metastases, resulting in a detection rate of
23.5% for isodense nodules (Fig.
2) with a maximum diameter of >2.0 cm and part of the lesion
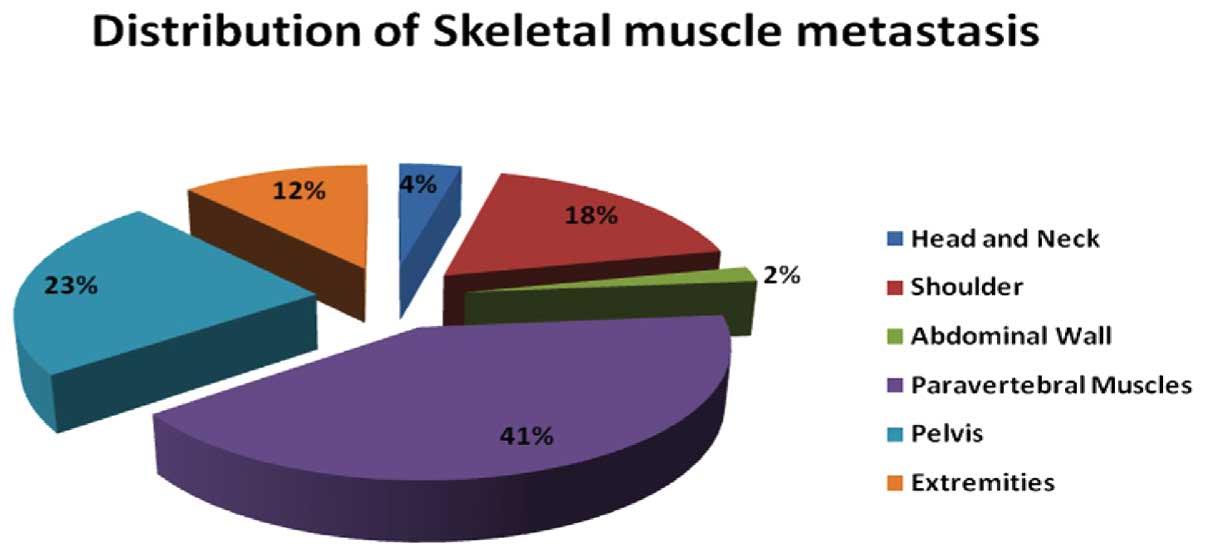
protruding from the surface. The paravertebral muscles were the
most common site of metastasis, accounting for 29.4% of the
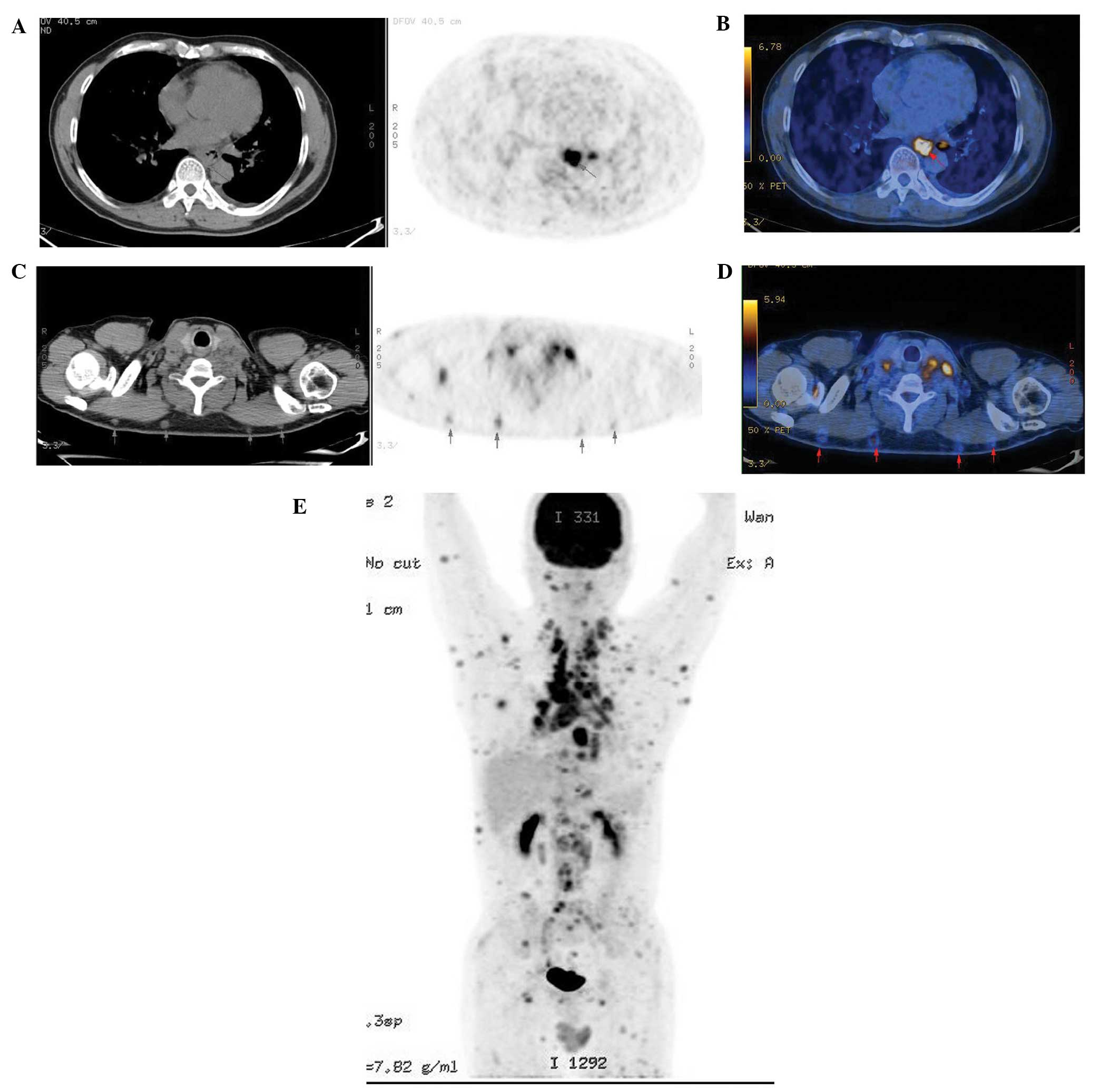
metastases in this study (21/51). PET/CT detected 170 subcutaneous
soft tissue nodules, whereas CT detected all 170 nodules with a
maximum diameter of 0.3–1.2 cm, demonstrating a 100% detection rate
(170/170); however, PET only detected 82 nodules with
SUVmax values of 1.25–3.52, accounting for 48.2%
(82/170) of the lesions (Fig. 3).
The subcutaneous lesions were easily identifiable as hyperdense
lesions on CT. No hyperdense skeletal muscle lesions were detected.
The sensitivities of PET and CT in the detection of SMM and
subcutaneous STM are shown in Fig.
4.
 | Table IDistribution of intramuscular
metastasis on positron emission tomography/computed tomography. |
Table I
Distribution of intramuscular
metastasis on positron emission tomography/computed tomography.
| Location | Muscles | Number of
metastases |
|---|
| Head and neck | External
pterygoid | 2 |
| Shoulder | Supraspinatus | 3 |
| Infraspinatus | 2 |
| Deltoid | 1 |
| Subscapular | 3 |
| Abdominal wall | Internal oblique | 1 |
| Paravertebral | Psoas | 6 |
| Erector spinae | 15 |
| Pelvis | Gluteus
maximus/medius | 10 |
| Pectineus | 1 |
| Adductor magnus | 1 |
| Extremities | Triceps brachii | 2 |
| Gastrocnemius | 1 |
| Biceps femoris,
semitendinosus | 3 |
| Total | | 51 |
Discussion
Distant metastasis to the soft tissues, defined as
metastasis to the skeletal muscle and subcutaneous tissues, has
been rarely reported in the literature. However, subclinical
metastases to the soft tissues may be more common than previously
acknowledged. The overall prevalence of STM in oncologic patients
may never be fully elucidated, as serial sectioning of all the soft
tissues in the body at autopsy is not practical. Autopsy studies
suggested that the frequency of muscle metastases is 0.8–16%
(9). Spencer and Helm reported
that the prevalence of skin metastasis of any cancer type varied
between 0.75 and 9% (10). STM is
generally more common during the progression of malignant tumours
and is often accompanied by multiple organ and lymph node
metastasis, whereas STM alone is quite rare. Metastasis occurs
mainly in the muscles and subcutaneous soft tissues, with muscle
metastasis being reportedly more common compared to subcutaneous
STM. In this study, the incidence of muscle metastasis among this
group of patients was 88.2%, while that of subcutaneous STM was
23.5%, similar to that reported by other studies (2).
Several factors are involved in STM, such as the pH
value, the accumulation of metabolites and the local temperature of
the soft tissue (3,11). In this study, the haematogenous
route was the most favoured pathway. Malignant tumours metastasise
into the musculature via venous vessels, particularly through the
paravertebral venous plexus. Paravertebral veins have multiple
connections with the inferior vena cava and the mesenteric venous
system (12). In our study, the
majority of SMM from pelvic and abdominal tumours were located in
the paravertebral musculature (29.4%). Recently, Magee and
Rosenthal (3) reported 8
biopsy-proven cases of skeletal metastases occurring in sites of
previously documented skeletal muscle trauma (28.7% of the total
patients) (13). It is possible
that skeletal muscle trauma results in focal hyperaemia, which
increases blood flow to the area, resulting in an increased
susceptibility to metastatic seeding by circulating malignant
cells. In the study by Magee and Rosenthal, metastases were
discovered at an average of 28 months after the trauma (range, 16
months-6 years post-trauma).
STM exhibits a broad spectrum of radiological
appearances. On plain radiographs STM generally presents as a soft
tissue shadow; however, in the case of metastatic gastric and
pancreatic carcinoma, calcifications within the soft tissue masses
have been observed (14).
Ossification within the muscle metastases of colorectal cancer has
also been reported (15). CT
provides a complete evaluation and definition of the extent of the
mass in the skeletal muscle and its association to the adjacent
bones, fascial planes, vessels and fat. Surov et al
(5) described 5 different types of
SMM based on the CT findings: focal intramuscular masses with
homogeneous contrast enhancement (type I), abscess-like
intramuscular lesions (type II), diffuse metastatic muscle
infiltration (type III), multifocal intramuscular calcification
(type IV) and intramuscular bleeding (type V), with type I being
the most common type in their study (52.5%). Magnetic resonance
imaging (MRI) is also able to detect metastatic lesions in skeletal
muscle and it is particularly useful for estimating tumour size and
location, as enhancement allows for differentiation between
metastatic lesions and the surrounding skeletal muscle. MRI has
been advocated as an indispensable tool for the diagnosis and
treatment planning of patients with malignant soft tissue masses.
However, MRI is not specific for STM. It was previously
demonstrated that 18F-FDG PET/CT exhibited a higher
sensitivity compared to MRI in detecting STM (16). However, there were false-positives
in a few cases, which should be taken into consideration. Most PET
facilities recommend at least 4 h of fasting prior to tracer
injection as a standard guideline. A longer fasting time may
increase the detection rate of STM.
The incidence of STM is quite low on conventional
imaging, mainly due to the following reasons: i) inadequate
understanding of clinicians, resulting from the low incidence of
STM; ii) the presence of STM does not alter the late staging of the
tumour and is hence undervalued by clinicians; and iii) lack of
experience in the traditional imaging characteristics of STM.
PET/CT performs anatomical as well as metabolic
imaging, is very sensitive in the detection of STM and provides
more information for clinical tumour staging. PET is more sensitive
in detecting lesions compared to CT before the density and
morphology of the soft tissues are altered, providing a clear view
of highly metabolic nodules within the soft tissue. In this study,
the detection rate of SMM by PET was 100% (51/51), while that of CT
was 23.5% (12/51), mainly due to the inability of CT to distinguish
between the densities of muscle and metastasis. The glucose uptake
of metastatic lesions is very high compared to normal muscle
uptake, making PET more sensitive in detecting abnormally highly
metabolic muscle metastases. A CT scan is able to clearly detect
subcutaneous STM due to the density difference between fat and soft
tissue lesions. Therefore, for subcutaneous STM, CT is able to
clearly identify lesions of abnormal density in the subcutaneous
fat. In this study, there were only 4 patients with subcutaneous
STM, which was significantly lower compared to the number of
patients with muscle metastasis; however, the number of
subcutaneous STM lesions was significantly higher compared to that
of muscle metastases. This is may be due to the limited spatial
resolution of PET; in addition, the majority of subcutaneous STM in
this study were small, with a maximum diameter of 0.3–1.2 cm. CT is
able to detect lesions <1.0 cm, which may give a false-negative
result on PET. Therefore, the detection rate of PET/CT for both
muscle and subcutaneous metastasis is significantly better compared
to that of PET or CT alone.
STM may present in a number of muscular and
subcutaneous sites across the body with a ratio higher than 1.5:1
(4) or 1.2:1 (15). In this study, the ratio was 1:3.3
(51/170), suggesting that subcutaneous STM may have been
underreported in the literature.
In oncology, whole-body PET/CT is typically
performed from the top of the skull to the upper thigh (LWB
18F-FDG PET/CT), since the majority of FDG-avid lesions
are expected to be within this field-of-view (FOV). If the primary
tumour or the suspected metastatic lesions are outside the LWB, the
FOV is then extended to cover this site, thus allowing a proper
diagnosis, staging and restaging. In this study, 2 patients with
palpable masses in their lower limbs underwent TWB PET/CT. The
results revealed that there were metastatic lesions in the lower
limbs. Nguyen et al (17)
reported that STM were detected with TWB 18F-FDG PET/CT
and compared the findings to those of the LWB FOV, indicating that
the LWB FOV may underestimate the true extent of STM by missing
lesions outside the FOV.
STM should be differentiated from primary soft
tissue sarcoma. Intramuscular metastases generally present as a
painful mass, which is commonly firm and tender (usually >5 cm
in diameter) (18, 19). This presentation may be an
important clue to suggest metastasis over the more common soft
tissue sarcoma, which presents as a soft, painless mass (11). Whole-body PET/CT imaging may reveal
the primary tumours of the STM, which is helpful for differential
diagnosis. In this study, 1 patient presented with squamous cell
carcinoma of the calf muscle and PET/CT identified oesophageal
carcinoma as the primary tumour. Therefore, the primary tumour
detection capability of PET/CT combined with pathological diagnosis
was helpful in determining the treatment and prognosis of the
patient. Although the majority of metastatic soft tissue tumours
have a history of primary tumour, it may be difficult to identify
the primary tumour, as explained by Plaza et al (2), who reported that 13.5% of soft tissue
STM had no primary tumours. If there are no primary tumours
identified, the differential diagnosis includes primary soft tissue
sarcoma, lymphoma, malignant melanoma and neurofibromatosis. The
differential diagnosis relies mainly on pathology and
immunochemistry (20). In this
study, PET/CT detected the primary tumours in all the patients,
combined with a pathological and clinical history for an accurate
diagnosis.
In conclusion, based on our study and the currently
available literature, the incidence of subcutaneous STM may have
been underreported in the literature. The prognosis of the patient
may vary depending on the accurate diagnosis of STM. PET/CT not
only detects STM, but also plays an important role in the detection
of the primary tumours during tumour staging and restaging.
Therefore, PET/CT is crucial for the differential diagnosis of
STM.
References
|
1
|
Molina-Garrido MJ and Guillén-Ponce C:
Muscle metastasis of carcinoma. Clin Transl Oncol. 13:98–101. 2011.
View Article : Google Scholar
|
|
2
|
Plaza JA, Perez-Montiel D, Mayerson J, et
al: Metastases to soft tissue: a review of 118 cases over a 30-year
period. Cancer. 112:193–203. 2008.PubMed/NCBI
|
|
3
|
Magee T and Rosenthal H: SMM at sites of
documented trauma. AJR Am J Roentgenol. 178:985–988. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koike Y, Hatori M and Kokubun S: Skeletal
muscle metastasis secondary to cancer - a report of seven cases.
Ups J Med Sci. 110:75–83. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Surov A, Hainz M, Holzhausen HJ, et al:
Skeletal muscle metastases: primary tumours, prevalence, and
radiological features. Eur Radiol. 20:649–658. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tuoheti Y, Okada K, Osanai T, et al:
Skeletal muscle metastases of carcinomas: a clinicopathological
study of 12 cases. Jpn J Clin Oncol. 34:210–214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nabeyama R, Tanaka K, Matsuda S and
Iwamoto Y: Multiple intramuscular metastases 15 years after radical
nephrectomy in a patient with stage IV renal cell carcinoma. J
Orthop Sci. 6:189–92. 2001.PubMed/NCBI
|
|
8
|
Hasegawa S, Sakurai Y, Imazu H, et al:
Metastasis to the forearm skeletal muscle from an adenocarcinoma of
the colon: report of a case. Surg Today. 30:1118–1123. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sudo A, Ogihara Y, Shiokawa Y, et al:
Intermuscular metastasis of carcinoma. Clin Orthop Relat Res.
296:213–217. 1993.
|
|
10
|
Spencer PS and Helm TN: Skin metastasis in
cancer patients. Cutis. 39:119–121. 1987.
|
|
11
|
Koike Y, Hatori M and Kokubun S: Skeletal
muscle metastasis secondary to cancer - a report of seven cases.
Ups J Med Sci. 110:75–83. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nabi G, Gupta NP and Gandhi D: Skeletal
muscle metastasis from transitional cell carcinoma of the urinary
bladder: clinicoradiological features. Clin Radiol. 58:883–885.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yiotakis J, Hantzakos A, Kostakopoulos A
and Adamopoulos G: Intramasseteric metastasis of renal cell
carcinoma. J Laryngol Otol. 115:65–67. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaira K, Ishizuka T, Yanagitani N, et al:
Forearm muscle metastasis as an initial clinical manifestation of
lung cancer. South Med J. 102:79–81. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stabler J: Case report: ossifying
metastases form carcinoma of the large bowel demonstrated by bone
scintigraphy. Clin Radiol. 50:730–731. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pfannenberg C1, Aschoff P, Schanz S, et
al: Prospective comparison of 18F-fluorodeoxyglucose
positron emission tomography/computed tomography and whole-body
magnetic resonance imaging in staging of advanced malignant
melanoma. Eur J Cancer. 43:557–564. 2007.
|
|
17
|
Nguyen NC, Chaar BT and Osman MM:
Prevalence and patterns of soft tissue metastasis: detection with
true whole-body F-18 FDG PET/CT. BMC Medical Imaging. 7:1471–2342.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Glockner JF, White LM, Sundaram M and
McDonald DJ: Unsuspected metastases presenting as solitary soft
tissue lesions: a fourteen-year review. Skeletal Radiol.
29:270–274. 2000.PubMed/NCBI
|
|
19
|
Kim CJ, Day S and Yeh KA: Metastatic soft
tissue squamous cell carcinoma. Am Surg. 67:111–114.
2001.PubMed/NCBI
|
|
20
|
Pretorius ES and Fishman EK: Helical CT of
skeletal muscle metastases from primary carcinomas. AJR Am J
Roentgenol. 174:401–404. 2000. View Article : Google Scholar : PubMed/NCBI
|