Introduction
Cancer-related fatigue (CRF) is a common phenomenon
in patients undergoing cytotoxic chemotherapy and radiotherapy,
with a prevalence of 59–100%, depending on the clinical status of
the disease (1). CRF is also
associated with other physical and psychological symptoms, such as
pain, sleep disturbance, reduced physical activity and depression
(2, 3). Therefore, CRF negatively affects the
functional status and quality of life of the patients (4).
Patients exhibiting moderate or severe fatigue may
benefit from non-pharmacological as well as pharmacological
interventions, including use of psychostimulants, such as
methylphenidate and dexmethylphenidate, modafinil and
erythropoietin-stimulating agents (5). However, the clinical management of
CRF remains unsatisfactory. For this reason, de Oliveira Campos
et al (6) performed a phase
II randomized, double-blind, placebo-controlled crossover study to
evaluated the effect of guarana (Paullinia cupana) against
CRF. Guarana is an Amazon fruit used since the pre-Columbian era
that is currently commercialized in herbal and energetic beverages
due to its stimulant properties (7).
A phase II randomized, double-blind,
placebo-controlled crossover study was conducted by de Oliveira
Campos et al (6) on breast
cancer patients undergoing systemic chemotherapy, with 100 mg/day
of guarana powder supplementation. The guarana supplementation
significantly decreased CRF. The study also reported no occurrence
of toxic adverse effects, sleep disturbance or anxiety and
depression in the patients receiving guarana supplementation. For
this reason, the authors suggested that guarana may be an
effective, non-toxic, cost-effective option for the treatment of
CRF (6). A complementary study was
also recently published, demonstrating that a purified dry extract
of guarana may be effective in treating CRF patients with various
solid tumors who undergo chemotherapy (8).
The guarana effect on CRF is possibly associated
with its chemical composition, which includes a higher content of
purinic alkaloid caffeine (1,3,7-trimethylxanthine) compared to
coffee (Coffea arabica), tea (Camellia sinensis) and
yerba mate (Ilex paraguariensis). Guarana also contains a
small proportion of other purinic alkaloids, including theobromine
and theophylline (6), as well as
other chemical bioactive molecules, such as tannins and
proanthocyanidins, with a higher content of catechins and
epicatechins (9).
Despite the results suggesting a beneficial effect
of guarana on breast cancer patients with CRF, the bioactive
molecules in guarana may also affect the chemotherapeutic efficacy.
The potential antitumor effect of catechins on breast cancer cells
has being extensively described in the literature (10, 11). However, the effect of caffeine is
more controversial (12). A
previous study suggested that caffeine may attenuate the MCF-7 cell
response to chemotherapy due its ability to intercalate into DNA
(13). By contrast, another study
performed on MCF-7 breast cancer cells treated with paclitaxel
reported that caffeine supplementation enhanced the apoptosis
induction triggered by the chemotherapeutic drug (14). A recent study also demonstrated
that co-treatment with anticancer agents and 6-selenocaffeine
decreased MCF-7 cell viability (15).
The abovementioned evidence prompted us to
investigate whether guarana affects the properties of antitumor
drugs when concomitantly administered to MCF-7 breast cancer cells.
Therefore, the present study aimed to evaluate the effect of
guarana on MCF-7 cell viability and proliferation, with and without
exposure to 7 chemotherapeutic agents currently used in the
treatment of breast cancer.
Materials and methods
Chemicals
Analytical grade chemicals and reagents were
obtained from Sigma-Aldrich (St. Louis, MO, USA). The MCF-7 cell
line was obtained from American Type Culture Collection (Manassas,
VA, USA). The RPMI-1640 culture medium, fetal bovine serum (FBS),
heat-inactivated equine serum, penicillin and streptomycin were
purchased from Gibco (Grand Island, NY, USA);
Vacutainer® tubes were provided by BD Diagnostics
(Plymouth, UK).
Guarana extract
The guarana powder used in the present study was
supplied by Western Agropecuary Research Brazilian Enterprise
(EMBRAPA), a non-profit Brazilian governmental sector that offers
technical support to the production of guarana in the Amazonas
state. The bioactive compounds present in guarana powder were
previously determined and described (16). The extract contained 12.240 mg/g
caffeine, 6.733 mg/g theobromine and 4.336 mg/g total catechins.
The concentration of condensed tannin was 16 mg/g. To perform the
in vitro assay, the lyophilized extract was diluted in
distilled water to a concentration of 200 mg/ml. The mixture was
infused for 7 min by boiling, centrifuged at 1,500 × g for 15 min
and filtered. The solution was sterilized by filtration (0.20 µM),
diluted in distilled water and added to cell culture medium to
obtain 1, 5 and 10 µg/ml guarana concentrations. These
concentrations were selected considering that in vivo
guarana supplementation of breast cancer patients was relatively
lower (100 mg/day).
Cell culture
MCF-7 cells were cultured in Dulbecco's modified
Eagle's medium supplemented with 10% FBS, 2 mM L-glutamine, 1 mM
sodium pyruvate, 0.1 mM non-essential amino acids, 100 U/ml
penicillin and 100 g/ml streptomycin (pH 7.2) in a 5%
CO2 incubator at 37°C. Cell viability was measured using
the MTT cell proliferation assay (17). Cells (1×105) were seeded
in a 96-well plate in 200 µl complete culture medium. Following
overnight adhesion, the medium was changed with media containing
the antitumor drugs and different guarana extract concentrations.
All the experiments were performed in triplicate.
Antitumor and guarana
co-administration
The effect of the three different guarana
concentrations on the cytotoxic and antiproliferative properties of
7 antitumor agents currently used in the treatment of breast cancer
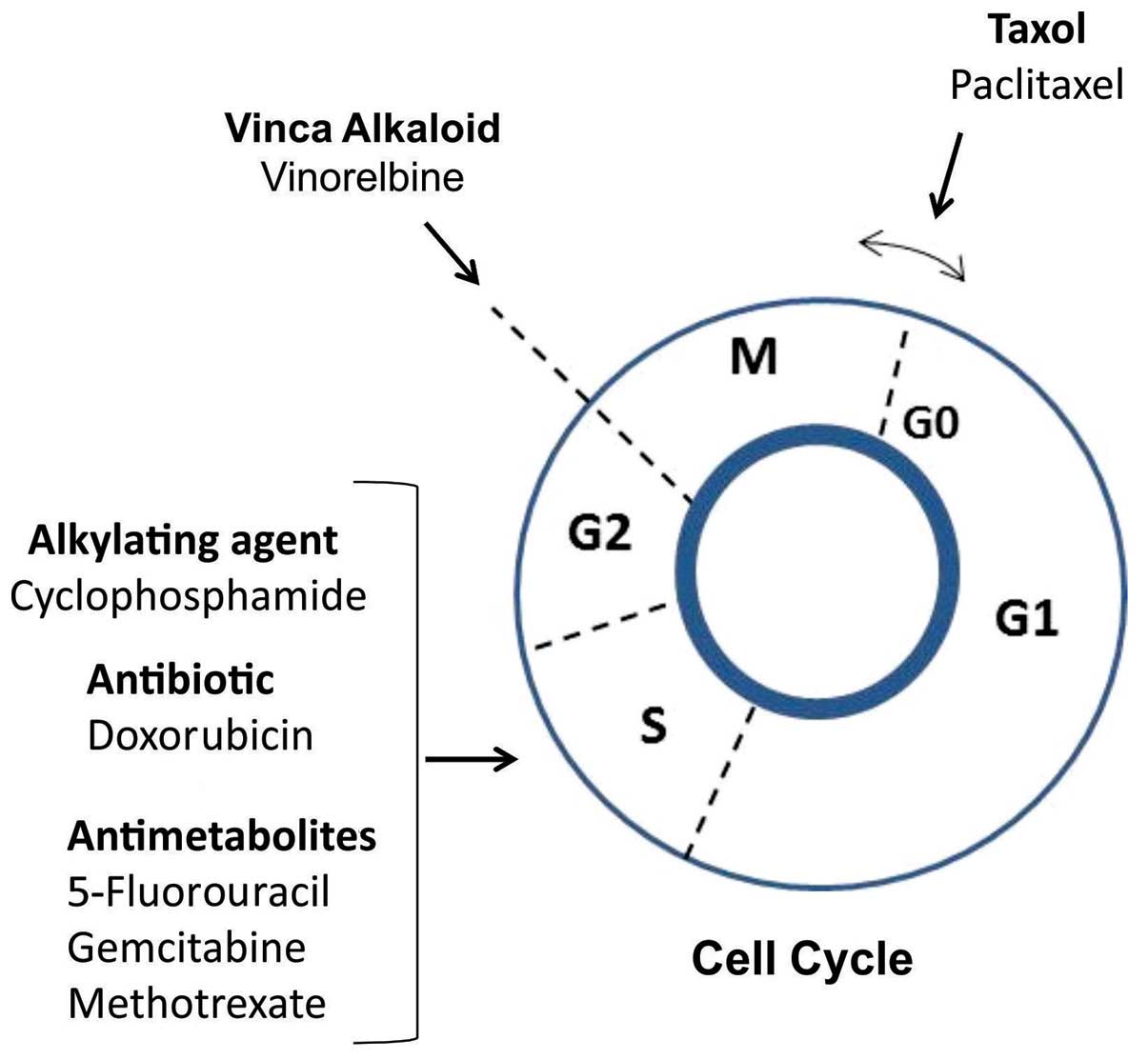
were tested on MCF-7 cells. The main actions of the
chemotherapeutic agents on the cell cycle are presented in Fig. 1.
The selection of the antitumor agents was based on
previous studies demonstrating their cytotoxic effect on MCF-7
breast cancer cells: Cyclophosphamide, a nitrogen mustard
alkylating agent that forms irreversible DNA crosslinks leading to
cell death (18); doxorubicin, an
anthracycline antibiotic with a DNA intercalating effect;
5-fluorouracil, a pyrimidine analog that belongs to the family of
antimetabolite drugs, causing irreversible inhibition of
thymidylate synthase and, consequently, cell cycle arrest and
apoptosis (19); paclitaxel, a
mitotic inhibitor targeting tubulin, causing defects in mitotic
spindle assembly and chromosome segregation (20); vinorelbine, a semi-synthetic vinca
alkaloid with an antimitotic effect (21); gemcitabine, a nucleoside analog
that adds a ‘faulty’ nucleoside during DNA synthesis, leading to
cell apoptosis (22); and
methotrexate, a folic acid analogue that prevents purine and
pyrimidine synthesis, leading to the inhibition of DNA, RNA and
protein synthesis (23). Based on
previous studies investigating the effects of these
chemotherapeutics on MCF-7 cells, their concentrations were as
follows: 10 µM gemcitabine (22),
vinorelbine and methotrexate (24); 2 µM 5-fluorouracil (25); 50 µM paclitaxel (26); 200 nM doxorubicin (19); and 5 mM cyclophosphamide (27).
Cell viability and proliferation
analysis
To evaluate the effect of the co-administration of
guarana and antitumor agents on cell viability and proliferation,
the MTT assay was used as previously described by Fukui et
al (26), who investigated the
effect of resveratrol and paclitaxel co-administration on the
viability of several cancer cell lines with slight modifications. A
total of 10 µl of MTT (at 5 mg/ml) was added to each well at a
final concentration of 500 µg/ml. Subsequently, the mixture in each
well was incubated for 1 h. The MTT is reduced by mitochondrial
dehydrogenase in living cells to produce insoluble purple formazan
crystals that are quantitatively measured following their removal
from the cells by the addition of 100 µl dimethyl sulfoxide (DMSO)
(18). However, prior to the
addition of DMSO, the treatment samples in the 96-well plate were
visualized by optic microscopy (magnification, x400) and
photographed. The images displayed living cells with purple
formazan crystals and dying cells without crystals. The absorbance
was read at 560 nm. The relative cell viability and/or
proliferation under antitumor agent and guarana treatment were
expressed as a percentage of the control well that was not treated
with chemotherapeutic drugs. To evaluate the guarana
co-administration effect on the properties of the chemotherapeutic
agents, the results were expressed as a percentage of each tumor
agent without guarana addition. All the experiments were performed
in triplicate.
Statistical analysis
The different treatments were compared using one-way
analysis of variance followed by Tukey's post hoc test. All the
tests with P<0.05 were considered to indicate statistically
significant differences.
Results
Effect of chemotherapeutic agents on
cell viability and proliferation
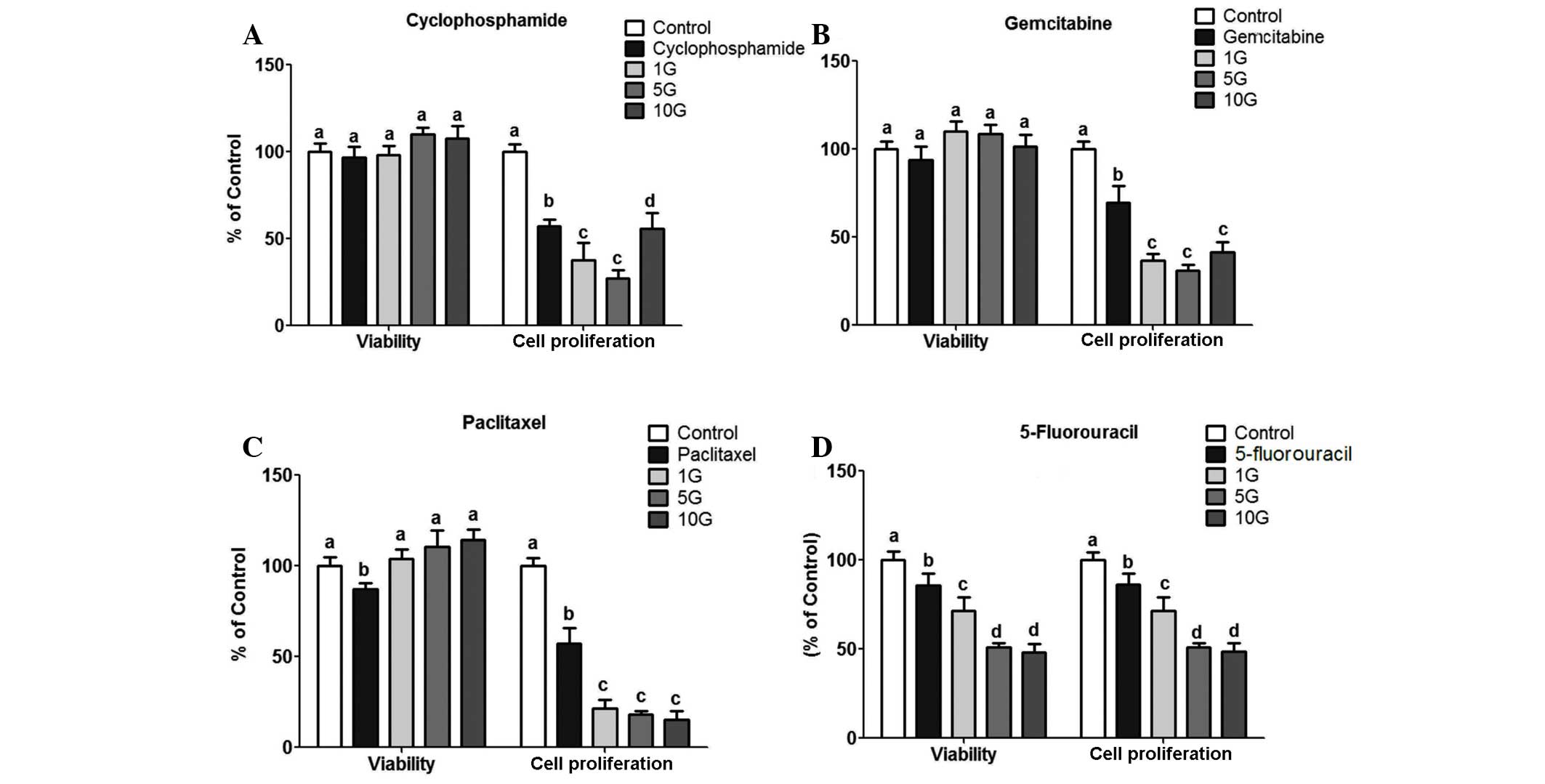
The effect of the chemotherapeutics on MCF-7 cells
was initially determined to confirm that their concentrations were
effective in decreasing cell viability and proliferation (Fig. 2). As expected, all the investigated
drugs significantly decreased MCF-7 cell viability and
proliferation (P<0.01). The effect on viability was similar
among all the chemotherapeutic agents used in the present study.
However, the effect on cell proliferation was drug-dependent.
Paclitaxel and cyclophosphamide were the chemotherapeutics that
most significantly inhibited MCF-7 cell proliferation (>70%)
compared to the control untreated cells and the cells treated by
the other 5 agents.
Effect of guarana on cell viability
and proliferation
The guarana extract produced from toasted seeds used
in all the protocols exhibited a high caffeine content (12.3 mg/g)
and was also rich in theobromine (6.8 mg/g) and total catechins
(4.3 mg/g) (Fig. 3A and B).
Therefore, three low concentrations of guarana (1, 5 and 10 µg/ml)
were selected to investigate the effect of this extract on
antitumor drugs. The isolated effect of guarana was evaluated prior
to testing its action on MCF-7 cell response to chemotherapeutic
drugs. As can be seen in Fig. 3,
the three guarana concentrations tested here did not affect MCF-7
cell viability at 24 h of exposure. However, a significant effect
on MCF-7 cell proliferation was observed in the cells exposed to
guarana at concentrations of 5 and 10 µg/ml (P<0.01).
Effect of guarana on the action of
chemotherapeutic agents
Based on these data, the effect of guarana on the
action of chemotherapeutics in MCF-7 cells was finally evaluated
and the results are presented in Figs.
4 and 5. Guarana did not
affect the viability of MCF-7 cells treated with cyclophosphamide,
gemcitabine and paclitaxel after 24 h of exposure (Fig. 4A-C). However, the presence of
different guarana concentrations significantly increased the
cytotoxicity of 5-fluorouracil after 24 h of exposure.
5-Fluorouracil plus guarana at concentrations of 5 or 10 µg/ml
killed ~50% of the MCF-7 cells compared to the untreated control
group (Fig. 4D).
By contrast, when compared to the negative and
positive control groups, guarana significantly increased MCF-7 cell
viability in the methotrexate- (Fig.
5A), doxorubicin- (Fig. 5B)
and vinorelbine-treated groups (Fig.
5C), mainly at concentrations of 5 and 10 µg/ml.
Different from the results at 24 h, the combination
of guarana with all the investigated chemotherapeutic drugs exerted
a strong antiproliferative effect on MCF-7 cells after 72 h of
exposure (P<0.01). This effect was more prominent when the cells
were exposed to all guarana concentrations and paclitaxel. The cell
proliferation was reduced by ~80% when compared to the untreated
control group. Furthermore, cyclophosphamide plus guarana at 5
µg/ml was also effective in decreasing the MCF-7 cell population by
>80% compared to the control group (Fig. 4A). As regards the other antitumor
drugs, the presence of guarana inhibited cell proliferation by
~40–50% when compared to the negative control group.
Discussion
Historically, natural products have provided
resources for the development of several antitumor molecules.
Plants, marine organisms and microorganisms are the origin of
>60% of the drugs currently used in cancer therapy (28). In addition, plants may also be used
to treat adverse effects caused by chemotherapeutics, such as CRF.
However, this effect has being less extensively investigated
compared to the anticancer action.
The present study demonstrated that guarana at low
concentrations is able to differentially modulate MCF-7 cell
proliferation, as well as affect the antitumor properties of 7
chemotherapeutic agents currently used in the treatment of breast
cancer. In general, guarana intensified the antiproliferative
effects of all the investigated drugs, although the initial effect
on cell viability was heterogeneous.
Guarana possesses biological properties described in
the literature as anti-inflammatory (29), antidepressant (30), panicolytic (31) and energetic (6), which may help minimize CRF, as
previously reported (7, 8). Other studies have also described the
antitumor activity of guarana using animal and cell experimental
models (32–34). It has been suggested that guarana
may be used to improve CRF caused by chemotherapy, which prompted
us to conduct the present study.
Considering that guarana is rich in caffeine and
also contains catechins, we performed a literature review regarding
the potential effect of caffeine and catechins on MCF-7 cells, as
well as the effect of these molecules on cell response to antitumor
drugs.
MCF-7 is a cell line derived from an invasive ductal
breast carcinoma, which expresses estrogen and progesterone
receptors and exhibits a proliferative response in the presence of
progesterone. This cell line may be used to investigate resistance
to antitumor agents, involving overexpression of the ABCG2 protein
that confers multidrug resistance to tumor cells by extruding a
variety of chemotherapeutic agents (35). Caffeine, the most widely used
neuroactive compound in the human diet, has antiproliferative
activity and the ability to induce cell cycle arrest and apoptosis
(10).
However, the effect of caffeine on antitumor drugs
appears to be cell line- and drug-dependent. As regards MCF-7, the
line used in the present study, previous studies demonstrated the
effect of caffeine on enhancing cell apoptosis caused by exposure
to paclitaxel (14) and increasing
the cytotoxic effect of alkylating drugs, such as cyclophosphamide
(36). In addition, caffeine and
other xanthines, including theophylline and dyphylline,
significantly decreased the expression of the ABCG2 protein in the
MCF-7/MX100 subline, which exhibits a high resistance to
anti-breast cancer drugs (37).
By contrast, Hill et al (13) reported that caffeine may attenuate
the cytotoxic effect of intercalating antitumor drugs, such as
doxorubicin. That study described a possible interceptor role of
caffeine, protecting cancer cell DNA from intercalation. In the
present study, we observed a significant increase in MCF-7 cell
viability after 24 h of exposure to doxorubicin plus guarana at
concentrations of 5 and 10 µg/ml. However, this potential
procarcinogenic effect was significantly attenuated after 72 h of
exposure. This contradictory effect may be caused by other
bioactive molecules present in guarana, such as catechins.
A previous study performed by Seeram et al
(38) described that several
catechin and anthocyanin molecules are able to inhibit the
proliferation of cancer cells, including the MCF-7 cell line
(39). The effect of catechins
appears to be associated with the ability of these molecules to
increase the expression of pro-apoptotic genes, such as caspase-3,
−8, −9, as well as other genes involved in the apoptotic pathway
(39).
A similar study that specifically evaluated the
effect of epigallocatechin-3-gallate (EGCG), the main catechin
present in green tea, on a breast carcinoma cell line resistant to
tamoxifen (MCF-7Tam cells) reported cell growth inhibition and
dose-dependent apoptosis. Following exposure to 100 µg/ml EGCG for
24 h, the expression of Bax was increased and the expression of
Bcl-2 was decreased (40). A
recent study also reported that microRNA expression in MCF-7 cells
may be affected by green tea, which is rich in catechins and
caffeine, resulting in inhibition of carcinogenesis (41). A recent study also reported that
microRNA overexpression in MCF-7 cells may be decreased following
treatment with polyphenon-60, a catechin included in green tea
(41). This mechanism of action
may explain the antitumor effect of these molecules on MCF-7 breast
cancer cells.
Despite the evidence on the effect of catechins on
MCF-7 cells, we were unable to identify previous studies
investigating the effect of these molecules on antitumor drug
efficacy. Therefore, a complementary investigation is required to
evaluate whether guarana exerts an effect on chemotherapeutic drug
action associated with the effect of catechins on apoptosis and
antitumor gene modulation.
Since all the investigated chemotherapeutic drugs
were affected by the addition of guarana, mostly by improving the
antiproliferative activity after 72 h of exposure, the therapeutic
use of guarana in the treatment of CRF apparently does not
compromise the effect of chemotherapy. However, in vitro
protocols present with methodological limitations that require
consideration in the interpretations of the results. Complementary
in vitro investigations evaluating the gene modulation of
the metabolic routes involved in carcinogenesis, as well as studies
using animal models are required to verify our results.
References
|
1
|
Weiss J: Cancer-related fatigue:
prevalence, assessment and treatment strategies. Expert Rev
Pharmacoecon Outcomes Res. 11:441–446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Byar KL, Berger AM, Bakken SL, et al:
Impact of adjuvant breast cancer chemotherapy on fatigue, other
symptoms, and quality of life. Oncol Nurs Forum. 33:E18–E26. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Jong N, Courtens AM, Abu-Saad HH, et
al: Fatigue in patients with breast cancer receiving adjuvant
chemotherapy: a review of the literature. Cancer Nurs. 25:283–297.
2002.
|
|
4
|
Saligan LN and Kim HS: A systematic review
of the association between immunogenomic markers and cancer-related
fatigue. Brain Behav Immun. 26:830–848. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Campos MP, Hassan BJ, Riechelmann R and
Del Giglio A: Cancer-related fatigue: a review. Rev Assoc Med Bras.
57:211–219. 2011.
|
|
6
|
de Oliveira Campos MP, Riechelmann R,
Martins LC, Hassan BJ, Casa FB and Del Giglio A: Guarana
(Paullinia cupana) improves fatigue in breast cancer
patients undergoing systemic chemotherapy. J Altern Complement Med.
17:505–512. 2011.
|
|
7
|
Schimpl FC, da Silva JF, Gonçalves JF and
Mazzafera P: Guaraná: revisiting a highly caffeinated plant from
the Amazon. J Ethnopharmacol. 150:14–31. 2013.
|
|
8
|
del Giglio AB, Cubero Dde I, Lerner TG,
Guariento RT, de Azevedo RG, Paiva H, Goldman C, Carelli B, Cruz
FM, Schindler F, Pianowski L, de Matos LL and del Giglio A:
Purified dry extract of Paullinia cupana (guaraná) (PC-18)
for chemotherapy-related fatigue in patients with solid tumors: an
early discontinuation study. J Diet Suppl. 10:325–334. 2013.
|
|
9
|
Basile A, Ferrara L, Pezzo MD, Mele G,
Sorbo S, Bassi P and Montesano D: Antibacterial and antioxidant
activities of ethanol extract from Paullinia cupana Mart. J
Ethnopharmacol. 102:32–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Valcic S, Timmermann BN, Alberts DS,
Wächter GA, Krutzsch M, Wymer J and Guillén JM: Inhibitory effect
of six green tea catechins and caffeine on the growth of four
selected human tumor cell lines. Anticancer Drugs. 7:461–468. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu AH and Butler LM: Green tea and breast
cancer. Mol Nutr Food Res. 55:921–930. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang W, Wu Y and Jiang X: Coffee and
caffeine intake and breast cancer risk: an updated dose-response
meta-analysis of 37 published studies. Gynecol Oncol. 129:620–629.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hill GM, Moriarity DM and Setzer WN:
Attenuation of cytotoxic natural product DNA intercalating agents
by caffeine. Sci Pharm. 79:729–747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saunders DE, Lawrence WD, Christensen C,
Wappler NL, Ruan H and Deppe G: Paclitaxel-induced apoptosis in
MCF-7 breast-cancer cells. Int J Cancer. 70:214–220. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Martins IL, Miranda JP, Oliveira NG,
Fernandes AS, Gonçalves S and Antunes AM: Synthesis and biological
activity of 6-selenocaffeine: potential modulator of
chemotherapeutic drugs in breast cancer cells. Molecules.
18:5251–5264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bittencourt LS, Machado DC, Machado MM, et
al: The protective effects of guaraná extract (Paullinia
cupana) on fibroblast NIH-3T3 cells exposed to sodium
nitroprusside. Food Chem Toxicol. 53:119–125. 2013.
|
|
17
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Trebunova M, Laputkova G, Slaba E,
Lacjakova K and Verebova A: Effects of docetaxel, doxorubicin and
cyclophosphamide on human breast cancer cell line MCF-7. Anticancer
Res. 32:2849–2854. 2012.PubMed/NCBI
|
|
19
|
Major PP, Egan EM, Sargent L and Kufe DW:
Modulation of 5-FU metabolism in human MCF-7 breast carcinoma
cells. Cancer Chemother Pharmacol. 8:87–91. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsuoka H, Furusawa M, Tomoda H and Seo
Y: Difference in cytotoxicity of paclitaxel against neoplastic and
normal cells. Anticancer Res. 14:163–167. 1994.PubMed/NCBI
|
|
21
|
Sugiyama K, Shimizu M, Akiyama T, Ishida
H, Okabe M, Tamaoki T and Akinaga S: Combined effect of navelbine
with medroxyprogesterone acetate against human breast carcinoma
MCF-7 cells in vitro. Br J Cancer. 77:1737–1743. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zeybek ND, Inan S, Ekerbicer N, Vatansever
HS, Karakaya J and Muftuoglu SF: The effects of gemcitabine and
vinorelbine on inducible nitric oxide synthase (iNOS) and
endothelial nitric oxide synthase (eNOS) distribution of MCF-7
breast cancer cells. Acta Histochem. 113:62–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barros S, Mencia N, Rodríguez L, Oleaga C,
Santos C, Noé V and Ciudad CJ: The redox state of cytochrome
c modulates resistance to methotrexate in human MCF7 breast
cancer cells. PLoS One. 8:e632762013.PubMed/NCBI
|
|
24
|
Hattangadi DK, DeMasters GA, Walker TD,
Jones KR, Di X, Newsham IF and Gewirtz DA: Influence of p53 and
caspase 3 activity on cell death and senescence in response to
methotrexate in the breast tumor cell. Biochem Pharmacol.
68:1699–1708. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Patterson AV, Zhang H, Moghaddam A,
Bicknell R, Talbot DC, Stratford IJ and Harris AL: Increased
sensitivity to the prodrug 5′;-deoxy-5-fluorouridine and modulation
of 5-fluoro-2′;-deoxyuridine sensitivity in MCF-7 cells transfected
with thymidine phosphorylase. Br J Cancer. 72:669–675. 1995.
|
|
26
|
Fukui M, Yamabe N and Zhu BT: Resveratrol
attenuates the anticancer efficacy of paclitaxel in human breast
cancer cells in vitro and in vivo. Eur J Cancer. 46:1882–1891.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singh N, Nigam M, Ranjan V, et al:
Resveratrol as an adjunct therapy in cyclophosphamide-treated MCF-7
cells and breast tumor explants. Cancer Sci. 102:1059–1067. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cragg GM and Newman DJ: Plants as a source
of anti-cancer agents. J Ethnopharmacol. 100:72–79. 2005.PubMed/NCBI
|
|
29
|
Campos AR, Barros AI, Santos FA and Rao
VS: Guaraná (Paullinia cupana Mart.) offers protection
against gastric lesions induced by ethanol and indomethacin in
rats. Phytother Res. 17:1199–1202. 2003.
|
|
30
|
Otobone FJ, Sanches AC, Nagae R, Martins
JV, Sela VR, de Mello JC and Audi EA: Effect of lyophilized
extracts from guaraná seeds [Paullinia cupana var.
sorbilis (Mart.) Ducke] on behavioral profiles in rats.
Phytother Res. 21:531–535. 2007.
|
|
31
|
Roncon CM, Biesdorf de Almeida C, Klein T,
de Mello JC and Audi EA: Anxiolytic effects of a semipurified
constituent of guaraná seeds on rats in the elevated T-maze test.
Planta Med. 77:236–241. 2011.PubMed/NCBI
|
|
32
|
Fukumasu H, Latorre AO and Zaidan-Dagli
ML: Paullinia cupana Mart. var. sorbilis, guarana,
increases survival of Ehrlich ascites carcinoma (EAC) bearing mice
by decreasing cyclin-D1 expression and inducing a G0/G1 cell cycle
arrest in EAC cells. Phytother Res. 25:11–16. 2011. View Article : Google Scholar
|
|
33
|
Fukumasu H, Avanzo JL, Nagamine MK,
Barbuto JA, Rao KV and Dagli ML: Paullinia cupana Mart var.
sorbilis, guaraná, reduces cell proliferation and increases
apoptosis of B16/F10 melanoma lung metastases in mice. Braz J Med
Biol Res. 41:305–310. 2008. View Article : Google Scholar
|
|
34
|
Fukumasu H, da Silva TC, Avanzo JL, et al:
Chemopreventive effects of Paullinia cupana Mart var.
sorbilis, the guaraná, on mouse hepatocarcinogenesis. Cancer
Lett. 233:158–164. 2006.
|
|
35
|
Ifergan I, Shafran A, Jansen G, Hooijberg
JH, Scheffer GL and Assaraf YG: Folate deprivation results in the
loss of breast cancer resistance protein (BCRP/ABCG2) expression. A
role for BCRP in cellular folate homeostasis. J Biol Chem.
279:25527–25534. 2004. View Article : Google Scholar
|
|
36
|
Teicher BA, Holden SA, Herman TS, Epelbaum
R, Pardee AB and Dezube B: Efficacy of pentoxifylline as a
modulator of alkylating agent activity in vitro and in vivo.
Anticancer Res. 11:1555–1560. 1991.PubMed/NCBI
|
|
37
|
Ding R, Shi J, Pabon K and Scotto KW:
Xanthines down-regulate the drug transporter ABCG2 and reverse
multidrug resistance. Mol Pharmacol. 81:328–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Seeram NP, Zhang Y and Nair MG: Inhibition
of proliferation of human cancer cells and cyclooxygenase enzymes
by anthocyanidins and catechins. Nutr Cancer. 46:101–106. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Alshatwi AA: Catechin hydrate suppresses
MCF-7 proliferation through TP53/caspase-mediated apoptosis. J Exp
Clin Cancer Res. 29(167)2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Farabegoli F, Papi A, Bartolini G, Ostan R
and Orlandi M: (-)-Epigallocatechin-3-gallate downregulates Pg-P
and BCRP in a tamoxifen resistant MCF-7 cell line. Phytomedicine.
17:356–362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fix LN, Shah M, Efferth T, Farwell MA and
Zhang B: MicroRNA expression profile of MCF-7 human breast cancer
cells and the effect of green tea polyphenon-60. Cancer Genomics
Proteomics. 7:261–277. 2010.PubMed/NCBI
|