Introduction
Head and neck squamous cell carcinoma (HNSCC) is a
devastating disease and the sixth most common cancer type
worldwide, responsible for >500,000 new cases annually. Despite
progression in diagnostic and therapeutic modalities, ~60% of
patients present with locally advanced disease, and the five-year
survival rate is ~50% (1–3). The main strategies of therapy are
chemoradiation therapy or surgery. Neoadjuvant or induction
chemotherapy, followed by local therapy, is often performed and it
was reported that the response to induction chemotherapy may be a
factor that can predict the efficacy of chemoradiation therapy
(4). However, no clinically validated
biomarkers of HNSCC exist for predicting prognosis or treatment
outcome. This may be one of the reasons for the low survival rate
or the occurrences of unsuccessful treatment. Therefore, it is
expected that the identification of novel biomarkers of HNSCC will
assist with determining prognosis more accurately or increase the
efficacy of treatment, which may provide the patients with more
options.
The pituitary homeobox 1 (PITX1) protein was
originally described as a member of the bicoid-associated homeobox
transcription factor family that is involved in the transcriptional
regulation of proopiomelanocortin gene in the adult pituitary gland
(5). PITX1 is important in the
development of hind limbs and determines morphological features of
the muscles, tendons and bones of the hind limbs (6). In addition, PITX1 has been identified as
a suppressor of RAS activity and tumorigenicity (7). Previously, it was reported that PITX1 is
a negative regulator of telomerase, due to transcriptional
repression of the promoter of telomerase reverse transcriptase
(TERT) (8). Downregulation of PITX1
is consistently associated with the malignancy of various human
cancer types, including oral squamous cell carcinoma (9), malignant melanoma (10), esophageal (11), gastric (8,12), lung
(13), breast (14), hepatic (15), colorectal (16), and prostate (17) cancer, suggesting that PITX1 may be a
tumor suppressor gene. By contrast, an association between PITX1
and HNSCC, including pharyngeal or laryngeal carcinoma, remains to
be identified.
In the present study, the expression of PITX1 and
its clinical significance in HNSCC was investigated. The present
study also examined the association of a frequently reported, but
not validated in HNSCC, p53 gene mutation (18–24). The
clinical utility of these biomarkers was then determined in
HNSCC.
Materials and methods
Tissue samples
Tissue samples from biopsies of 47 HNSCCs were
collected prior to the administration of any treatment. All cases
were diagnosed as HNSCC for the first time at the Department of
Otolaryngology, Head and Neck Surgery, Faculty of Medicine, Tottori
University (Yonago, Japan) between January 2008 and December 2012.
The cases included 21 laryngeal carcinomas, 16 hypopharyngeal
carcinomas, 5 oropharyngeal carcinomas and 5 oral carcinomas. Of
these 47 cases, 41 were at stage III or IV, and 6 were at stage I
or II. All the patients received induction chemotherapy comprising
cisplatin, 5-fluorouracil and docetaxel, followed by a local
therapy. In the case of patients >65-years-old, nedaplatin was
used instead of cisplatin. A total of 4 normal samples of
oropharyngeal tissue served as controls. Approval for the present
study was obtained from the Institutional Review Board of the
Faculty of Medicine, Tottori University (Approval No. 2334). All
specimens were fixed in 10% formalin (Wako Pure Chemical
Industries, Ltd., Osaka, Japan) and embedded in paraffin (Wako Pure
ChemicalIndustries, Ltd.). Subsequently, 4 µm-thick sections were
prepared with a microtome and examined using
immunohistochemistry.
Immunohistochemistry
Dewaxed paraffin-embedded sections were
immunostained using the streptavidin-biotin peroxidase complex
method with a HISTOFINE SAB-PO Immunohistochemical Staining kit
(Nichirei Biosciences, Inc., Tokyo, Japan). The sections were
immersed in 3% hydrogen peroxide in phosphate-buffered saline for
60 min to bleach the melanin dye and block endogenous peroxidase
activity. Antigen retrieval was performed by autoclaving with 10 mM
citrate buffer (pH 6.0) for 10 min. The primary antibodies used
were as follows: Rabbit polyclonal anti-PITX1 (1:500, Abcam,
Cambridge, UK) and mouse monoclonal anti-p53 (DO7; 1:50; Dako,
Glostrup, Denmark), which is thought to detect the p53 gene
mutation. Immunoreactions were visualized with diaminobenzidine
(DakoCytomation, Glostrup, Denmark) and the sections were
counterstained with hematoxylin (Wako Pure Chemical Industries,
Ltd.). Evaluation of the immunoreactive cells was performed using a
light microscope (Eclipse E400; Nikon, Tokyo, Japan; magnification,
×400) in four random visual fields in each slide. In the HNSCC
cases, the percentage of immunoreactive cells among the tumor cells
was calculated, and in the control cases, the percentage of those
among normal squamous cells was calculated. The percentage of
immunoreactive cells was defined as the labeling index (LI) for
each primary antibody.
Evaluation of the response to
chemotherapy
Following one cycle of neoadjuvant chemotherapy,
computed tomography or magnetic resonance imaging was performed.
The response to chemotherapy was subsequently determined, according
to the RECIST guidelines (25) and
classified as a complete response (CR), partial response (PR), or
stable disease (SD) or progressive disease (PD).
Statistical analysis
The Mann-Whitney U test was used to compare the
expression of PITX1 in HNSCC with that of the control cases and to
evaluate the association between the expression of PITX1 or p53,
and clinical factors, including the response to chemotherapy,
prognosis and degree of differentiation. Kaplan-Meier analysis was
also used to assess the overall survival and disease-free survival.
The differences in survival rates were assessed by means of the
log-rank test. The data are presented as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of PITX1 and p53 in
HNSCC
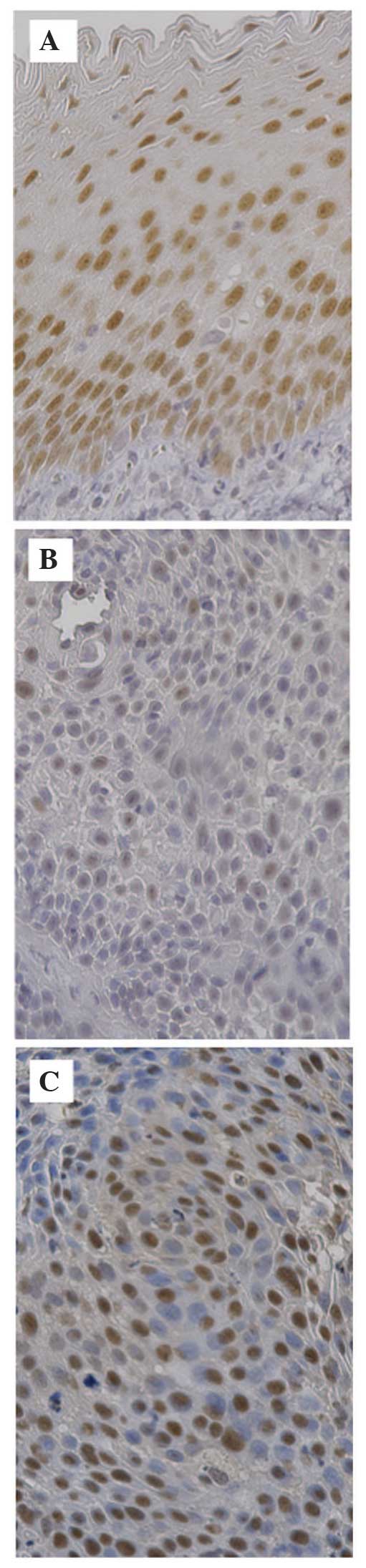
Immunohistochemical analysis revealed that the
expression of PITX1 in the samples of normal pharyngeal tissue was
clearly visible as a brown nucleus in the squamous-epithelium
cells, particularly in the basal cell layer (Fig. 1A). By contrast, PITX1 expression in
the HNSCC samples was detected less in a smaller percentage of
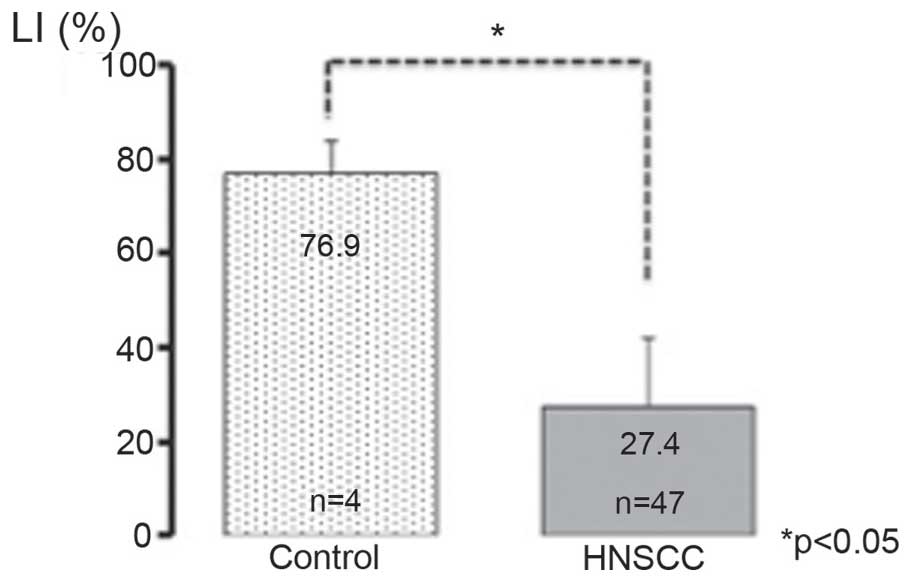
cells compared with in the normal control (Fig. 1B). The PITX1 LI was 76.9±6.97% in the
control pharyngeal samples and 27.4±14.5% in the HNSCC samples
(Fig. 2). This difference was
statistically significant (P<0.05).
Immunohistochemical analysis also revealed that p53
expression was clearly observed in the nuclei of HNSCC cells
(Fig. 1C), however, not in normal
pharyngeal epithelial cells. The p53 LI was 23.0±15.4% in the HNSCC
samples.
Correlation between the expression
levels of PITX1 or p53 and the response to chemotherapy
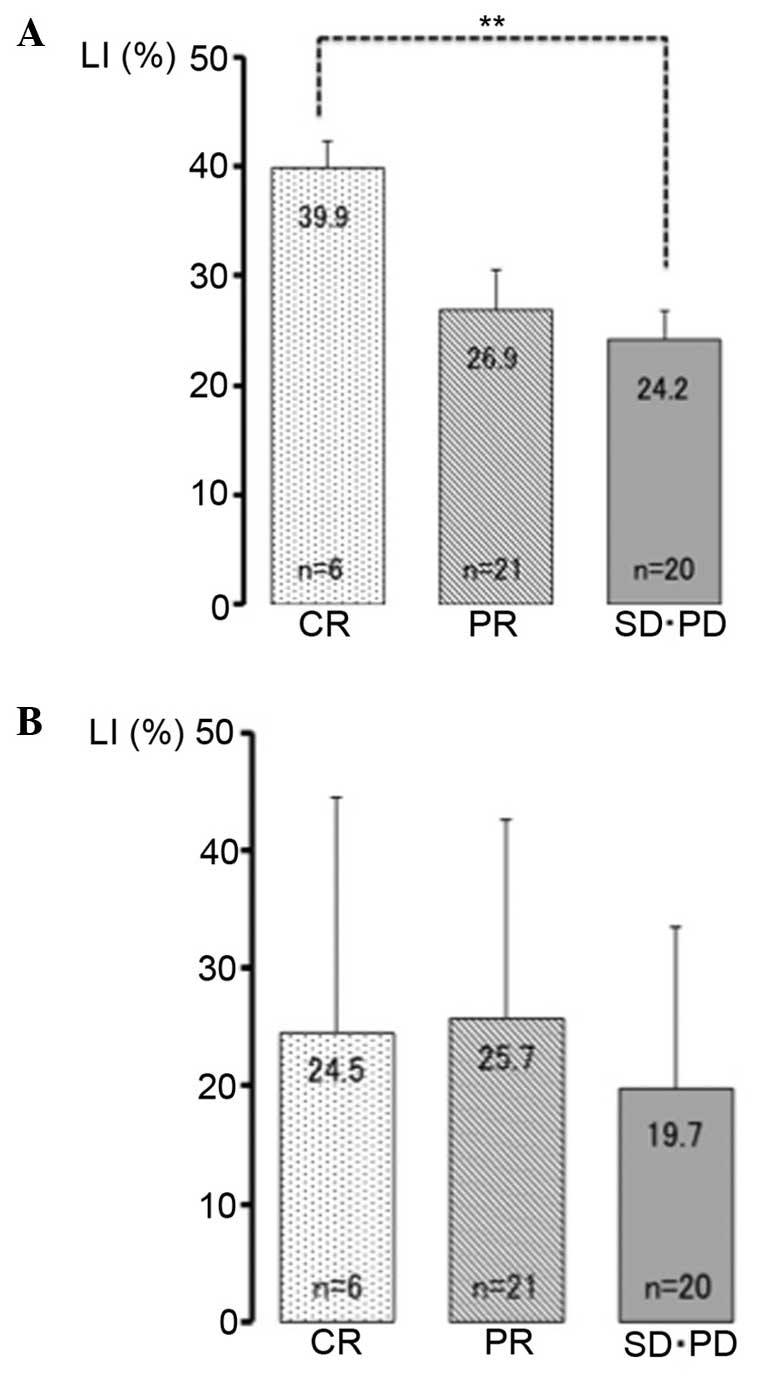
Of the 47 HNSCC patients, 6 patients exhibited a CR,
21 exhibited a PR, 19 exhibited a SD and 1 exhibited a PD. The
PITX1 LIs were 39.9±6.2, 26.9±16.9 and 24.2±11.8% in the CR, PR and
SD/PD groups, respectively (Fig. 3A).
The PITX1 LI in the CR group exhibited the highest result among all
groups and was significantly higher compared with that of the SD/PD
groups (P<0.01). The p53 LIs were 24.5±19.9, 25.7±16.9 and
19.8±13.8% in the CR, PR and SD/PD groups, respectively (Fig. 3B). No significant differences were
observed between the groups.
Correlation between the expression of
PITX1 and the degree of differentiation
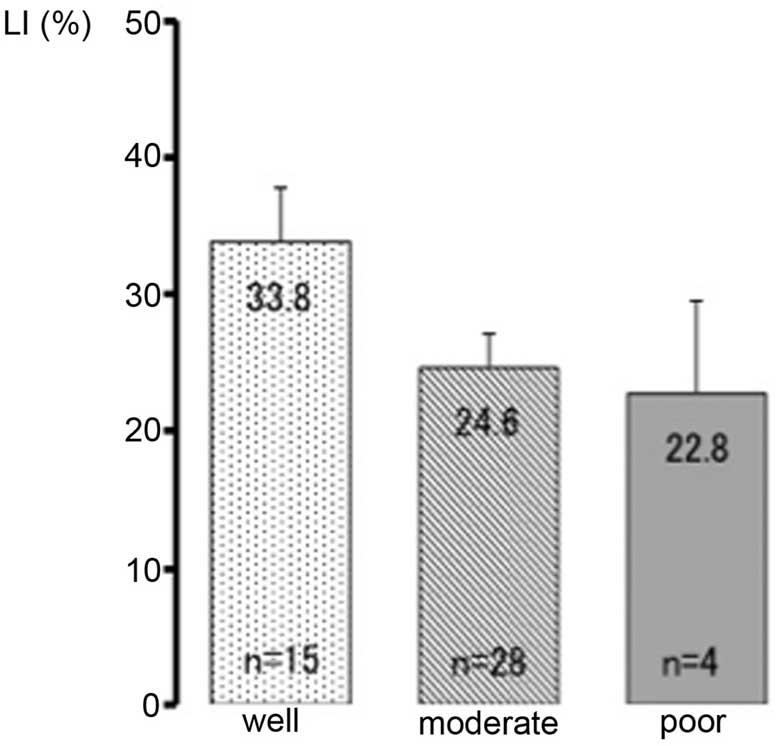
Of the 47 cases, 15 exhibited well differentiated
carcinoma, 28 exhibited moderately differentiated carcinoma and 4
exhibited poorly differentiated carcinoma. The PITX1 LIs were
33.8±15.2, 24.6±13.6 and 22.8±13.4% in the well differentiated,
moderately differentiated, and poorly differentiated groups,
respectively (Fig. 4). No significant
differences were observed between these subgroups.
Correlation between the expression of
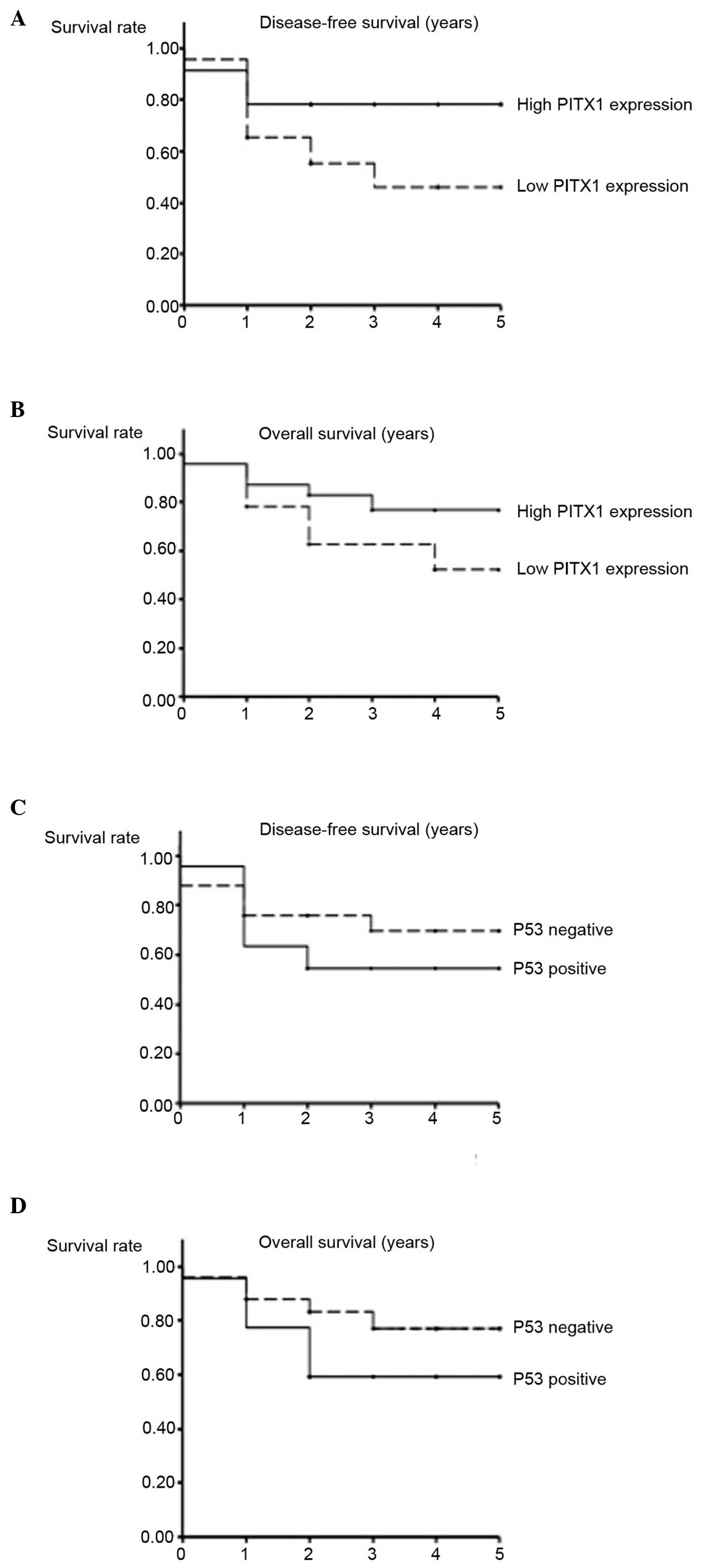
either PITX1 or p53 and prognosis
To compare the differences in disease-free survival
and overall survival, the HNSCC samples were separated into two
groups by PITX1 LIs. The samples whose LIs were <28.9 (the
median LI of all HNSCC samples) were designated as the low PITX1
expression group, and the other samples were designated as the high
PITX1 expression group. Although the higher expression of PITX1
tended to be associated with an improved prognosis, no significant
differences were observed between the two groups (χ2=3.06 and
P=0.08 for disease-free survival and χ2=2.20 and P=0.14 for overall
survival; Fig. 5A and B).
Additionally, the samples whose LI was <20.0 were designated as
the p53 mutation negative (n=25) group, and the others
termed the p53 mutation positive (n=22) group. No
significant differences were observed between these two groups with
regards to disease-free survival (χ2=1.08; P=0.30) overall survival
(χ2=2.07; P=0.15; Fig. 5C and D).
Discussion
The present study demonstrated that the expression
of PITX1 was downregulated in HNSCC samples compared with that in
normal pharyngeal samples, suggesting that PITX1 may act as a tumor
suppressor and a possible novel biomarker in HNSCC and other human
malignancies (8–17). A previous study demonstrated that a
low expression of PITX1 is associated with a poor prognosis in
colorectal carcinoma (16) and
hepatocellular carcinoma (15). It
was reported that a low expression of PITX1 is also associated with
poor differentiation of gastric cancer (12) and oral squamous cell carcinoma
(9). However, the clinical
significance of PITX1 in HNSCC remains unclear. Therefore, the
present study assessed this significance and the potential use of
PITX1 as a novel biomarker of HNSCC.
The present results revealed that the expression of
PITX1 was significantly higher in the CR group compared with the
SD/PD group, suggesting that higher expression of PITX1 is
associated with higher chemosensitivity of HNSCC. It is known that
histological differentiation is associated with the
chemosensitivity of HNSCC (26) and
that the level of PITX1 expression correlates with differentiation
of oral squamous cell carcinoma (9)
and human gastric cancer (12).
Accordingly, the correlation between the expression of PITX1 and
the degree of differentiation was assessed. No statistically
significant result was obtained. These data markedly suggested that
PITX1 is a possible biomarker for predicting chemosensitivity,
independently from histological differentiation of HNSCC.
It is known that p53 is a commonly mutated
tumor suppressor gene in HNSCC (18).
Several previous studies have shown that a p53 gene mutation
is a prognostic biomarker of HNSCC (19–21) and
that a p53 mutation is associated with the risk of a poor
response to chemotherapy (22,23). By
contrast, it was also reported that a p53 mutation is not
significantly associated with prognosis (24) or chemosensitivity (27) in HNSCC. According to the present
results, neither PITX1 nor p53 are statistically significant
predictors of overall survival and disease-free survival. The
p53 mutation is not significantly associated with
chemosensitivity in the present study, although the differences
from the previous reports in the design of chemotherapy must be
taken into consideration. Therefore, the expression of p53 as a
clinical biomarker in HNSCC remains controversial.
The present study is the first, to the best of our
knowledge, to describe the expression of PITX1 and a correlation
between the expression of PITX1 and the chemosensitivity of HNSCC.
However, the mechanisms that underlie the association between PITX1
and chemosensitivity remain unclear. Previously, PITX1 was
identified as a tumor suppressor that downregulates the RAS pathway
by acting on RASAL1, a member of the RAS-GTPase activating protein
family (7). The protein expression of
wild-type KRAS2 is a major determinant of the proliferation of
HNSCC cells. Amplification of unmutated KRAS2 in HNSCC
contributes to tumor growth (28). It
was also reported that PITX1 suppresses TERT by directly binding to
the TERT promoter (8), which is known
to be a component of the telomerase enzyme and is necessary for
cancer cell immortalization and proliferation (29). Previously, Ohira et al
(30) reported that the mRNA
expression level of PITX1 is directly suppressed by microRNA-19b,
followed by the upregulation of TERT expression in melanoma cells
(30). In addition, certain previous
reports have shown that upregulated telomerase activation, as well
as the mRNA expression of TERT, correlates with poor
chemosensitivity (31–33). These previous reports appear to
support the hypothesis that attenuating the expression of PITX1 by
miR-19b may be associated with malignancy and may assist with
chemoresistance via telomerase activation in HNSCC cells.
The present study is the first, to the best of our
knowledge, to report an association between PITX1 and HNSCC. The
downregulation of PITX1 in head and neck squamous epithelial cells
may be involved in the carcinogenesis of HNSCC. Therefore, PITX1 is
considered a candidate tumor suppressor gene. Additionally, PITX1
may serve as a novel biomarker for predicting the response to
chemotherapy in HNSCC. Future investigaitons are required to
determine the potential role of PITX1 for modulating
chemosensitivity in HNSCC cells.
Acknowledgements
The present study was supported by JSPS KAKENHI (no.
22501012).
References
|
1.
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Al-Sarraf M: Treatment of locally advanced
head and neck cancer: Historical and critical review. Cancer
Control. 9:387–399. 2002.PubMed/NCBI
|
|
3.
|
Seiwert TY and Cohen EEW: State-of-the-art
management of locally advanced head and neck cancer. Br J Cancer.
92:1341–1348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Urba S, Wolf G, Eisbruch A, Worden F, Lee
J, Bradford C, Teknos T, Chepeha D, Prince M, Hogikyan N and Taylor
J: Single-cycle induction chemotherapy selects patients with
advanced laryngeal cancer for combined chemoradiation: A new
treatment paradigm. J Clin Oncol. 24:593–598. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lamonerie T, Tremblay JJ, Lanctôt C,
Therrien M, Gauthier Y and Drouin J: Ptx1, a bicoid-related homeo
box transcription factor involved in transcription of the
pro-opiomelanocortin gene. Genes Dev. 10:1284–1295. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
DeLaurier A, Schweitzer R and Logan M:
Pitx1 determines the morphology of muscle, tendon and bones of the
hindlimb. Dev Biol. 299:22–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kolfschoten IGM, van Leeuwen B, Berns K,
Mullenders J, Beijersbergen RL, Bernards R, Voorhoeve PM and Agami
R: A genetic screen identifies PITX1 as a suppressor of RAS
activity and tumorigenicity. Cell. 121:849–858. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Qi DL, Ohhira T, Fujisaki C, Inoue T, Ohta
T, Osaki M, Ohshiro E, Seko T, Aoki S, Oshimura M and Kugoh H:
Identification of PITX1 as a TERT suppressor gene located on human
chromosome 5. Mol Cell Biol. 31:1624–1636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Libório TN, Acquafreda T,
Matizonkas-Antonio LF, Silva-Valenzuela MG, Ferraz AR and Nunes FD:
In situ hybridization detection of homeobox genes reveals distinct
expression patterns in oral squamous cell carcinomas.
Histopathology. 58:225–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Osaki M, Chinen H, Yoshida Y, Ohhira T,
Sunamura N, Yamamoto O, Ito H, Oshimura M and Kugoh H: Decreased
PITX1 gene expression in human cutaneous malignant melanoma and its
clinicopathological significance. Eur J Dermatol. 23:344–349.
2013.PubMed/NCBI
|
|
11.
|
Lord RV, Brabender J, Wickramasinghe K,
DeMeester SR, Holscher A, Schneider PM, Danenberg PV and DeMeester
TR: Increased CDX2 and decreased PITX1 homeobox gene expression in
Barrett's esophagus and Barrett's-associated adenocarcinoma.
Surgery. 138:924–931. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Chen YN, Chen H, Xu Y, Zhang X and Luo Y:
Expression of pituitary homeobox 1 gene in human gastric
carcinogenesis and its clinicopathological significance. World J
Gastroenterol. 14:292–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Chen Y, Knösel T, Ye F, Pacyna-Gengelbach
M, Deutschmann N and Petersen I: Decreased PITX1 homeobox gene
expression in human lung cancer. Lung Cancer. 55:287–294. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Stender JD, Stossi F, Funk CC, Charn TH,
Barnett DH and Katzenellenbogen BS: The estrogen-regulated
transcription factor PITX1 coordinates gene-specific regulation by
estrogen receptor-alpha in breast cancer cells. Mol Endocrinol.
25:1699–1709. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Calvisi DF, Ladu S, Conner EA, Seo D,
Hsieh JT, Factor VM and Thorgeirsson SS: Inactivation of Ras
GTPase-activating proteins promotes unrestrained activity of
wild-type Ras in human liver cancer. J Hepatol. 54:311–319. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Knösel T, Chen Y, Hotovy S, Settmacher U,
Altendorf-Hofmann A and Petersen I: Loss of desmocollin 1–3 and
homeobox genes PITX1 and CDX2 are associated with tumor progression
and survival in colorectal carcinoma. Int J Colorectal Dis.
27:1391–1399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kwok SC, Liu XM, Mangel P and Daskal I:
PTX1 (ERGIC2)-VP22 fusion protein upregulates interferon-beta in
prostate cancer cell line PC-3. DNA Cell Biol. 25:523–529. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Somers KD, Merrick MA, Lopez ME, Incognito
LS, Schechter GL and Casey G: Frequent p53 mutations in head and
neck cancer. Cancer Res. 52:5997–6000. 1992.PubMed/NCBI
|
|
19.
|
Poeta M, Manola J, Goldwasser MA,
Forastiere A, Benoit N, Califano JA, Ridge JA, Goodwin J, Kenady D,
Saunders J, et al: TP53 mutations and survival in squamous-cell
carcinoma of the head and neck. N Engl J Med. 357:2552–2561. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Warnakulasuriya S, Jia C, Johnson N and
Houghton J: p53 and P-glycoprotein expression are significant
prognostic markers in advanced head and neck cancer treated with
chemo/radiotherapy. J Pathol. 191:33–38. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Mannarini L, Bertino G, Morbini P, Villa C
and Benazzo M: Markers of chemoradiation resistance in patients
with locally advanced head and neck squamous cell carcinoma,
treated by intra-arterial carboplatin and concurrent radiation.
Acta Otorhinolaryngol Ital. 27:173–180. 2007.PubMed/NCBI
|
|
22.
|
Temam S, Flahault A, Périé S, Monceaux G,
Coulet F, Callard P, Bernaudin JF, St Guily JL and Fouret P: P53
gene status as a predictor of tumor response to induction
chemotherapy of patients with locoregionally advanced squamous cell
carcinomas of the head and neck. J Clin Oncol. 18:385–394.
2000.PubMed/NCBI
|
|
23.
|
Okumura K, Hasegawa Y, Harada H, Ishizaki
K and Murakami S: A comparative study of p53 status and clinical
response to chemotherapy in head and neck cancer. Nagoya Med J.
46:171–180. 2003.
|
|
24.
|
Szentkúti G, Dános K, Brauswetter D,
Kiszner G, Krenács T, Csákó L, Répássy G and Tamás L: Correlations
between prognosis and regional biomarker profiles in head and neck
squamous cell carcinomas. Pathol Oncol Res. 21:643–650. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweji J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guldelines to
evaluate the response to treatment in solod tumors. J Natl Cancer
Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Nakashima T, Maehara Y, Kohnoe S, Hayashi
I and Katsuta Y: Histologic differentiation and chemosensitivity of
human head and neck squamous cell carcinomas. Head Neck.
12:406–410. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Hoffmann TK, Sonkoly E, Hauser U, van
Lierop A, Whiteside TL, Klussmann JP, Hafner D, Schuler P,
Friebe-Hoffmann U, Scheckenbach K, et al: Alterations in the p53
pathway and their association with radio- and chemosensitivity in
head and neck squamous cell carcinoma. Oral Oncol. 44:1100–1109.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Hoa M, Davis SL, Ames SJ and Spanjaard RA:
Amplification of wild-type K -ras promotes growth of head and neck
squamous. Cancer Res. 62:7154–7156. 2002.PubMed/NCBI
|
|
29.
|
Blagoev KB: Cell proliferation in the
presence of telomerase. PLoS One. 4:e46222009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Ohira T, Naohiro S, Nakayama Y, Osaki M,
Okada F, Oshimura M and Kugoh H: miR-19b regulates hTERT mRNA
expression through targeting PITX1 mRNA in melanoma cells. Sci Rep.
5:82012015. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Ueda Y, Hiyama E, Kamimatsuse A, Kamei N,
Ogura K and Sueda T: Wnt signaling and telomerase activation of
hepatoblastoma: Correlation with chemosensitivity and surgical
resectability. J Pediatr Surg. 46:2221–2227. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Wang L, Li PF, Geng M, Cao YC and Yin YC:
Correlation between chemosensitivity to anticancer drugs and
telomerase reverse transcriptase mRNA expression in gastric cancer.
Diagn Pathol. 8:332013. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Guo X, Wang W, Zhou F, Lu Z, Fang R, Jia
F, Bu X, Li R, Zhang B, Wu M and Wei L: siRNA-mediated inhibition
of hTERT enhances chemosensitivity of hepatocellular carcinoma.
Cancer Biol Ther. 7:1555–1560. 2008. View Article : Google Scholar : PubMed/NCBI
|