Introduction
Primitive neuroectodermal tumors (PNETs) are a rare
group of malignant tumors of neuroectodermal origin. PNETs are most
commonly encountered in pediatric patients with diverse clinical
manifestations, and are classified into three groups based on the
tissue of origin: Central nervous system (CNS) PNETs, neuroblastoma
and peripheral PNEs (pPNETs) derived from tissues outside the CNS
and autonomic nervous system (1).
pPNETs occur predominantly during infancy or childhood, and are
located in the abdomen (2,3), thoracopulmonary region (4,5) and,
rarely, in the head and neck region (6). pPNETs are extremely rare in adults,
particularly in the hip muscles with bone metastasis. Metastasis is
considered to be the most significant prognostic factor, and
patients without metastasis may have a relatively better prognosis
and longer survival time. Early correct diagnosis and accurate
staging are crucial for selecting the optimal therapeutic regimens
(7).
Case report
A 44-year-old woman was referred to our hospital on
April 8th, 2015, with a 1-year history of progressive pain,
swelling and a mass near the left hip, which had worsened over the
previous month. The patient had no history of trauma, surgery, bone
fracture, or accident. There was no other significant family or
past medical history. Physical examination revealed a solid soft
mass near the upper 1/4 of the left thigh, ~8 cm in diameter.
Increased skin temperature and limited hip mobility were also
observed. Tumor markers, including carcinoembryonic antigen,
thyroglobulin, neuron-specific enolase, cytokeratin 19 fragment and
cancer antigen 19-9 were all within the normal range. A plain CT
scan of the pelvis and upper thigh was performed, which revealed a
soft tissue mass lesion in the hip muscles measuring 4.3×4.3×4.4 cm
(Fig. 2A). The lesion was an
ill-defined, aggressive, heterogeneous, hypodense mass with
multiple internal lower-density areas and mild post-contrast
enhancement (Fig. 2B and C). There
was a large number of bent neovessels and several branches from the
left internal iliac artery and deep femoral artery on enhanced CT
angiography (Fig. 2D). Three-phase
dynamic imaging was performed immediately after the injection of
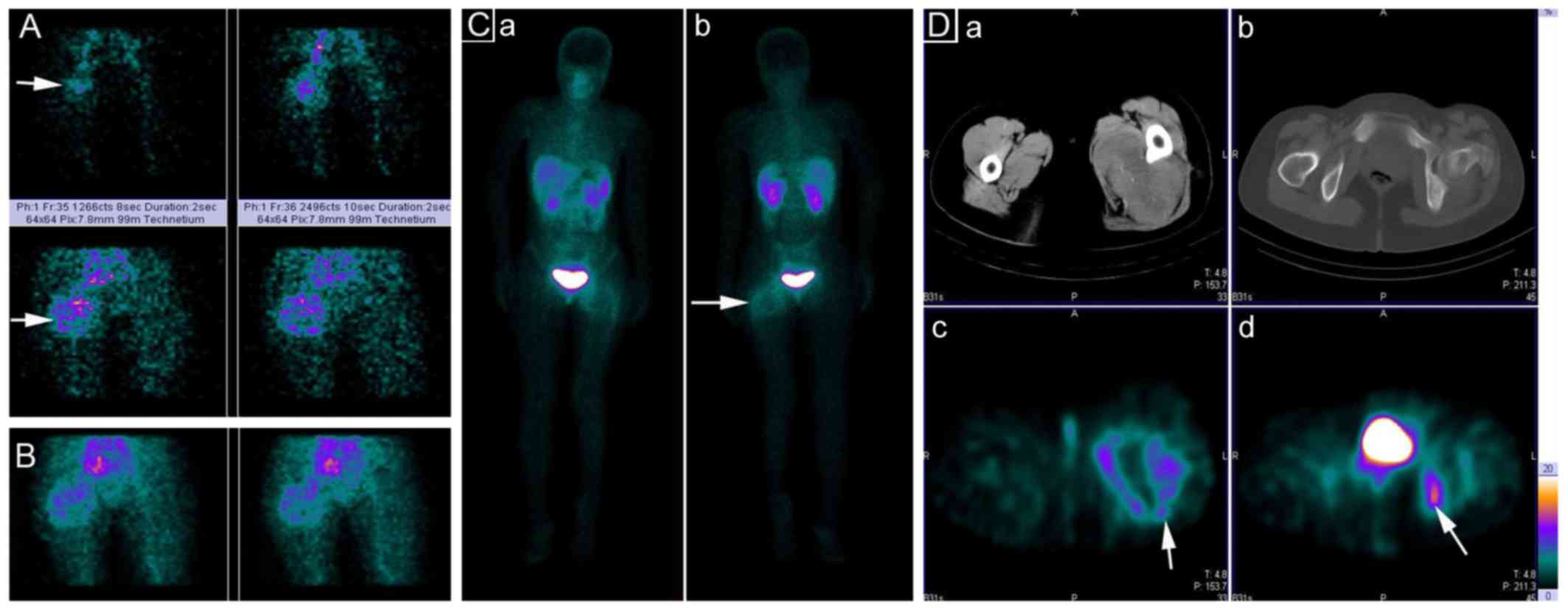
99mTc-3P-RGD2 via the right ulnar vein. Blood
perfusion (Fig. 1A, posterior view)
and blood pooling phase (Fig. 1B,
posterior view) images revealed increased blood perfusion and blood
pooling in the soft mass. Delayed planar (Fig. 1C, anterior and posterior view) and
single-photon emission (SPECT)-CT images acquired at 1 h showed a
non-uniformly increased uptake of integrin
αvβ3-targeting
99mTc-3P-RGD2 with a central core of
decreased uptake (Fig. 1D). The soft
mass was localized at the posteromedial muscle group of the left
hip. The imaging results of the SPECT-CT were the same as those on
plain CT imaging. Regional accumulation of
99mTc-3P-RGD2 was observed in the ischium and
ilium near the left hip joint and the suspected diagnosis was soft
tissue sarcoma or pPNET with bone metastasis.
Histopathological examination of the biopsy sample
(hematoxylin and eosin staining) revealed a highly cellular tumor
composed of a diffuse, compact sheet of tumor cells displaying
monomorphic, small round, finely vesicular nuclei and occasional
necrosis (Fig. 2E). The
immunohistochemical (IHC) staining for CD99 and FLI-1 yielded
highly positive (+++) and moderately positive (++) results,
respectively (Fig. 2F).
Chemoembolization and anti-angiogenic treatment were performed via
the left internal iliac artery and left deep femoral artery,
respectively.
Discussion
From a pathological perspective, pPNETs and Ewing
sarcomas (ESs) are both composed of small round cells with
identical histopathological, IHC and molecular characteristics.
They are currently classified into the same tumor family with
identical chromosomal translocations, and they generally represent
different manifestations of the same tumor with similar genetic
alterations. ES occurs predominately in the bone, while pPNETs are
more common in soft tissues, such as the kidney (2,6),
masseter muscle (3) and lungs
(5). Furthermore, pPNETs mainly
occur in children. We herein report a case of pPNET in an adult
patient, originating in the hip muscles with bone metastasis.
Unambiguous differentiation of pPNETs from other small round cell
tumors, such as CNS PNETs, poorly differentiated carcinoma,
lymphoma, desmoplastic small round cell tumor and rhabdomyosarcoma,
is difficult but of vital significance. These tumors may be
difficult to diagnose due to their primitive morphology. Cell
membranous immunoexpression of CD99 (F) and FLI1 (G) are highly
reliable markers for the definitive diagnosis of pPNET (8–10).
Tumor site, volume and the presence of metastasis,
are significant prognostic factors for pPNETs. Radiology may offer
important information for the diagnosis and prognosis of pPNETs. As
in the present case, pPNETs commonly present as large, ill-defined,
aggressive, heterogeneous, hypodense masses, exhibiting
heterogeneous enhancement with varying internal lower-density
regions (11,12). Due to the high incidence of
metastasis at presentation, a full metastatic workup is warranted
in cases with suspected pPNET. Furthermore, as pPNET is mainly
diagnosed via pathological examination of biopsy samples or
surgical specimens, more extensive investigations, such as nuclear
medicine imaging, including whole-body technetium 99m bone scan or
positron emission tomography imaging, are indicated (13). 99mTc-3P-RGD2,
an integrin αvβ3-targeted radiotracer, has
completed a phase III clinical study for solid tumor imaging in
China; it offers a major advantage for the differential diagnosis
of solid tumors and metastasis, as well as for patient selection
and evaluation for anti-angiogenic therapy (14–18). The
mechanism underlying this imaging technique is the high expression
of integrin αvβ3 on the endothelial cells of
neovessels, tumor cells, and osteoclasts. PNET, which is reported
to be integrin αv-positive, has an integrin profile almost
identical to that of ES, which is integrin
αvβ3-positive (19). We herein present what is, to the best
of our knowledge, the first report of triple-phase
99mTc-3P-RGD2 imaging (plain and SPECT-CT)
for the diagnosis and staging of a patient with pPNET. The blood
perfusion results of the lesion were consistent with that from
enhanced CT. In addition to the CT data,
99mTc-3P-RGD2 SPECT-CT imaging also
demonstrated bone metastasis, which was negative on CT in this
case. The regional aggressive nature and metastatic lesions
precluded complete surgical excision. Triple-phase
99mTc-3P-RGD2 imaging supported the use of
transarterial chemoembolization and anti-angiogenic treatment
(20). With the development of
therapeutic radionuclide labeling RGD, such as
177Lu-RGD2, integrin
αvβ3-targeted radiotherapy appears to be
promising for the future treatment of pPNETs with bone metastasis
(21).
References
|
1
|
Batsakis JG, Mackay B and el-Naggar AK:
Ewing's sarcoma and peripheral primitive neuroectodermal tumor: An
interim report. Ann Otol Rhinol Laryngol. 105:838–843. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gupta S, Majumder K, Chahal A, Saini AK
and Gupta A: management of primitive neuroectodermal tumor of the
kidney with inferior vena cava thrombus. Curr Urol. 9:47–50. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seth A, Mahapatra SK, Nayak B, Saini AK
and Biswas B: Primitive neuroectodermal tumors of kidney: Our
experience in a tertiary care center. Indian J Cancer. 53:109–112.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Purkayastha A, Pathak A, Sharma N,
Viswanath S and Dutta V: Primitive neuroectodermal tumor of lungs
in adults: A rare series of three cases treated with upfront
chemo-radiation. Transl Lung Cancer Res. 5:350–355. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jin X, Cao J, Liu Y, Bian F, Zhao Q, Wang
Y, Lv X and Huang Y: Primitive neuroectodermal tumor originating
from the lung: A case report. Oncol Lett. 12:2692–2695.
2016.PubMed/NCBI
|
|
6
|
Yazc H, Yiğit B, Doğan S, Sunter AV and
Behzatoğlu K: Peripheral primitive neuroectodermal tumor in
masseter muscle. J Craniofac Surg. 24:872–874. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Blank PM, Ostrom QT, Rouse C, Wolinsky
Y, Kruchko C, Salcido J and Barnholtz-Sloan JS: Years of life lived
with disease and years of potential life lost in children who die
of cancer in the United States, 2009. Cancer Med. 4:608–619. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Delattre O, Zucman J, Melot T, Garau XS,
Zucker JM, Lenoir GM, Ambros PF, Sheer D, TurcCarel C, Triche TJ,
et al: The Ewing family of tumors-a subgroup of small-round-cell
tumors defined by specific chimeric transcripts. N Engl J Med.
331:294–299. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ijichi K, Tsuzuki T, Adachi M and Murakami
S: A peripheral primitive neuroectodermal tumor in the larynx: A
case report and literature review. Oncol Lett. 11:1120–1124.
2016.PubMed/NCBI
|
|
10
|
Li Q, Cui W, Abulajiang G, Ma Y, Liu X,
Zhang W and Li X: Application of immunohistochemistry in the
diagnosis of small round blue-cell tumors of soft tissue. Clin Lab.
60:1383–1392. 2014.PubMed/NCBI
|
|
11
|
Ba L, Tan H, Xiao H, Guan Y, Gao J and Gao
X: Radiologic and clinicopathologic findings of peripheral
primitive neuroectodermal tumors. Acta Radiol. 56:820–828. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiao H, Bao F, Tan H, Wang B, Liu W, Gao J
and Gao X: CT and clinical findings of peripheral primitive
neuroectodermal tumour in children. Br J Radiol. 89:201404502016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dhull VS, Sharma P, Khangembam BC, Bal C
and Kumar R: Intradiploic epidermoid cyst mimicking skull
metastasis in a patient with primitive neuroectodermal tumor:
Correct diagnosis with 99mTc-MDP hybrid SPECT-CT. Rev
Esp Med Nucl Imagen Mol. 33:120–121. 2014.PubMed/NCBI
|
|
14
|
Shao Y, Liang W, Kang F, Yang W, Ma X, Li
G, Zong S, Chen K and Wang J: A direct comparison of tumor
angiogenesis with 68Ga-labeled NGR and RGD peptides in
HT-1080 tumor xenografts using microPET imaging. Amino Acids.
46:2355–2364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin X, Liang N, Wang M, Meng Y, Jia B, Shi
X, Li S, Luo J, Luo Y, Cui Q, et al: Integrin Imaging with
(99m)Tc-3PRGD2 SPECT/CT Shows High Specificity in the Diagnosis of
Lymph Node Metastasis from Non-Small Cell Lung Cancer. Radiology.
281:958–966. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kazmierczak PM, Schneider M, Habereder T,
HirnerEppeneder H, Eschbach RS, Moser M, Reiser MF, Lauber K,
Nikolaou K and Cyran CC: αvß3-Integrin-Targeted Magnetic Resonance
Imaging for the Assessment of Early Antiangiogenic Therapy Effects
in Orthotopic Breast Cancer Xenografts. Invest Radiol. 51:746–755.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miao W, Zheng S, Dai H, Wang F, Jin X, Zhu
Z and Jia B: Comparison of 99mTc-3PRGD2
integrin receptor imaging with 99mTc-MDP bone scan in
diagnosis of bone metastasis in patients with lung cancer: A
multicenter study. PLoS One. 9:e1112212014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu Z, Miao W, Li Q, Dai H, Ma Q, Wang F,
Yang A, Jia B, Jing X, Liu S, et al: 99mTc-3PRGD2 for integrin
receptor imaging of lung cancer: A multicenter study. J Nucl Med.
53:716–722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barth T, Möller P and Mechtersheimer G:
Differential expression of beta 1, beta 3 and beta 4 integrins in
sarcomas of the small, round, blue cell category. Virchows Arch.
426:19–25. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bargellini I, Turini F, Bozzi E, Lauretti
D, Cicorelli A, Lunardi A, Cioni R and Bartolozzi C: Image fusion
of preprocedural CTA with real-time fluoroscopy to guide proper
hepatic artery catheterization during transarterial
chemoembolization of hepatocellular carcinoma: A feasibility study.
Cardiovasc Intervent Radiol. 36:526–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi J, Fan D, Dong C, Liu H, Jia B, Zhao
H, Jin X, Liu Z, Li F and Wang F: Anti-tumor effect of integrin
targeted (177)Lu-3PRGD2 and combined therapy with
Endostar. Theranostics. 4:256–266. 2014. View Article : Google Scholar : PubMed/NCBI
|