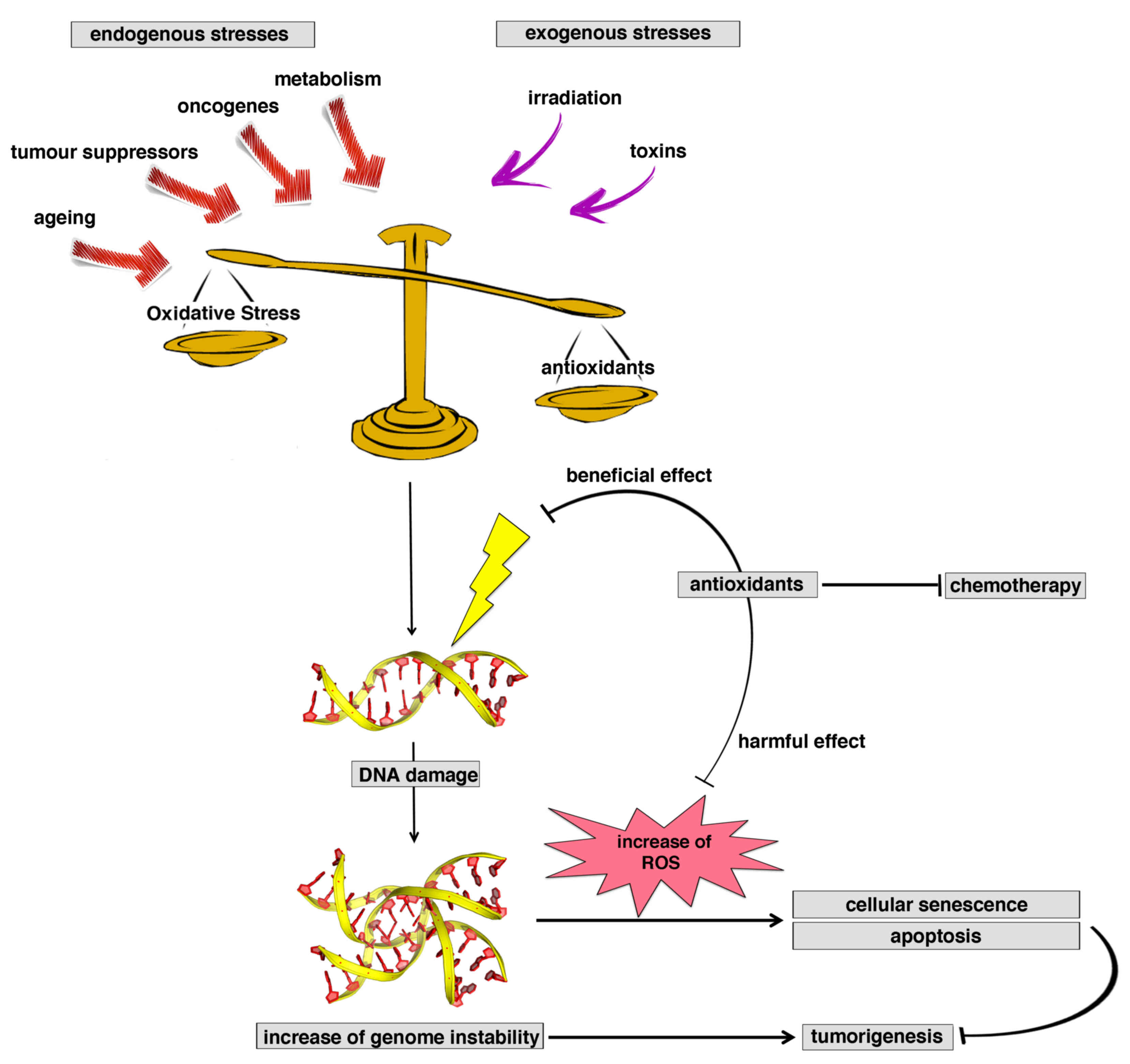
Life in an oxygen-rich environment has to deal with
the danger of oxidative stress. Oxidative stress represents a
biochemical state characterized by an excessive presence of free
radicals and reactive metabolites potentially harmful for the
organism (1,2). Free radicals are highly reactive
chemical species, typically with a short half-life, consisting of
an atom or a molecule containing one or more unpaired electrons.
These electrons give a significant reactivity to the radical,
making it able to bind to other radicals or subtract an electron
from other molecules nearby. Reactive oxygen species (ROS) are the
most important class of free radicals that are produced by
organisms: Elevated levels result from an imbalance between the
production of oxidants and their elimination by the antioxidant
system protecting the organism. The superoxide anion radical (O2=)
is one of the best known ROS. Its metabolites, such as the hydroxyl
radical (•OH) and hydrogen peroxide (H2O2),
are very reactive (3). Reactive
nitrogen species (RNS) are another family of free radicals (with
antimicrobial action), derived from nitric oxide (•NO) and
superoxide anion (O2=), that, acting together with ROS, can damage
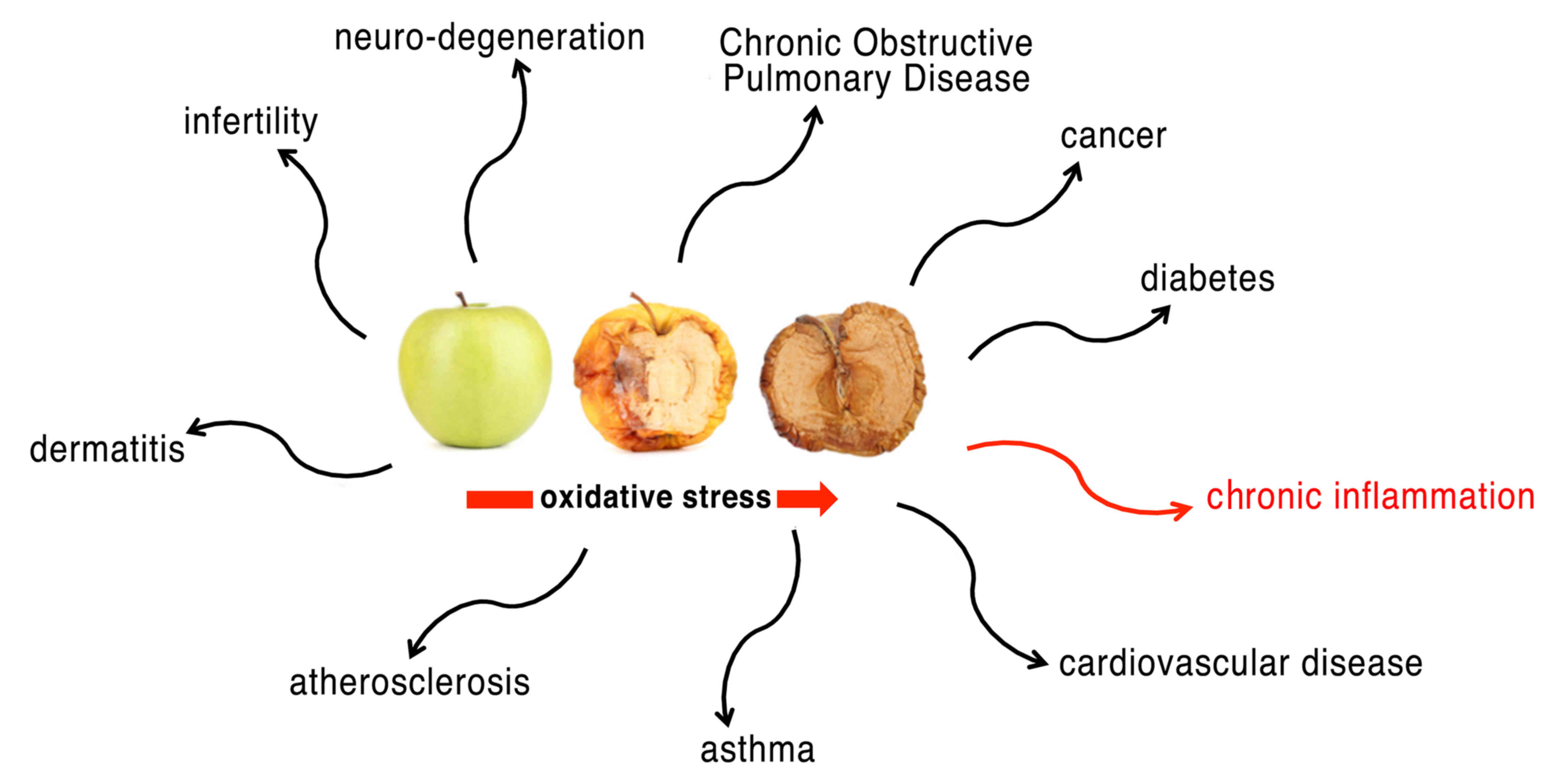
cells. Not surprisingly, in humans, associations between the level
of oxidative stress and serious diseases, including diabetes
mellitus, atherosclerosis, hypertension, inflammatory diseases,
neurodegenerative disorders and cancer, are well known (1,2,4) (Fig.
1).
During normal cell metabolism, the production of ATP
by aerobic respiration in mitochondria constantly produces ROS and
RNS, such as the by-products of oxidative phosphorylation.
At low to moderate concentrations, ROS exert an
important positive role in several physiological processes,
including defense against infectious agents and cell signaling
(5), although at high concentrations
they are able to react with many cellular components, such as
nucleic acids, proteins and lipids, causing DNA damage that escapes
the DNA repair system. For this reason, their concentration needs
to be strictly controlled. In numerous organisms, ROS, and
especially H2O2, are signaling molecules able
to cross the membranes, to function as second messengers inside the
cell, and to induce specific signal transduction pathways.
Furthermore, ROS and RNS are able to control the activity of
enzymes by triggering several post-translational modifications,
such as disulfide bond formation, thiol oxidation to
sulfenic/sulfinic/sulfonic acid, glutathionylation, nitrosylation
and carbonylation. Several studies have reported that cell
stimulation by a variety of growth factors, cytokines and
G-protein-coupled receptors generates intracellular
H2O2, e.g. (6), so that it may be difficult to resolve
whether the cell is being subjected to
H2O2-dependent signaling or to oxidative
stress. Aerobic organisms are equipped with nonenzymatic
(ascorbate, glutathione, tocopherol and carotenoid) or enzymatic
[catalase (CAT), ascorbate peroxidase, superoxide dismutase (SOD),
glutathione peroxidase (GPx) and peroxiredoxin] antioxidant systems
to neutralize ROS and RNS in the cells and to finely control their
concentrations.
One of the most important enzyme systems that,
together with SOD, CAT and GPx, act in the defense against
oxidative stress is the “peroxiredoxin” (PRDX) family (7,8).
PRDXs have been identified in numerous organisms and
constitute a ubiquitous family of thiol-dependent peroxidases,
catalyzing the reduction of H2O2, alkyl
hydroperoxides and peroxynitrite to water, the corresponding
alcohol and nitrite, respectively (9–11),
emerging as arguably the most important and widespread peroxide and
peroxynitrite scavenging enzymes in all of biology (12,13).
Their role was long overshadowed by well-studied oxidative stress
defense enzymes such as catalase and glutathione peroxidase,
considered for a long time to be the major enzymes responsible for
protecting cells against hydroperoxides.
Unlike heme-dependent catalase and the
selenium-dependent glutathione peroxidase, PRDXs do not require
cofactors. They were identified approximately 27 years ago in yeast
(14) and 25 years ago in mammals
(15), and are functionally
conserved in all three phylogenetic domains: Archaea, Bacteria and
Eukaryota, stressing the importance of the existence of systems
protecting against ROS for the evolution of living organisms
(16) (Table I).
PRDXs have been classified into the following
subgroups on the basis of functional site sequence similarity
(17): Prx1/PRDX1, Prx5/PRDX5 and
Prx6/PRDX6 (18). The phylogenetic
distribution of PRDXs demonstrates the widest biological
distribution for the Prx1/PRDX1 and Prx6/PRDX6 subfamilies;
Prx5/PRDX5 members are apparently lost in archaea (Table I).
PRDXs of different subgroups vary in their
oligomerization states, conformational flexibility, and certain
secondary structural elements. In addition, most organisms possess
multiple isoforms (19): In humans,
for example, six different isoforms of PRDX are present (20), four PRDX1 subtypes, one PRDX5 subtype
and one PRDX6 subtype.
PRDX1, PRDX2, PRDX3, PRDX5 and PRDX6 are localized
in the cytosol, in the mitochondria, in the nuclei and in the
peroxisomes (21–23), whereas PRDX4 is mainly present in the
endoplasmic reticulum, or it is secreted (24).
The catalytic activity of PRDXs is crucially
dependent on a conserved peroxidatic Cys (Cp) residue
contained within a universally conserved Pxxx(T/S)xxC active-site
motif in the amino-terminal portion of the protein (17), which corresponds to Cys-47 in yeast
cytosolic thioredoxin peroxidase I (cTPx I) (15). Five out of six human PRDXs also
contain an additional conserved Cys in the carboxy-terminal region,
which corresponds to Cys-170 in yeast thiol-specific antioxidant
(TSA) (15), termed resolving
cysteine (Cr). Depending on the PRDX, the Cr
may be located within the same chain of Cp or in the
chain of another subunit, therefore human PRDXs are classified into
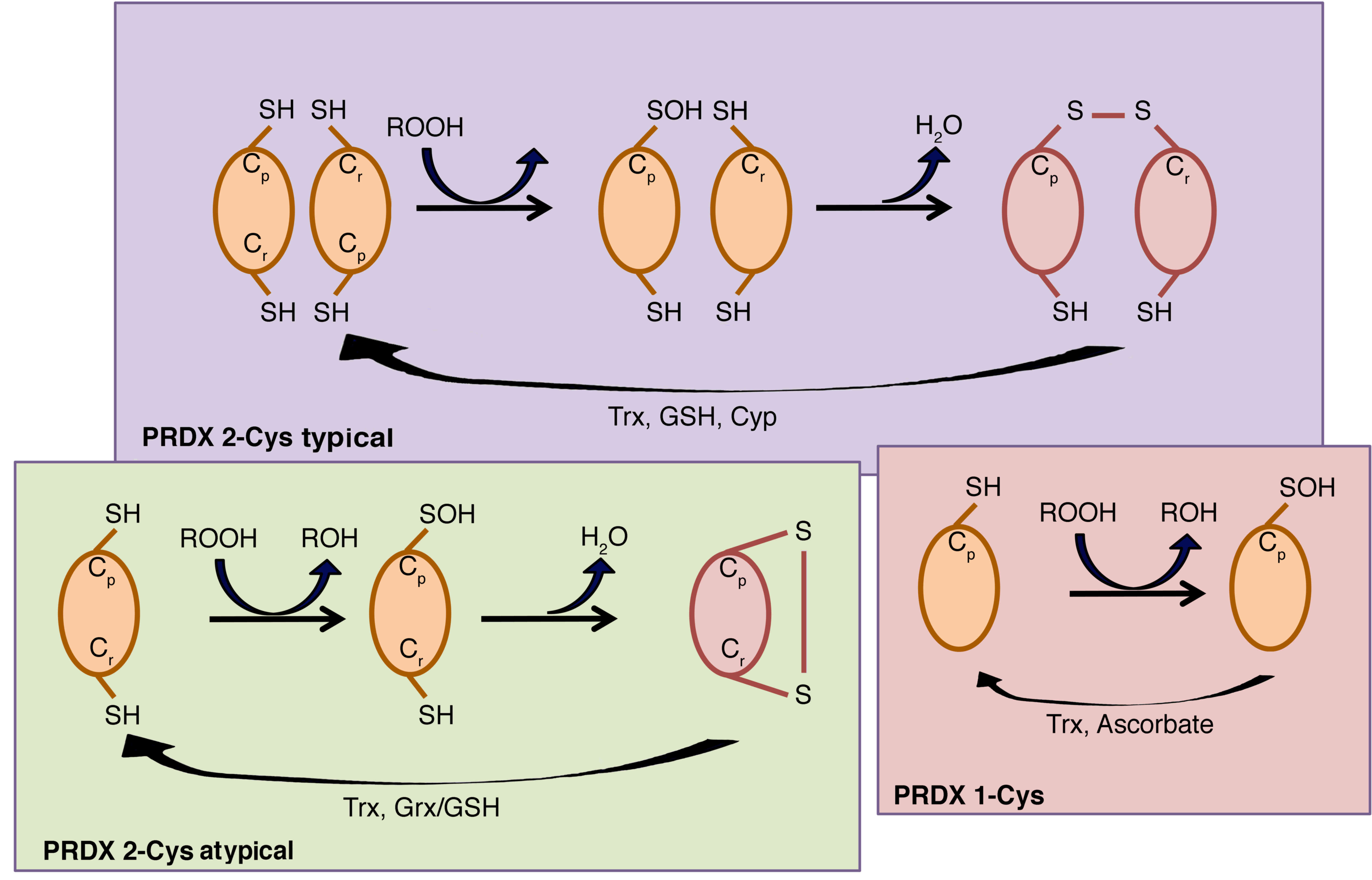
three classes: i) Typical 2-Cys PRDXs, which include PRDX1-4, ii)
atypical 2-Cys PRDX and PRDX5, and iii) 1-Cys PRDX and PRDX6
(25). The typical 2-Cys PRDXs are
obligate homodimers containing two identical active sites, bringing
the two redox-active cysteines (Cp and Cr)
into close proximity (26). By
contrast, atypical 2-Cys PRDXs form an intramolecular disulfide
intermediate by reacting the amino-terminal sulfenic acid (Cys-47)
with a carboxy-terminal Cys-SH (Cys-151) of the same molecule that
is able to be reduced by thioredoxin (27).
PRDX6 is the only known mammalian member of the
1-Cys subgroup. The mechanism by which its sulfenic acid form is
reduced has yet to be fully elucidated (27), but Monteiro et al (28) have unequivocally demonstrated that
1-Cys PRDXs are reduced by ascorbate (Fig. 2).
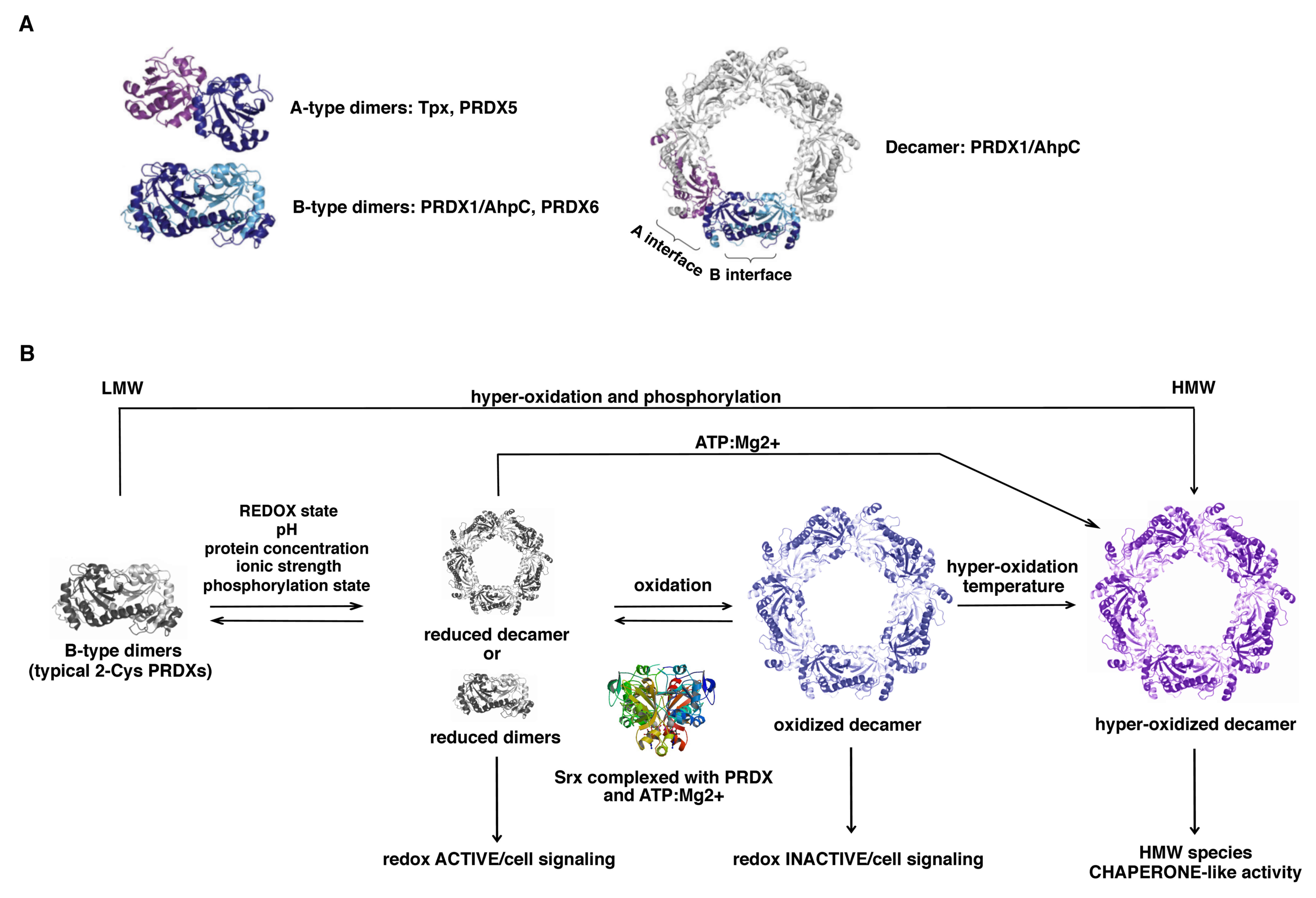
Members of the Prx1/PRDX1 and Prx6/PRDX6 subfamilies
dimerize using the ‘B interface’ (denoting the β-strand
interactions) to form an extended 10 to 14-strand β-sheet (29) (Fig.
3), whereas members of the Prx5/PRDX5 subfamily typically
dimerize and associate across the ‘A interface’ (denoting either
‘alternate’ or ‘ancestral’). In addition, a large number of PRDXs
that form dimers across their B interface can show a further
redox-sensitive dependent oligomerization to form octamers,
decamers or dodecamers across their A interface (19).
Typical 2-Cys PRDXs (PRDX1-4) form decamers or
dodecamers in the reduced or hyperoxidized state, acquiring the
ability to exercise other functions as chaperones, binding
partners, enzyme activators and/or redox sensors, while the
oxidized form is preferentially present as dimers (30) (Fig.
3). Atypical 2-Cys PRDXs are able to undergo protein-protein
interactions with functional implications, although their level of
polymerization is less compared with that of typical 2-Cys
PRDXs.
By contrast, 1-Cys PRDXs are not able to form
decamers, and this is probably the reason why they serve mainly an
antioxidant function rather than a molecular chaperone function,
despite their enzymatic mechanism being very similar to that of
2-Cys PRDXs (31). Concerning the
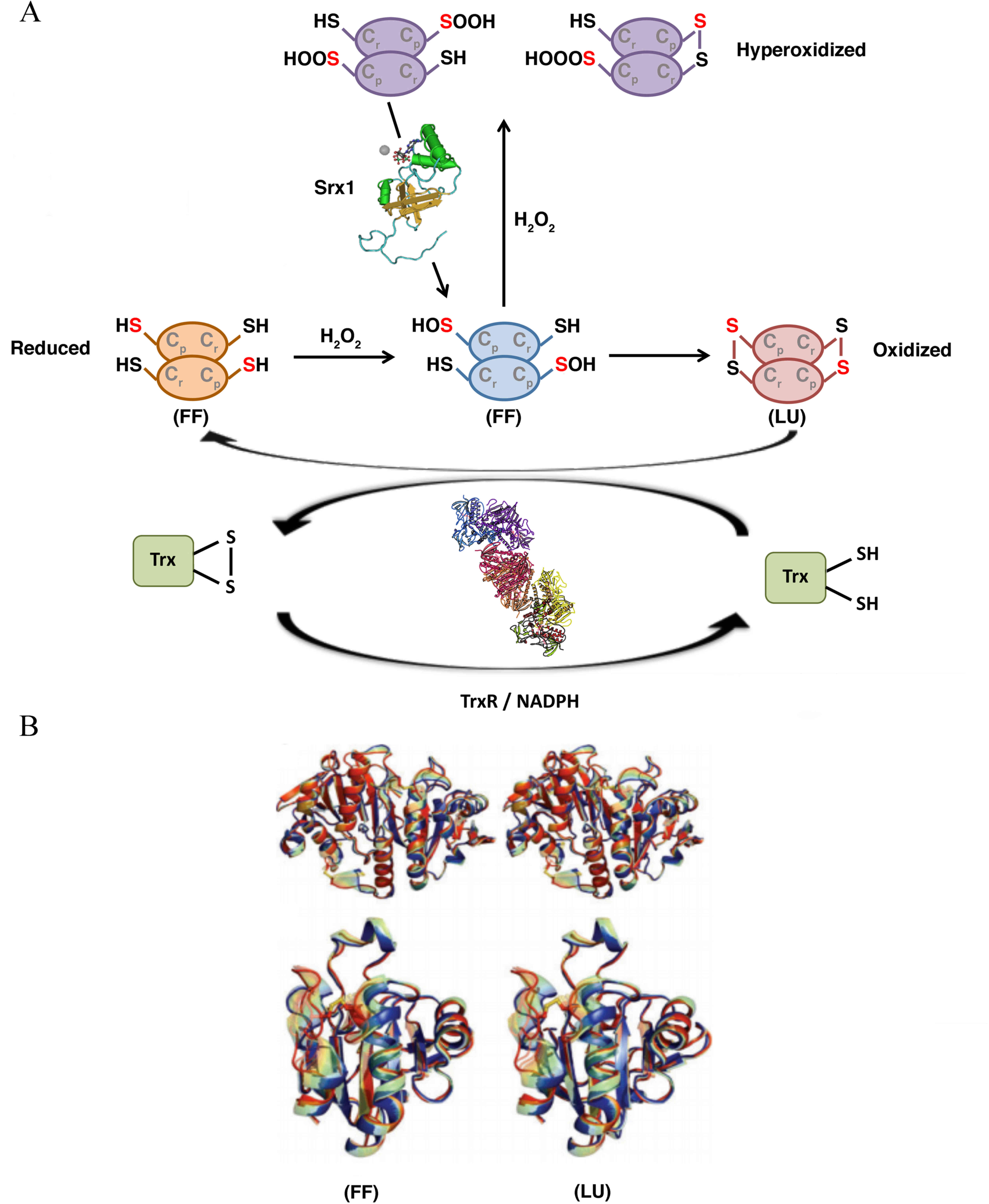
catalytic function, PRDXs tune the sensitivity to hyperoxidation
switching from a fully folded (FF) conformation, in which
Cp can react with the peroxide, to a locally unfolded
(LU) conformation, in which the Cp is exposed and can
form a disulfide bridge with the Cr (Fig. 4).
PRDXs are highly involved in the control of cellular
physiological functions, including growth, differentiation,
apoptosis, embryonic development, lipid metabolism, the immune
response, as well in the maintenance of cellular homeostasis
(32) (Fig. 5). Over the course of the last few
years, a large body of evidence has suggested their involvement in
carcinogenesis and in the development of drug resistance. This
review focuses on the complex relationships between oxidant balance
and cancer, and it provides a brief account of the involvement of
PRDXs in tumorigenesis and in the development of
chemoresistance.
Moreover, the tendency of cancer cells to undergo
profound changes in their own intrinsic metabolism (Warburg
metabolic reprogramming), characterized by increased activity in
aerobic glycolysis and by lipid metabolism deregulation, is also
largely modulated by oxidative stress. Therefore, ROS may promote
numerous aspects of tumor onset and progression towards a malignant
phenotype (45–47). Nevertheless, it should not be
overlook that high levels of ROS can be lethal for the cancer
cells. This could be one of the reasons why cancer cells, in order
to defend themselves, potentiate their antioxidant capacity
(48,49).
Cells are endowed with several overlapping
peroxide-degrading systems, the relative importance of which is a
matter of debate. PRDXs are a fascinating group of thiol-dependent
peroxidases that, under physiological conditions, are responsible
for divergent functions, such as protecting cells against oxidative
DNA damage and genomic instability, regulating cell signaling
associated with H2O2, and influencing cell
differentiation and proliferation, immune responses and apoptosis
(50) (Fig. 6). A number of studies have
demonstrated that cancer cells exhibit an increased production of
ROS, in part caused by a loss in proper redox control (35,36–39).
Therefore, over the course of the last few years, much attention
has been paid to exploring the role of PRDXs in cancerogenesis.
Increased or decreased levels of PRDXs have been demonstrated in
many human cancers. Studies performed in vitro or in
vivo models have demonstrated that overexpression of PRDXs may
either inhibit cancer development or promote cancer growth
(48), depending on the specific
PRDX family member and on the cancer context.
In the following chapters, the most recent findings
regarding the dual action of PRDXs in tumorigenesis are reviewed
and discussed. Table II summarizes
different types of cancer in which the expression of an individual
member of the PRDX family is altered.
Amongst the PRDX family members, PRDX1 possess the
widest cellular distribution and show the highest abundance in
various tissues (51). Its cellular
expression is controlled at the transcriptional level by nuclear
factor (erythroid-derived 2)-related factor 2 (NRF2) (52), and at the post-transcriptional level,
through degradation and deadenylation/polyadenylation processes
(53).
A tumor suppressor function of PRDX1 was first
demonstrated in a knockout-mouse model, where its deficiency
generated mice suffering from hemolytic anemia and multiple tumors,
including mammary carcinomas (54–56).
These studies suggested that the tumor suppressive effects of PRDX1
were mediated by a reduction of c-Myc transcriptional activity
(55) or of phosphatase and tensin
homolog (PTEN/AKT) activity (56).
In particular, PRDX1 exerts its protective effect by oxidation of
the Cys residue located within the active site of PTEN phosphatase,
thereby reducing the predisposition of PRDX1-deficient mice to
develop Ras-induced mammary tumors (56).
In breast cancer (estrogen-receptor-positive cases),
PRDX1 prevents oxidative stress-mediated estrogen receptor α
reduction. Its overexpression in these cancer tissues may be
considered as a biomarker of favorable prognosis (57). In lung cancer, the tumor suppressant
effect is mediated by the modulation of the ROS-mediated activation
of the K-Ras/extracellular signal-regulated kinase (ERK) pathway
(58). Similarly, in human acute
myeloid leukemia (AML), PRDX1 promotes the reactivation of the
protein tyrosine phosphatase DEP-1, a tumor suppressor that
counteracts the action of the transforming kinase, FLT3 ITD
(59).
The tumor-promoting function of PRDX1 has been
demonstrated in numerous types of human cancer, and appears to be
mediated through its interaction with several cancer-associated
signal pathways. An increased level of PRDX1 has been described in
lung cancer (60), in bladder cancer
(61), in ovarian carcinoma
(62), in aggressive esophageal
squamous carcinomas (63), in hilar
cholangiocarcinoma (64), in liver
cancer (65), in pancreatic cancer
(66), in mesothelioma (67) and in glioblastoma (68). In prostate cancer, PRDX1
overexpression induces tumor growth and tumor progression through
the Toll-like receptor 4 (TLR4)-dependent regulation of tumor
vasculature, increasing the expression of the vascular endothelial
growth factor (VEGF) (69). In
certain lung cancer cellular models, it has become well established
that the pro-oncogenic role of PRDX1 is mediated by the activation
of c-Jun and AP-1 (70), and that
the transforming growth factor β1 (TGFβ1)-induced EMT is caused by
direct inhibition of E-cadherin expression (71). In addition, PRDX1 may exerts its
tumor-promoting function by affecting intracellular signaling
pathways that affect apoptosis. In thyroid cancer cells, it
inhibits apoptosis through the inhibition of apoptosis
signal-regulating kinase 1 (ASK1) activity (72), whereas in human hepatoma it
suppresses the redox-dependent activation of caspases, inducing
tumor necrosis factor-α (TNFα)-related apoptosis-inducing ligand
(TRAIL) resistance (73).
PRDX2 is another member of the typical 2-Cys
subgroup, and it is mainly present in the cytosol (76). In red blood cells (RBCs), the
oxidation-reduction cycle of PRDX2 correlates with a robust
temperature-entrainable and temperature-compensated circadian
rhythm, the oscillations of which result in circadian
rhythm-dependent oligomerization of PRDX2 (77). Notably, the fluctuations in levels of
hyperoxidized PRDX2 are not affected at the transcriptional level,
considering the absence of a nucleus in the RBCs (77); neither are they controlled by
sulfiredoxin (Srx), but are rather controlled by hemoglobin
autoxidation and the 20S proteasome (78). Circadian rhythms are highly conserved
time-tracking systems regulating important biological processes at
the systemic and the cellular level (76). Interestingly, it has been recently
demonstrated that the nuclear levels of PRDX2 oscillate
rhythmically over two entire 24-h long cycles in HaCaT
keratinocytes, contributing to the regulation of the redox balance
of human keratinocytes. These findings open new perspectives for an
understanding of circadian-pathophysiological processes in the skin
(79). It is not yet clear whether
the PRDXs are essential for circadian rhythmicity, although it is
evident that their deletion has generally deleterious cellular
consequences (77).
Depending on the tumor type and the stage of tumor
progression, PRDX2 may exhibit strong tumor-suppressive or
tumor-promoting functions. A decreased expression of PRDX2 has been
demonstrated in only a few types of cancer, among which are the
melanomas. The function of PRDX2 in melanoma cell growth and
metastasis has not yet been fully elucidated. To date, it has been
demonstrated, both in vitro in melanoma cell lines and in
vivo in metastatic melanoma models, that a downregulation of
PRDX2 correlates with increased proliferative and migratory
activities, and with the acquisition of a metastatic potential. In
particular, it appears that the PRX2-mediated signaling pathway for
suppression of melanoma metastasis involves a synergistic
collaboration of the processes of ERK-dependent E-cadherin
expression and the Src-dependent retention of β-catenin in the
adherens junctions (85). In a
colorectal cancer (CRC) cellular model, PRDX2 inhibits
TGFβ1-induced EMT, reducing the invasive phenotype through the
modulation of the transcription factors, Twist1, Snail, ZEB1 and
ZEB2 (86).
Over the course of the last few years, a number of
studies, instead, have revealed that PRDX2 is increased in various
human malignancies, suggesting a possible role for PRDX2 as a tumor
promoter. High levels of PRDX2 and 4 were observed in ovarian
borderline cancer compared with the other benign ovarian lesions,
allowing a hypothesis to be made for the potential use of these in
determining a differential diagnosis between benign and borderline
epithelial ovarian tumors (87).
Elevated expression levels of PRDX2 have also been found in vaginal
carcinoma (88), in cervical cancer
(89), in prostate cancer (90), in esophageal cancer (91) and, more recently, in B cell-derived
primary lymphoma cells (92). In
breast cancer, PRDX2 works like a ‘metabolic adaptor’ driver
protein that specifically induces the selective growth of
metastatic cells in the lung by protecting them against oxidative
stress (93). The dual action of
PRDX2 in tumorigenesis has been demonstrated in a CRC model. Lu
et al (94), in contrast with
what was reported by Feng et al (86), demonstrated that PRDX2 overexpression
in CRC tissues was strongly correlated with a more aggressive
cancer behavior, tumor metastasis and the tumor-node-metastasis
(TNM) stage, indicating a possible role for PRDX2 in CRC
progression. Taken together, these data suggest that the mechanism
by which PRDX2 exerts its oncogenic action is still poorly
understood, and further studies are required.
PRDX3 is a mitochondrial member of the antioxidant
family of thioredoxin peroxidases that uses mitochondrial
thioredoxin 2 (Trx2) as a source of reducing equivalents to
scavenge hydrogen peroxide (95).
Its specific localization to the mitochondria suggests that PRDX3,
together with its mitochondrion-specific electron suppliers, Trx2
and Trx reductase 2 (TrxR2), may provide a primary line of defense
against H2O2 produced by the mitochondrial
respiratory chain (96). PRDX3 is
highly sensitive to the oxidative state. The regulation of its
expression involves sirtuin 1 (SIRT1), a class III histone
deacetylase that is not cell-type-specific, in bovine aortic
endothelial cells. SIRT1 positively controls PRDX3 expression by an
enhancement of the formation of the PGC-1α/FoxO3a transcriptional
complex (97). Depending on the
cancer type, the regulation of PRDX3 expression may be mediated by
different factors. In colon cancer stem cells (CSCs), the forkhead
box protein 1, FOXM1, activates transcription of PRDX3 and the
expression of CD133 (98). In
medulloblastoma tumor tissue samples and cell lines, the level of
the microRNA, miR-383, is a modulator of PRDX3 expression (99), whereas in human prostate cancer
cells, miR-23b directly regulates PRDX3 expression under normal and
hypoxic conditions (100). Finally,
in von Hippel-Lindau (VHL)-deficient clear cell renal cell
carcinoma (CCRCC), the transcription factor, hypoxia-inducible
factor 1 (HIF-1), downregulates the level of PRDX3 (101).
A high level of PRDX3 expression has been reported
in hepatocellular carcinomas (102), in malignant mesothelioma (67), in breast carcinoma (103), in prostate cancer (104), in lung cancer (105) and in cervical carcinoma (106). All these studies have demonstrated
that PRDX3 overexpression in cancer cells correlates with a more
aggressive phenotype.
PRDX4 is another member of the typical 2-Cys PRDX
family, homologous with other typical 2-Cys PRDXs, such as PRDX1
and PRDX2, which share the same catalytic mechanism. It is located
predominantly in the endoplasmic reticulum (ER) and extracellular
spaces, with the highest expression occurring in the pancreas,
liver and heart, and lowest expression in blood leukocytes and the
brain (107). A distinctive
hydrophobic amino-terminus in PRDX4 functions as a signal sequence
involved in the process of secretion from the cells (108). No solid evidence is available
regarding the role of extracellular PRDX4 as a biomarker in certain
types of disease (24). It has been
suggested that the secretable form of PRDX4 may be involved in the
interactions between carcinoma cells and the extracellular
environment (109).
As described above for the other PRDXs,
modifications in PRDX4 levels have been associated with invasion,
recurrence, prognosis, and other characteristics of cancer
(112).
In pancreatic cancer, several reports have described
the downregulation or upregulation of PRDX4 (113,114),
although it is not yet clear whether the PRDX4 expression level may
be considered to be a cause or an effect of pancreatic cancer.
PRDX4 is overexpressed in prostate cancer (90), where it enhances the rate of cell
proliferation (115). In other
types of epithelial cancers, such as oral cavity squamous cell
carcinoma (116), breast cancer
(117), ovarian cancer (118), CRC (119) or lung cancer (70), overexpression of PRDX4 correlates
with the metastatic potential. In particular, in lung cancer A549
cells, the Srx-PRDX4 complex significantly contributes to the
maintenance of anchorage-independent colony formation, cell
migration and invasion (120). It
is noteworthy that PRDX4 is overexpressed in the majority of
cancers where Srx is also overexpressed (113), contributing to cell proliferation
by the activation of the RAS-RAF-MEK signaling pathway (120). On the other hand, its marked
downregulation has been reported in acute promyelocytic leukemia
(APL) (121).
PRDX5 was the last member to be identified among the
six mammalian PRDXs. It is the unique atypical 2-Cys PRDX in
mammals, widely expressed in tissues at different levels, with a
large subcellular distribution including the mitochondria, the
peroxisomes, the cytosol and the nucleus (22). PRDX5 is a thioredoxin peroxidase that
acts mainly by reducing alkyl hydroperoxides and peroxynitrite via
cytosolic or mitochondrial thioredoxins. Its crystal structures
highlight the unconventional enzymatic mechanism, involving two
catalytic Cys residues that provide an opportunity for reaction
with an additional molecule of H2O2, leading
to overoxidation of Cp (122). PRDX5 is a cytoprotective
antioxidant enzyme rather than a redox sensor, able to act against
endogenous or exogenous peroxide attacks. Its overexpression in
different subcellular compartments defends cells against death
provoked by nitro-oxidative stresses, while its silencing makes the
cells more susceptible to oxidative damage and apoptosis (122). It is constitutively expressed at a
high level in different mammalian cell lines and normal tissues,
but the specific transcription factors involved in the regulation
of its expression have not yet been completely identified. It is
known that transcription factors such as AP-1, nuclear factor-κB
(NF-κB), antioxidant response element (ARE), insulin response
element (InRE), glucocorticoid response element (GRE) (123), and also c-Myc (124), may directly modulate PRDX5
expression by interacting with putative responsive elements in the
5′-flanking region of the gene. Other transcription factors, such
as nuclear respiratory factor 1 (NRF-1) and nuclear respiratory
factor 2 (NRF-2; GABPA), involved in the response of mammalian
cells to oxidative stress and in the biogenesis of mitochondria,
are also able to modulate PRDX5 expression in an indirect way
(123,125). c-Myc not only directly controls
PRDX5 transcription, but also contributes in the maintenance of ROS
homeostasis through its ability to selectively induce the
transcription of specific PRDXs when the function of one of them is
compromised (124). Up- or
downregulation of PRDX5 has been reported in different types of
cancer. An upregulation of transcriptional activity of PRDX5,
mediated by E-twenty-six transcription factor 1 and 2 (Ets1/2) and
high-mobility-group protein B1 (HMGB1), has been described in human
prostate and epidermoid cancer cells exposed to
H2O2 or hypoxia (126). Increased levels of PRDX5 have been
reported in aggressive Hodgkin's lymphomas (127), in malignant mesothelioma (67), in breast carcinoma (103), in ovarian carcinoma (87) and in thyroid cancer (128). Reduced levels of PRDX5 expression
have been described only in adrenocortical carcinoma (129).
PRDX6 is the prototype and the only mammalian 1-Cys
member of the PRDX family. Homologous 1-Cys proteins are widely
distributed throughout all kingdoms, and they have been described
in archaea, bacteria, parasites, yeast, insects, mollusks,
amphibians, birds and other orders (130) (Table
I). Although PRDX6 shares structural and functional properties
with other members of the family, it has important and unique
characteristics: It has a single conserved Cys residue causing a
different catalytic cycle, and it uses glutathione (GSH) instead of
thioredoxin as the physiological reductant. Furthermore, PRDX6 is
able to bind and reduce phospholipid hydroperoxides, serving an
important role in the repair of membrane damage caused by oxidative
stress and, finally, it is a bifunctional enzyme with both
phospholipase A2 (PLA2) activity and peroxidase function (130). Its catalytic Cys residue is buried
at the base of a narrow pocket, differently from the other PRDXs,
which renders PRDX6 unable to dimerize through disulfide formation
in the native configuration, although it can homodimerize and
multimerize through hydrophobic interactions (131).
PRDX6 has a widespread distribution in all organs,
and essentially in all cell types (130). Its expression is regulated by the
mainly redox-active regulators, such as Nrf2, Nrf3 (132,133),
NF-κB (134), Sp1 (135), c-Jun, c-Myc (130) and HSF1 (136), which are able to interact with the
ARE and the putative GRE localized in the PRDX6 gene promoter
region. PRDX6 has been reported to be implicated in the development
and progression of several human diseases, such as Alzheimer's
disease (137), Parkinson's
dementia (138), diabetes (139), cataractogenesis (140) and cancer. Concerning the neoplastic
diseases, elevated levels of PRDX6 have been described in breast
cancer (141), in malignant
mesothelioma (67), in bladder
cancer (61), in esophageal cancer
(142), in lung (143), ovarian (87) and pancreas (144) cancer, in cancer of the
gingivo-buccal area (145), and in
lymphoma (146). Elevated
expression levels of PRDX6 have been associated with a more
invasive phenotype and metastatic potential of breast cancer
(147), and with a worse prognosis
of clinically localized prostate cancer following radical
prostatectomy (148).
By contrast, studies performed using a thyroid
proteomic approach have highlighted the reduction of PRDX6 in
follicular adenomas (149),
suggesting a possible role for this protein as a complementary
marker to distinguish between different follicular neoplasms. More
recently, a marked reduction in PRDX6 levels has been demonstrated
in a cohort of PTCs (75). Taken
together, all these studies have demonstrated that PRDX6 has a
pro-tumorigenic function, promoting cell proliferation by its
peroxidase activity, and facilitating invasiveness by means of its
PLA2 activity (150).
To date, the association between PRDX6 single
nucleotide polymorphisms (SNPs) and cancer has yet to be fully
elucidated. In esophageal cancer, no association was identified
between the risk of cancer and clinicopathological characteristics,
including the tumor grade and stage, and the presence of SNPs
(91). However, preliminary studies
in breast cancer have demonstrated that the survival of carriers of
the PRDX6 SNPs, rs4916362 and rs7314, was consistently less
favorable (151).
Cancer cells, compared with normal cells, have a
high rate of ROS production as by-products of their metabolism
(152), and to survive with this
redox status, the levels of antioxidant proteins, such as CAT, SOD,
glutaredoxin and PRDXs, are increased (152–154).
This unique capability of cancer cells may serve an important role
also in the development of resistance to chemo- or radiotherapy, as
these treatments are strongly dependent on ROS-induced
cytotoxicity. A search of the literature demonstrates that
increased levels of PRDXs are often associated with radioresistance
or chemoresistance to numerous drugs. High levels of PRDX2
correlate with radioresistance in breast cancer and glioma cells
(155), as well as with cisplatin
chemoresistance in gastric cancer cell lines (156) and in human erythroleukemia K652 and
human ovarian carcinoma SKOV-3 cells (157), since increased levels of this
antioxidant inhibit apoptosis. Furthermore, in head-and-neck cancer
and in gastric carcinoma cells, PRDX2-specific antisense vectors
restore the induction of pro-apoptotic pathways following radiation
or cisplatin treatment, confirming the important role of PRDX2 in
the resistance process (158).
Several other types of cancer, including erythroleukemia, breast
carcinoma and human ovarian carcinoma, develop cisplatin resistance
through a significant increase in the levels of PRDX1, PRDX3 and
PRDX6 (157,159). In addition, the upregulation of
PRDX2 is also involved in the development of gefitinib resistance
in a non-small cell lung carcinoma model, where it is responsible
for the induction of tumor cell growth via activation of
phosphorylated c-Jun N-terminal kinase (JNK) and the suppression of
apoptosis signaling (160). In
breast cancer cell lines, increased PRDX3 levels correlate with
resistance to the chemotherapeutic drug, doxorubicin (161). PRDX3 controls the apoptotic
signaling pathway through the regulation of cytochrome c
release from the mitochondria, as well as through interaction with
the complex of leucine zipper-bearing kinase (LZK) and IKB kinase
(IKK). Therefore, it is conceivable that drugs targeting PRDX3 and
the mitochondrion-specific electron suppliers, Trx2, TrxR2 and Srx,
could represent a good strategy for improving the response to
various chemotherapeutic agents, including cisplatin, paclitaxel
and etoposide (162,163). Finally, there is evidence that
PRDX5 is also involved in the chemoresistance to adriamycin,
bleomycin, vinblastine and dacarbazine in patients affected with
aggressive Hodgkin's lymphomas (127) and in vitro in the lung
carcinoma U1810 cell line (164),
always by inhibiting chemotherapeutic-induced apoptosis.
Chemoresistance is a complex phenomenon caused by
multiple and heterogeneous mechanisms of action, which are
orchestrated not only by the tumor microenvironment, but also by
the biology of the tumor itself. The modulation of endogenous
antioxidant levels may be a determining factor for the sensitivity
of certain tumors to various chemotherapeutic agents. In addition,
it is important to highlight that the regulation of intracellular
antioxidant concentration is a ‘double-edged sword’: On the one
hand, enhanced antioxidant activity represents an advantageous
protection of the cells from ROS, whereas, on the other hand, the
depletion of antioxidants represents an important strategy to
sensitize cancer cells to chemotherapy (chemosensitization)
(50).
PRDXs serve a critical role in several
physiological, as well as pathological, conditions involving redox
signaling. Although their protective role in cardiovascular and
neurological diseases is clear, their role in cancer remains
controversial: Different PRDX isoforms may have a tumor-suppressor
or an oncogenic role, depending on the cancer type. Considering the
peroxidase-dependent and -independent secondary functions and the
fine balance involved in the regulation of the oligomeric state and
the function of the PRDX, more has been learnt about these
antioxidants and their involvement in the control of cell growth
and survival, particularly as a part of normal growth and
development. To date, it remains to be clarified how the levels of
peroxide and the peroxidases interplay, and how the regulatory
behavior may change depending on the different developmental stages
of the tissue, or on the disease states. Several studies have
hypothesized that the ROS resistance of the cancer cells is
sustained, at least in part, by overexpression of the PRDXs
responsible for the antioxidant activity increase and/or the
alteration in growth and activation of the death pathways (112,165).
On the other hand, in certain cases PRDXs have been suggested to
function as tumor preventers, rather than as tumor suppressors
(166), in that, via detoxification
of the ROS, they contribute to the maintenance of genomic
integrity. In conclusion, in the future it will be crucial to
clarify the exact role of PRDXs in cellular homeostasis, as well as
in cancer development and drug resistance, in order to develop new
target therapeutic strategies for cancer treatment or
prevention.
|
1
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duracková Z: Some current insights into
oxidative stress. Physiol Res. 59:459–469. 2010.PubMed/NCBI
|
|
3
|
Xing M: Oxidative stress: A new risk
factor for thyroid cancer. Endocr Relat Cancer. 19:C7–C11. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dröge W: Free radicals in the
physiological control of cell function. Physiol Rev. 82:47–95.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giorgio M, Trinei M, Migliaccio E and
Pelicci PG: Hydrogen peroxide: A metabolic by-product or a common
mediator of ageing signals? Nat Rev Mol Cell Biol. 8:722–728. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rhee SG, Bae YS, Lee SR and Kwon J:
Hydrogen peroxide: A key messenger that modulates protein
phosphorylation through cysteine oxidation. Sci STKE.
2000:pe12000.PubMed/NCBI
|
|
7
|
Cha MK, Kim HK and Kim IH:
Thioredoxin-linked ‘thiol peroxidase’ from periplasmic space of
Escherichia coli. J Biol Chem. 270:28635–28641. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Y, Wan XY, Wang HL, Yan ZY, Hou YD
and Jin DY: Bacterial scavengase p20 is structurally and
functionally related to peroxiredoxins. Biochem Biophys Res Commun.
233:848–852. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nogoceke E, Gommel DU, Kiess M, Kalisz HM
and Flohé L: A Unique cascade of oxidoreductases catalyses
trypanothione-mediated peroxide metabolism in Crithidia
fasciculata. Biol Chem. 378:827–836. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bryk R, Griffin P and Nathan C:
Peroxynitrite reductase activity of bacterial peroxiredoxins.
Nature. 407:211–215. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hillas PJ, del Alba FS, Oyarzabal J, Wilks
A and De Montellano PR Ortiz: The AhpC and AhpD antioxidant defense
system of Mycobacterium tuberculosis. J Biol Chem. 275:18801–18809.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karplus PA: A primer on peroxiredoxin
biochemistry. Free Radic Biol Med. 80:183–190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Winterbourn CC: Reconciling the chemistry
and biology of reactive oxygen species. Nat Chem Biol. 4:278–286.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim K, Kim IH, Lee KY, Rhee SG and
Stadtman ER: The isolation and purification of a specific
‘protector’ protein which inhibits enzyme inactivation by a
thiol/Fe(III)/O2 mixed-function oxidation system. J Biol Chem.
263:4704–4711. 1988.PubMed/NCBI
|
|
15
|
Chae HZ, Robison K, Poole LB, Church G,
Storz G and Rhee SG: Cloning and sequencing of thiol-specific
antioxidant from mammalian brain: Alkyl hydroperoxide reductase and
thiol-specific antioxidant define a large family of antioxidant
enzymes. Proc Natl Acad Sci USA. 91:7017–7021. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edgar RS, Green EW, Zhao Y, van Ooijen G,
Olmedo M, Qin X, Xu Y, Pan M, Valekunja UK, Feeney KA, et al:
Peroxiredoxins are conserved markers of circadian rhythms. Nature.
485:459–464. 2012.PubMed/NCBI
|
|
17
|
Nelson KJ, Knutson ST, Soito L, Klomsiri
C, Poole LB and Fetrow JS: Analysis of the peroxiredoxin family:
Using active-site structure and sequence information for global
classification and residue analysis. Proteins. 79:947–964. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perkins A, Gretes MC, Nelson KJ, Poole LB
and Karplus PA: Mapping the active site helix-to-strand conversion
of CxxxxC peroxiredoxin Q enzymes. Biochemistry. 51:7638–7650.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hall A, Nelson K, Poole LB and Karplus PA:
Structure-based insights into the catalytic power and
conformational dexterity of peroxiredoxins. Antioxid Redox Signal.
15:795–815. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dammeyer P and Arnér ES: Human protein
atlas of redox systems-what can be learnt? Biochim Biophys Acta.
1810:111–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rhee SG, Chae HZ and Kim K:
Peroxiredoxins: A historical overview and speculative preview of
novel mechanisms and emerging concepts in cell signaling. Free
Radic Biol Med. 38:1543–1552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seo MS, Kang SW, Kim K, Baines IC, Lee TH
and Rhee SG: Identification of a new type of mammalian
peroxiredoxin that forms an intramolecular disulfide as a reaction
intermediate. J Biol Chem. 275:20346–20354. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang SW, Baines IC and Rhee SG:
Characterization of a mammalian peroxiredoxin that contains one
conserved cysteine. J Biol Chem. 273:6303–6311. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fujii J, Ikeda Y, Kurahashi T and Homma T:
Physiological and pathological views of peroxiredoxin 4. Free Radic
Biol Med. 83:373–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rhee SG, Kang SW, Chang TS, Jeong W and
Kim K: Peroxiredoxin, a novel family of peroxidases. IUBMB Life.
52:35–41. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hofmann B, Hecht HJ and Flohé L:
Peroxiredoxins. Biol Chem. 383:347–364. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Declercq JP, Evrard C, Clippe A, Stricht
DV, Bernard A and Knoops B: Crystal structure of human
peroxiredoxin 5, a novel type of mammalian peroxiredoxin at 1.5 A
resolution. J Mol Biol. 311:751–759. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Monteiro G, Horta BB, Pimenta DC, Augusto
O and Netto LE: Reduction of 1-Cys peroxiredoxins by ascorbate
changes the thiol-specific antioxidant paradigm, revealing another
function of vitamin C. Proc Natl Acad Sci USA. 104:4886–4891. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Karplus PA and Hall A: Structural survey
of the peroxiredoxins. Subcell Biochem. 44:41–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wood ZA, Poole LB, Hantgan RR and Karplus
PA: Dimers to Doughnuts: Redox-sensitive oligomerization of
2-cysteine peroxiredoxins. Biochemistry. 41:5493–5504. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barranco-Medina S, Lázaro JJ and Dietz KJ:
The oligomeric conformation of peroxiredoxins links redox state to
function. FEBS Lett. 583:1809–1816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fujii J and Ikeda Y: Advances in our
understanding of peroxiredoxin, a multifunctional, mammalian redox
protein. Redox Rep. 7:123–130. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pisoschi AM and Pop A: The role of
antioxidants in the chemistry of oxidative stress: A review. Eur J
Med Chem. 97:55–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fulda S, Gorman AM, Hori O and Samali A:
Cellular stress responses: Cell survival and cell death. Int J Cell
Biol. 2010:2140742010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kamiguti AS, Serrander L, Lin K, Harris
RJ, Cawley JC, Allsup DJ, Slupsky JR, Krause KH and Zuzel M:
Expression and activity of NOX5 in the circulating malignant B
cells of hairy cell leukemia. J Immunol. 175:8424–8430. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsao SM, Yin MC and Liu WH: Oxidant stress
and B vitamins status in patients with non-small cell lung cancer.
Nutr Cancer. 59:8–13. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khandrika L, Kumar B, Koul S, Maroni P and
Koul HK: Oxidative stress in prostate cancer. Cancer Lett.
282:125–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Patel BP, Rawal UM, Dave TK, Rawal RM,
Shukla SN, Shah PM and Patel PS: Lipid peroxidation, total
antioxidant status, and total thiol levels predict overall survival
in patients with oral squamous cell carcinoma. Integr Cancer Ther.
6:365–372. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Szatrowski TP and Nathan CF: Production of
large amounts of hydrogen peroxide by human tumor cells. Cancer
Res. 51:794–798. 1991.PubMed/NCBI
|
|
40
|
Nishikawa M: Reactive oxygen species in
tumor metastasis. Cancer Lett. 266:53–59. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Clerkin JS, Naughton R, Quiney C and
Cotter TG: Mechanisms of ROS modulated cell survival during
carcinogenesis. Cancer Lett. 266:30–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Krstić J, Trivanović D, Mojsilović S and
Santibanez JF: Transforming Growth Factor-Beta and Oxidative Stress
Interplay: Implications in Tumorigenesis and cancer progression.
Oxid Med Cell Longev. 2015:6545942015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ushio-Fukai M and Nakamura Y: Reactive
oxygen species and angiogenesis: NADPH oxidase as target for cancer
therapy. Cancer Lett. 266:37–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fiaschi T and Chiarugi P: Oxidative
stress, tumor microenvironment, and metabolic reprogramming: A
diabolic liaison. Int J Cell Biol. 2012:7628252012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Weinberg F and Chandel NS: Reactive oxygen
species-dependent signaling regulates cancer. Cell Mol Life Sci.
66:3663–3673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Park MH, Jo M, Kim YR, Lee CK and Hong JT:
Roles of peroxiredoxins in cancer, neurodegenerative diseases and
inflammatory diseases. Pharmacol Ther. 163:1–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Taguchi K, Motohashi H and Yamamoto M:
Molecular mechanisms of the Keap1-Nrf2 pathway in stress response
and cancer evolution. Genes Cells. 16:123–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kwee JK: A paradoxical chemoresistance and
tumor suppressive role of antioxidant in solid cancer cells: A
strange case of Dr. Jekyll and Mr. Hyde. Biomed Res Int.
2014:2098452014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Neumann CA, Cao J and Manevich Y:
Peroxiredoxin 1 and its role in cell signaling. Cell Cycle.
8:4072–4078. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kim YJ, Ahn JY, Liang P, Ip C, Zhang Y and
Park YM: Human prx1 gene is a target of Nrf2 and is up-regulated by
hypoxia/reoxygenation: Implication to tumor biology. Cancer Res.
67:546–554. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Thélie A, Papillier P, Pennetier S,
Perreau C, Traverso JM, Uzbekova S, Mermillod P, Joly C, Humblot P
and Dalbiès-Tran R: Differential regulation of abundance and
deadenylation of maternal transcripts during bovine oocyte
maturation in vitro and in vivo. BMC Dev Biol. 7:1252007.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Neumann CA, Krause DS, Carman CV, Das S,
Dubey DP, Abraham JL, Bronson RT, Fujiwara Y, Orkin SH and Van
Etten RA: Essential role for the peroxiredoxin Prdx1 in erythrocyte
antioxidant defence and tumour suppression. Nature. 424:561–565.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Egler RA, Fernandes E, Rothermund K,
Sereika S, de Souza-Pinto N, Jaruga P, Dizdaroglu M and Prochownik
EV: Regulation of reactive oxygen species, DNA damage, and c-Myc
function by peroxiredoxin 1. Oncogene. 24:8038–8050. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cao J, Schulte J, Knight A, Leslie NR,
Zagozdzon A, Bronson R, Manevich Y, Beeson C and Neumann CA: Prdx1
inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J.
28:1505–1517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
O'Leary PC, Terrile M, Bajor M, Gaj P,
Hennessy BT, Mills GB, Zagozdzon A, O'Connor DP, Brennan DJ, Connor
K, et al: Peroxiredoxin-1 protects estrogen receptor α from
oxidative stress-induced suppression and is a protein biomarker of
favorable prognosis in breast cancer. Breast Cancer Res.
16:R792014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Park YH, Kim SU, Lee BK, Kim HS, Song IS,
Shin HJ, Han YH, Chang KT, Kim JM, Lee DS, et al: Prx I suppresses
K-ras-driven lung tumorigenesis by opposing redox-sensitive
ERK/cyclin D1 pathway. Antioxid Redox Signal. 19:482–496. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Godfrey R, Arora D, Bauer R, Stopp S,
Müller JP, Heinrich T, Böhmer SA, Dagnell M, Schnetzke U, Scholl S,
et al: Cell transformation by FLT3 ITD in acute myeloid leukemia
involves oxidative inactivation of the tumor suppressor
protein-tyrosine phosphatase DEP-1/PTPRJ. Blood. 119:4499–4511.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kim JH, Bogner PN, Baek SH, Ramnath N,
Liang P, Kim HR, Andrews C and Park YM: Up-regulation of
peroxiredoxin 1 in lung cancer and its implication as a prognostic
and therapeutic target. Clin Cancer Res. 14:2326–2333. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Quan C, Cha EJ, Lee HL, Han KH, Lee KM and
Kim WJ: Enhanced expression of peroxiredoxin I and VI correlates
with development, recurrence and progression of human bladder
cancer. J Urol. 175:1512–1516. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chung KH, Lee DH, Kim Y, Kim TH, Huh JH,
Chung SG, Lee S, Lee C, Ko JJ and An HJ: Proteomic identification
of overexpressed PRDX 1 and its clinical implications in ovarian
carcinoma. J Proteome Res. 9:451–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ren P, Ye H, Dai L, Liu M, Liu X, Chai Y,
Shao Q, Li Y, Lei N, Peng B, et al: Peroxiredoxin 1 is a
tumor-associated antigen in esophageal squamous cell carcinoma.
Oncol Rep. 30:2297–2303. 2013.PubMed/NCBI
|
|
64
|
Zhou J, Shen W, He X, Qian J, Liu S and Yu
G: Overexpression of Prdx1 in hilar cholangiocarcinoma: A predictor
for recurrence and prognosis. Int J Clin Exp Pathol. 8:9863–9874.
2015.PubMed/NCBI
|
|
65
|
Sun YL, Cai JQ, Liu F, Bi XY, Zhou LP and
Zhao XH: Aberrant expression of peroxiredoxin 1 and its clinical
implications in liver cancer. World J Gastroenterol.
21:10840–10852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cai CY, Zhai LL, Wu Y and Tang ZG:
Expression and clinical value of peroxiredoxin-1 in patients with
pancreatic cancer. Eur J Surg Oncol. 41:228–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kinnula VL, Lehtonen S, Sormunen R,
Kaarteenaho-Wiik R, Kang SW, Rhee SG and Soini Y: Overexpression of
peroxiredoxins I, II, III, V, and VI in malignant mesothelioma. J
Pathol. 196:316–323. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Svendsen A, Verhoeff JJ, Immervoll H,
Brøgger JC, Kmiecik J, Poli A, Netland IA, Prestegarden L,
Planagumà J, Torsvik A, et al: Expression of the progenitor marker
NG2/CSPG4 predicts poor survival and resistance to ionising
radiation in glioblastoma. Acta Neuropathol. 122:495–510. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Riddell JR, Maier P, Sass SN, Moser MT,
Foster BA and Gollnick SO: Peroxiredoxin 1 stimulates endothelial
cell expression of VEGF via TLR4 dependent activation of HIF-1α.
PLoS One. 7:e503942012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jiang H, Wu L, Mishra M, Chawsheen HA and
Wei Q: Expression of peroxiredoxin 1 and 4 promotes human lung
cancer malignancy. Am J Cancer Res. 4:445–460. 2014.PubMed/NCBI
|
|
71
|
Ha B, Kim EK, Kim JH, Lee HN, Lee KO, Lee
SY and Jang HH: Human peroxiredoxin 1 modulates TGF-β1-induced
epithelial-mesenchymal transition through its peroxidase activity.
Biochem Biophys Res Commun. 421:33–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Du ZX, Yan Y, Zhang HY, Liu BQ, Gao YY,
Niu XF, Guan Y, Meng X and Wang HQ: Suppression of MG132-mediated
cell death by peroxiredoxin 1 through influence on ASK1 activation
in human thyroid cancer cells. Endocr Relat Cancer. 17:553–560.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Song IS, Kim SU, Oh NS, Kim J, Yu DY,
Huang SM, Kim JM, Lee DS and Kim NS: Peroxiredoxin I contributes to
TRAIL resistance through suppression of redox-sensitive caspase
activation in human hepatoma cells. Carcinogenesis. 30:1106–1114.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yanagawa T, Ishikawa T, Ishii T, Tabuchi
K, Iwasa S, Bannai S, Omura K, Suzuki H and Yoshida H:
Peroxiredoxin I expression in human thyroid tumors. Cancer Lett.
145:127–132. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nicolussi A, D'Inzeo S, Mincione G,
Buffone A, Di Marcantonio MC, Cotellese R, Cichella A, Capalbo C,
Di Gioia C, Nardi F, et al: PRDX1 and PRDX6 are repressed in
papillary thyroid carcinomas via BRAF V600E-dependent and
-independent mechanisms. Int J Oncol. 44:548–556. 2014.PubMed/NCBI
|
|
76
|
Wood ZA, Poole LB and Karplus PA:
Peroxiredoxin evolution and the regulation of hydrogen peroxide
signaling. Science. 300:650–653. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
O'Neill JS and Reddy AB: Circadian clocks
in human red blood cells. Nature. 469:498–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Cho CS, Yoon HJ, Kim JY, Woo HA and Rhee
SG: Circadian rhythm of hyperoxidized peroxiredoxin II is
determined by hemoglobin autoxidation and the 20S proteasome in red
blood cells. Proc Natl Acad Sci USA. 111:12043–12048. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Avitabile D, Ranieri D, Nicolussi A,
D'Inzeo S, Capriotti AL, Genovese L, Proietti S, Cucina A, Coppa A,
Samperi R, et al: Peroxiredoxin 2 nuclear levels are regulated by
circadian clock synchronization in human keratinocytes. Int J
Biochem Cell Biol. 53:24–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sobotta MC, Liou W, Stöcker S, Talwar D,
Oehler M, Ruppert T, Scharf AN and Dick TP: Peroxiredoxin-2 and
STAT3 form a redox relay for H2O2 signaling. Nat Chem Biol.
11:64–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Rhee SG and Woo HA: Multiple functions of
peroxiredoxins: Peroxidases, sensors and regulators of the
intracellular messenger H2O2, and protein
chaperones. Antioxid Redox Signal. 15:781–794. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Huo YY, Li G, Duan RF, Gou Q, Fu CL, Hu
YC, Song BQ, Yang ZH, Wu DC and Zhou PK: PTEN deletion leads to
deregulation of antioxidants and increased oxidative damage in
mouse embryonic fibroblasts. Free Radic Biol Med. 44:1578–1591.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Barbosa AC, Funato N, Chapman S, McKee MD,
Richardson JA, Olson EN and Yanagisawa H: Hand transcription
factors cooperatively regulate development of the distal midline
mesenchyme. Dev Biol. 310:154–168. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Furuta J, Nobeyama Y, Umebayashi Y, Otsuka
F, Kikuchi K and Ushijima T: Silencing of Peroxiredoxin 2 and
aberrant methylation of 33 CpG islands in putative promoter regions
in human malignant melanomas. Cancer Res. 66:6080–6086. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lee DJ, Kang DH, Choi M, Choi YJ, Lee JY,
Park JH, Park YJ, Lee KW and Kang SW: Peroxiredoxin-2 represses
melanoma metastasis by increasing E-Cadherin/β-Catenin complexes in
adherens junctions. Cancer Res. 73:4744–4757. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Feng J, Fu Z, Guo J, Lu W, Wen K, Chen W,
Wang H, Wei J and Zhang S: Overexpression of peroxiredoxin 2
inhibits TGF-β1-induced epithelial-mesenchymal transition and cell
migration in colorectal cancer. Mol Med Rep. 10:867–873.
2014.PubMed/NCBI
|
|
87
|
Pylväs M, Puistola U, Kauppila S, Soini Y
and Karihtala P: Oxidative stress-induced antioxidant enzyme
expression is an early phenomenon in ovarian carcinogenesis. Eur J
Cancer. 46:1661–1667. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hellman K, Alaiya AA, Becker S, Lomnytska
M, Schedvins K, Steinberg W, Hellström AC, Andersson S, Hellman U
and Auer G: Differential tissue-specific protein markers of vaginal
carcinoma. Br J Cancer. 100:1303–1314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kim K, Yu M, Han S, Oh I, Choi YJ, Kim S,
Yoon K, Jung M and Choe W: Expression of human peroxiredoxin
isoforms in response to cervical carcinogenesis. Oncol Rep.
21:1391–1396. 2009.PubMed/NCBI
|
|
90
|
Basu A, Banerjee H, Rojas H, Martinez SR,
Roy S, Jia Z, Lilly MB, De León M and Casiano CA: Differential
expression of peroxiredoxins in prostate cancer: Consistent
upregulation of PRDX3 and PRDX4. Prostate. 71:755–765. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhang B, Wang K, He G, Guan X, Liu B, Liu
Y and Bai Y: Polymorphisms of peroxiredoxin 1, 2 and 6 are not
associated with esophageal cancer. J Cancer Res Clin Oncol.
138:621–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Trzeciecka A, Klossowski S, Bajor M,
Zagozdzon R, Gaj P, Muchowicz A, Malinowska A, Czerwoniec A,
Barankiewicz J, Domagala A, et al: Dimeric peroxiredoxins are
druggable targets in human Burkitt lymphoma. Oncotarget.
7:1717–1731. 2016.PubMed/NCBI
|
|
93
|
Stresing V, Baltziskueta E, Rubio N,
Blanco J, Arriba MC, Valls J, Janier M, Clézardin P, Sanz-Pamplona
R, Nieva C, et al: Peroxiredoxin 2 specifically regulates the
oxidative and metabolic stress response of human metastatic breast
cancer cells in lungs. Oncogene. 32:724–735. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Lu W, Fu Z, Wang H, Feng J, Wei J and Guo
J: Peroxiredoxin 2 is upregulated in colorectal cancer and
contributes to colorectal cancer cells' survival by protecting
cells from oxidative stress. Mol Cell Biochem. 387:261–270. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Watabe S, Hiroi T, Yamamoto Y, Fujioka Y,
Hasegawa H, Yago N and Takahashi SY: SP-22 is a
thioredoxin-dependent peroxide reductase in mitochondria. Eur J
Biochem. 249:52–60. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Miranda-Vizuete A, Damdimopoulos AE and
Spyrou G: The mitochondrial thioredoxin system. Antioxid Redox
Signal. 2:801–810. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Olmos Y, Sánchez-Gómez FJ, Wild B,
García-Quintans N, Cabezudo S, Lamas S and Monsalve M: SirT1
regulation of antioxidant genes is dependent on the formation of a
FoxO3a/PGC-1α complex. Antioxid Redox Signal. 19:1507–1521. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Song IS, Jeong YJ, Jeong SH, Heo HJ, Kim
HK, Bae KB, Park YH, Kim SU, Kim JM, Kim N, et al: FOXM1-Induced
PRX3 regulates stemness and survival of colon cancer cells via
maintenance of mitochondrial function. Gastroenterology.
149:1006–1016.e9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li KK, Pang JC, Lau KM, Zhou L, Mao Y,
Wang Y, Poon WS and Ng HK: MiR-383 is downregulated in
medulloblastoma and targets peroxiredoxin 3 (PRDX3). Brain Pathol.
23:413–425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
He HC, Zhu JG, Chen XB, Chen SM, Han ZD,
Dai QS, Ling XH, Fu X, Lin ZY, Deng YH, et al: MicroRNA-23b
downregulates peroxiredoxin III in human prostate cancer. FEBS
Lett. 586:2451–2458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Xi H, Gao YH, Han DY, Li QY, Feng LJ,
Zhang W, Ji G, Xiao JC, Zhang HZ and Wei Q: Hypoxia inducible
factor-1α suppresses Peroxiredoxin 3 expression to promote
proliferation of CCRCC cells. FEBS Lett. 588:3390–3394. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Choi JH, Kim TN, Kim S, Baek SH, Kim JH,
Lee SR and Kim JR: Overexpression of mitochondrial thioredoxin
reductase and peroxiredoxin III in hepatocellular carcinomas.
Anticancer Res. 22:3331–3335. 2002.PubMed/NCBI
|
|
103
|
Karihtala P, Mäntyniemi A, Kang SW,
Kinnula VL and Soini Y: Peroxiredoxins in breast carcinoma. Clin
Cancer Res. 9:3418–3424. 2003.PubMed/NCBI
|
|
104
|
Ummanni R, Barreto F, Venz S, Scharf C,
Barett C, Mannsperger HA, Brase JC, Kuner R, Schlomm T, Sauter G,
et al: Peroxiredoxins 3 and 4 are overexpressed in prostate cancer
tissue and affect the proliferation of prostate cancer cells in
vitro. J Proteome Res. 11:2452–2466. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kim YS, Lee HL, Lee KB, Park JH, Chung WY,
Lee KS, Sheen SS, Park KJ and Hwang SC: Nuclear factor E2-related
factor 2 dependent overexpression of sulfiredoxin and peroxiredoxin
III in human lung cancer. Korean J Intern Med. 26:304–313. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hu JX, Gao Q and Li L: Peroxiredoxin 3 is
a novel marker for cell proliferation in cervical cancer. Biomed
Rep. 1:228–230. 2013.PubMed/NCBI
|
|
107
|
Schulte J: Peroxiredoxin 4: A
multifunctional biomarker worthy of further exploration. BMC Med.
9:1372011. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Okado-Matsumoto A, Matsumoto A, Fujii J
and Taniguchi N: Peroxiredoxin IV is a secretable protein with
heparin-binding properties under reduced conditions. J Biochem.
127:493–501. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Roumes H, Pires-Alves A, Gonthier-Maurin
L, Dargelos E and Cottin P: Investigation of peroxiredoxin IV as a
calpain-regulated pathway in cancer. Anticancer Res. 30:5085–5089.
2010.PubMed/NCBI
|
|
110
|
Ikeda Y, Nakano M, Ihara H, Ito R,
Taniguchi N and Fujii J: Different consequences of reactions with
hydrogen peroxide and t-butyl hydroperoxide in the hyperoxidative
inactivation of rat peroxiredoxin-4. J Biochem. 149:443–453. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zhu L, Yang K, Wang X, Wang X and Wang CC:
A novel reaction of peroxiredoxin 4 towards substrates in oxidative
protein folding. PLoS One. 9:e1055292014. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Ishii T, Warabi E and Yanagawa T: Novel
roles of peroxiredoxins in inflammation, cancer and innate
immunity. J Clin Biochem Nutr. 50:91–105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Mishra M, Jiang H, Wu L, Chawsheen HA and
Wei Q: The sulfiredoxin-peroxiredoxin (Srx-Prx) axis in cell signal
transduction and cancer development. Cancer Lett. 366:150–159.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chen JH, Ni RZ, Xiao MB, Guo JG and Zhou
JW: Comparative proteomic analysis of differentially expressed
proteins in human pancreatic cancer tissue. Hepatobiliary Pancreat
Dis Int. 8:193–200. 2009.PubMed/NCBI
|
|
115
|
Pritchard C, Mecham B, Dumpit R, Coleman
I, Bhattacharjee M, Chen Q, Sikes RA and Nelson PS: Conserved gene
expression programs integrate mammalian prostate development and
tumorigenesis. Cancer Res. 69:1739–1747. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Chang KP, Yu JS, Chien KY, Lee CW, Liang
Y, Liao CT, Yen TC, Lee LY, Huang LL, Liu SC, et al: Identification
of PRDX4 and P4HA2 as metastasis-associated proteins in oral cavity
squamous cell carcinoma by comparative tissue proteomics of
microdissected specimens using iTRAQ technology. J Proteome Res.
10:4935–4947. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Karihtala P, Kauppila S, Soini Y and
Arja-Jukkola-Vuorinen: Oxidative stress and counteracting
mechanisms in hormone receptor positive, triple-negative and
basal-like breast carcinomas. BMC Cancer. 11:2622011. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Karihtala P, Soini Y, Vaskivuo L, Bloigu R
and Puistola U: DNA adduct 8-hydroxydeoxyguanosine, a novel
putative marker of prognostic significance in ovarian carcinoma.
Int J Gynecol Cancer. 19:1047–1051. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Yi N, Xiao MB, Ni WK, Jiang F, Lu CH and
Ni RZ: High expression of peroxiredoxin 4 affects the survival time
of colorectal cancer patients, but is not an independent
unfavorable prognostic factor. Mol Clin Oncol. 2:767–772.
2014.PubMed/NCBI
|
|
120
|
Wei Q, Jiang H, Xiao Z, Baker A, Young MR,
Veenstra TD and Colburn NH: Sulfiredoxin-Peroxiredoxin IV axis
promotes human lung cancer progression through modulation of
specific phosphokinase signaling. Proc Natl Acad Sci USA.
108:7004–7009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Palande KK, Beekman R, van der Meeren LE,
Beverloo HB, Valk PJ and Touw IP: The antioxidant protein
peroxiredoxin 4 is epigenetically down regulated in acute
promyelocytic leukemia. PLoS One. 6:e163402011. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Knoops B, Goemaere J, Van der Eecken V and
Declercq JP: Peroxiredoxin 5: Structure, mechanism, and function of
the mammalian atypical 2-Cys peroxiredoxin. Antioxid Redox Signal.
15:817–829. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Nguyên-Nhu NT, Berck J, Clippe A,
Duconseille E, Cherif H, Boone C, Van der Eecken V, Bernard A,
Banmeyer I and Knoops B: Human peroxiredoxin 5 gene organization,
initial characterization of its promoter and identification of
alternative forms of mRNA. Biochim Biophys Acta. 1769:472–483.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Graves JA, Metukuri M, Scott D, Rothermund
K and Prochownik EV: Regulation of reactive oxygen species
homeostasis by peroxiredoxins and c-Myc. J Biol Chem.
284:6520–6529. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kropotov A, Usmanova N, Serikov V,
Zhivotovsky B and Tomilin N: Mitochondrial targeting of human
peroxiredoxin V protein and regulation of PRDX5 gene expression by
nuclear transcription factors controlling biogenesis of
mitochondria. FEBS J. 274:5804–5814. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Shiota M, Izumi H, Miyamoto N, Onitsuka T,
Kashiwagi E, Kidani A, Hirano G, Takahashi M, Ono M, Kuwano M, et
al: Ets regulates peroxiredoxin1 and 5 expressions through their
interaction with the high-mobility group protein B1. Cancer Sci.
99:1950–1959. 2008.PubMed/NCBI
|
|
127
|
Bur H, Haapasaari KM, Turpeenniemi-Hujanen
T, Kuittinen O, Auvinen P, Marin K, Koivunen P, Sormunen R, Soini Y
and Karihtala P: Oxidative stress markers and mitochondrial
antioxidant enzyme expression are increased in aggressive Hodgkin
lymphomas. Histopathology. 65:319–327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Gérard AC, Many MC, Daumerie Ch, Knoops B
and Colin IM: Peroxiredoxin 5 expression in the human thyroid
gland. Thyroid. 15:205–209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Fernandez-Ranvier GG, Weng J, Yeh RF,
Shibru D, Khafnashar E, Chung KW, Hwang J, Duh QY, Clark OH and
Kebebew E: Candidate diagnostic markers and tumor suppressor genes
for adrenocortical carcinoma by expression profile of genes on
chromosome 11q13. World J Surg. 32:873–881. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Fisher AB: Peroxiredoxin 6: A bifunctional
enzyme with glutathione peroxidase and phospholipase A2
activities. Antioxid Redox Signal. 15:831–844. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Choi HJ, Kang SW, Yang CH, Rhee SG and Ryu
SE: Crystal structure of a novel human peroxidase enzyme at 2.0 A
resolution. Nat Struct Biol. 5:400–406. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Chowdhury I, Fisher AB,
Christofidou-Solomidou M, Gao L, Tao JQ, Sorokina EM, Lien YC,
Bates SR and Feinstein SI: Keratinocyte growth factor and
glucocorticoid induction of human peroxiredoxin 6 gene expression
occur by independent mechanisms that are synergistic. Antioxid
Redox Signal. 20:391–402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Chowdhury I, Mo Y, Gao L, Kazi A, Fisher
AB and Feinstein SI: Oxidant stress stimulates expression of the
human peroxiredoxin 6 gene by a transcriptional mechanism involving
an antioxidant response element. Free Radic Biol Med. 46:146–153.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Fatma N, Kubo E, Takamura Y, Ishihara K,
Garcia C, Beebe DC and Singh DP: Loss of NF-kappaB control and
repression of Prdx6 gene transcription by reactive oxygen
species-driven SMAD3-mediated transforming growth factor beta
signaling. J Biol Chem. 284:22758–22772. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Chhunchha B, Fatma N, Bhargavan B, Kubo E,
Kumar A and Singh DP: Specificity protein, Sp1-mediated increased
expression of Prdx6 as a curcumin-induced antioxidant defense in
lens epithelial cells against oxidative stress. Cell Death Dis.
2:e2342011. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Wu X, Ji P, Zhang L, Bu G, Gu H, Wang X,
Xiong Y and Zuo B: The expression of porcine Prdx6 gene is
up-regulated by C/EBPβ and CREB. PLoS One. 10:e01448512015.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Power JH, Asad S, Chataway TK, Chegini F,
Manavis J, Temlett JA, Jensen PH, Blumbergs PC and Gai WP:
Peroxiredoxin 6 in human brain: Molecular forms, cellular
distribution, and association with Alzheimer's disease pathology.
Acta Neuropathol. 115:611–622. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Yun HM, Choi DY, Oh KW and Hong JT: PRDX6
exacerbates dopaminergic neurodegeneration in a MPTP mouse model of
Parkinson's disease. Mol Neurobiol. 52:422–431. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
El Eter E, Al Masri A, Habib S, Al Zamil
H, Al Hersi A, Al Hussein F and Al Omran M: Novel links among
peroxiredoxins, endothelial dysfunction, and severity of
atherosclerosis in type 2 diabetic patients with peripheral
atherosclerotic disease. Cell Stress Chaperones. 19:173–181. 2014.
View Article : Google Scholar
|
|
140
|
Kubo E, Fatma N, Akagi Y, Beier DR, Singh
SP and Singh DP: TAT-mediated PRDX6 protein transduction protects
against eye lens epithelial cell death and delays lens opacity. Am
J Physiol Cell Physiol. 294:C842–C855. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Thongwatchara P, Promwikorn W, Srisomsap
C, Chokchaichamnankit D, Boonyaphiphat P and Thongsuksai P:
Differential protein expression in primary breast cancer and
matched axillary node metastasis. Oncol Rep. 26:185–191.
2011.PubMed/NCBI
|
|
142
|
Zhang J, Wang K, Zhang J, Liu SS, Dai L
and Zhang JY: Using proteomic approach to identify tumor-associated
proteins as biomarkers in human esophageal squamous cell carcinoma.
J Proteome Res. 10:2863–2872. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Schremmer B, Manevich Y, Feinstein SI and
Fisher AB: Peroxiredoxins in the lung with emphasis on
peroxiredoxin VI. Subcell Biochem. 44:317–344. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Park JY, Kim SA, Chung JW, Bang S, Park
SW, Paik YK and Song SY: Proteomic analysis of pancreatic juice for
the identification of biomarkers of pancreatic cancer. J Cancer Res
Clin Oncol. 137:1229–1238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Shukla S, Pranay A, D'Cruz AK, Chaturvedi
P, Kane SV and Zingde SM: Immunoproteomics reveals that cancer of
the tongue and the gingivobuccal complex exhibit differential
autoantibody response. Cancer Biomark. 5:127–135. 2009.PubMed/NCBI
|
|
146
|
Kuusisto ME, Haapasaari KM,
Turpeenniemi-Hujanen T, Jantunen E, Soini Y, Peroja P, Bloigu R,
Karihtala P and Kuittinen O: High intensity of cytoplasmic
peroxiredoxin VI expression is associated with adverse outcome in
diffuse large B-cell lymphoma independently of international
prognostic index. J Clin Pathol. 68:552–556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Chang XZ, Li DQ, Hou YF, Wu J, Lu JS, Di
GH, Jin W, Ou ZL, Shen ZZ and Shao ZM: Identification of the
functional role of peroxiredoxin 6 in the progression of breast
cancer. Breast Cancer Res. 9:R762007. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Raatikainen S, Aaaltomaa S, Kärjä V and
Soini Y: Increased peroxiredoxin 6 expression predicts biochemical
recurrence in prostate cancer patients after radical prostatectomy.
Anticancer Res. 35:6465–6470. 2015.PubMed/NCBI
|
|
149
|
Sofiadis A, Becker S, Hellman U,
Hultin-Rosenberg L, Dinets A, Hulchiy M, Zedenius J, Wallin G,
Foukakis T, Höög A, et al: Proteomic profiling of follicular and
papillary thyroid tumors. Eur J Endocrinol. 166:657–667. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Ho JN, Lee SB, Lee SS, Yoon SH, Kang GY,
Hwang SG and Um HD: Phospholipase A2 activity of peroxiredoxin 6
promotes invasion and metastasis of lung cancer cells. Mol Cancer
Ther. 9:825–832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Seibold P, Hall P, Schoof N, Nevanlinna H,
Heikkinen T, Benner A, Liu J, Schmezer P, Popanda O, Flesch-Janys D
and Chang-Claude J: Polymorphisms in oxidative stress-related genes
and mortality in breast cancer patients-potential differential
effects by radiotherapy? Breast. 22:817–823. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Glasauer A and Chandel NS: Targeting
antioxidants for cancer therapy. Biochem Pharmacol. 92:90–101.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Gorrini C, Harris IS and Mak TW:
Modulation of oxidative stress as an anticancer strategy. Nat Rev
Drug Discov. 12:931–947. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Yang Y, Karakhanova S, Werner J and Bazhin
AV: Reactive oxygen species in cancer biology and anticancer
therapy. Curr Med Chem. 20:3677–3692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Wang T, Diaz AJ and Yen Y: The role of
peroxiredoxin II in chemoresistance of breast cancer cells. Breast
Cancer (Dove Med Press). 6:73–80. 2014.PubMed/NCBI
|
|
156
|
Chung YM, Yoo YD, Park JK, Kim YT and Kim
HJ: Increased expression of peroxiredoxin II confers resistance to
cisplatin. Anticancer Res. 21:1129–1133. 2001.PubMed/NCBI
|
|
157
|
Kalinina EV, Berezov TT, Shtil AA, Chernov
NN, Glazunova VA, Novichkova MD and Nurmuradov NK: Expression of
peroxiredoxin 1, 2, 3, and 6 genes in cancer cells during drug
resistance formation. Bull Exp Biol Med. 153:878–881. 2012.(In
English, Russian). View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Park SH, Chung YM, Lee YS, Kim HJ, Kim JS,
Chae HZ and Yoo YD: Antisense of human peroxiredoxin II enhances
radiation-induced cell death. Clin Cancer Res. 6:4915–4920.
2000.PubMed/NCBI
|
|
159
|
Pak JH, Choi WH, Lee HM, Joo WD, Kim JH,
Kim YT, Kim YM and Nam JH: Peroxiredoxin 6 overexpression
attenuates cisplatin-induced apoptosis in human ovarian cancer
cells. Cancer Invest. 29:21–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Kwon T, Rho JK, Lee JC, Park YH, Shin HJ,
Cho S, Kang YK, Kim BY, Yoon DY and Yu DY: An important role for
peroxiredoxin II in survival of A549 lung cancer cells resistant to
gefitinib. Exp Mol Med. 47:e1652015. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
McDonald C, Muhlbauer J, Perlmutter G,
Taparra K and Phelan SA: Peroxiredoxin proteins protect MCF-7
breast cancer cells from doxorubicin-induced toxicity. Int J Oncol.
45:219–226. 2014.PubMed/NCBI
|
|
162
|
Song IS, Kim HK, Jeong SH, Lee SR, Kim N,
Rhee BD, Ko KS and Han J: Mitochondrial peroxiredoxin III is a
potential target for cancer therapy. Int J Mol Sci. 12:7163–7185.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Li L and Yu AQ: The functional role of
peroxiredoxin 3 in reactive oxygen species, apoptosis, and
chemoresistance of cancer cells. J Cancer Res Clin Oncol.
141:2071–2077. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Kropotov A, Gogvadze V, Shupliakov O,
Tomilin N, Serikov VB, Tomilin NV and Zhivotovsky B: Peroxiredoxin
V is essential for protection against apoptosis in human lung
carcinoma cells. Exp Cell Res. 312:2806–2815. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Butterfield LH, Merino A, Golub SH and
Shau H: From cytoprotection to tumor suppression: The
multifactorial role of peroxiredoxins. Antioxid Redox Signal.
1:385–402. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Neumann CA and Fang Q: Are peroxiredoxins
tumor suppressors? Curr Opin Pharmacol. 7:375–380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Woo HA, Jeong W, Chang TS, Park KJ, Park
SJ, Yang JS and Rhee SG: Reduction of cysteine sulfinic acid by
sulfiredoxin is specific to 2-cys peroxiredoxins. J Biol Chem.
280:3125–3128. 2005. View Article : Google Scholar : PubMed/NCBI
|