Introduction
Skull base surgery is a new cross edge discipline
which has been formed in recent years and primary skull base tumor
has less incidence in clinical relatively. Most of the skull base
tumors treated by oral and maxillofacial surgery mainly involve the
lateral skull base (LSB). The anatomy of this area had been
described in detail: This area is a well-concealed and complex
anatomical region with significant functions and narrow surgery
view (1–3). All of these account for the diversity
of surgical approaches for removal of lateral skull base tumors
(LSBTs). Most LSBTs are benign (65–75%), and they usually originate
from the salivary glands comprising 40–50% of the total. The rest
are neurogenic (20%) or otherwise (20%) (3–7).
Manifestations of LSBTs include a mass in the oropharynx or the
upper neck, changes in voice, cranial nerve deficits, and so forth.
However, in some cases, LSBTs may go undetected for a long time,
and they usually present as an asymptomatic mass (6,8,9). A variety of surgical approaches have
been described for management of LSBTs, the most common among them
including the transmandibular, the transmaxillary,
transparotid-transcervical and the transcervical approaches. The
decisive factor that affects the option of the surgical approach is
which one will maximize exposure for intact tumor excision while
minimizing functional and aesthetic deficits. This article
describes our 8-year experience of managing these tumors, mainly
concerning which current diagnostic evaluation is used for the
determination of the operational plan, and our surgical approaches
for the removal of LSBTs.
Patients and methods
Patient population
Between January 2007 and August 2015, 21 patients
(13 male and 8 female) ranging in age from 25–70 years (mean, 46
years) underwent operations for lesions involving the LSB. Patients
were evaluated by an oral maxillofacial surgeon and all were
operated on by the same surgical team. Patients were followed for
an average of 36 months. The medical charts were analyzed
retrospectively.
Clinical presentation
Patients mainly presented with local symptoms and
signs. Maxillofacial pain and facial mass were the most common
types experienced by the patients. Other manifestations, including
facial paralysis, trismus, dysphonia, dysphagia, foreign body
sensation, hoarseness, visual change, rhinocleisis, and so forth,
can also be observed in certain cases. If lesions invade the
cavernous sinus, palsies of partial cranial nerves may occur.
Imaging
Imaging modalities such as computer tomography (CT)
and magnetic resonance imaging (MRI) are of great importance for
determining the extent of the lesion, to depict its relationship
with vital vessels, to rule out any intracranial involvement, to
assess its resectability, and to guide the operating surgeon
through planning the right surgical approach (2,5). We make
a comparison between a contrast computerized tomographic (CT) scan
and a basic magnetic resonance imaging (MRI), and we consider that
MRI with gadolinium is preferred to CT in terms of diagnosing LSBTs
with the exception of its cost. In addition, angiography is
recommended for all enhancing lesions or vascularized masses,
particularly if imaging shows a widening of the carotid
bifurcation. Visualization of a vascular flow void on a CT or MRI
study is usually adequate, but computerized tomographic angiography
(CTA) or magnetic resonance angiography (MRA) may occasisionally be
added for supportive evidence for vascular tumors. Moreover, 3D CT
or 3D MRI reconstruction makes it convenient to observe neoplasm
invasion by multiplanar graphics. So broadly speaking,
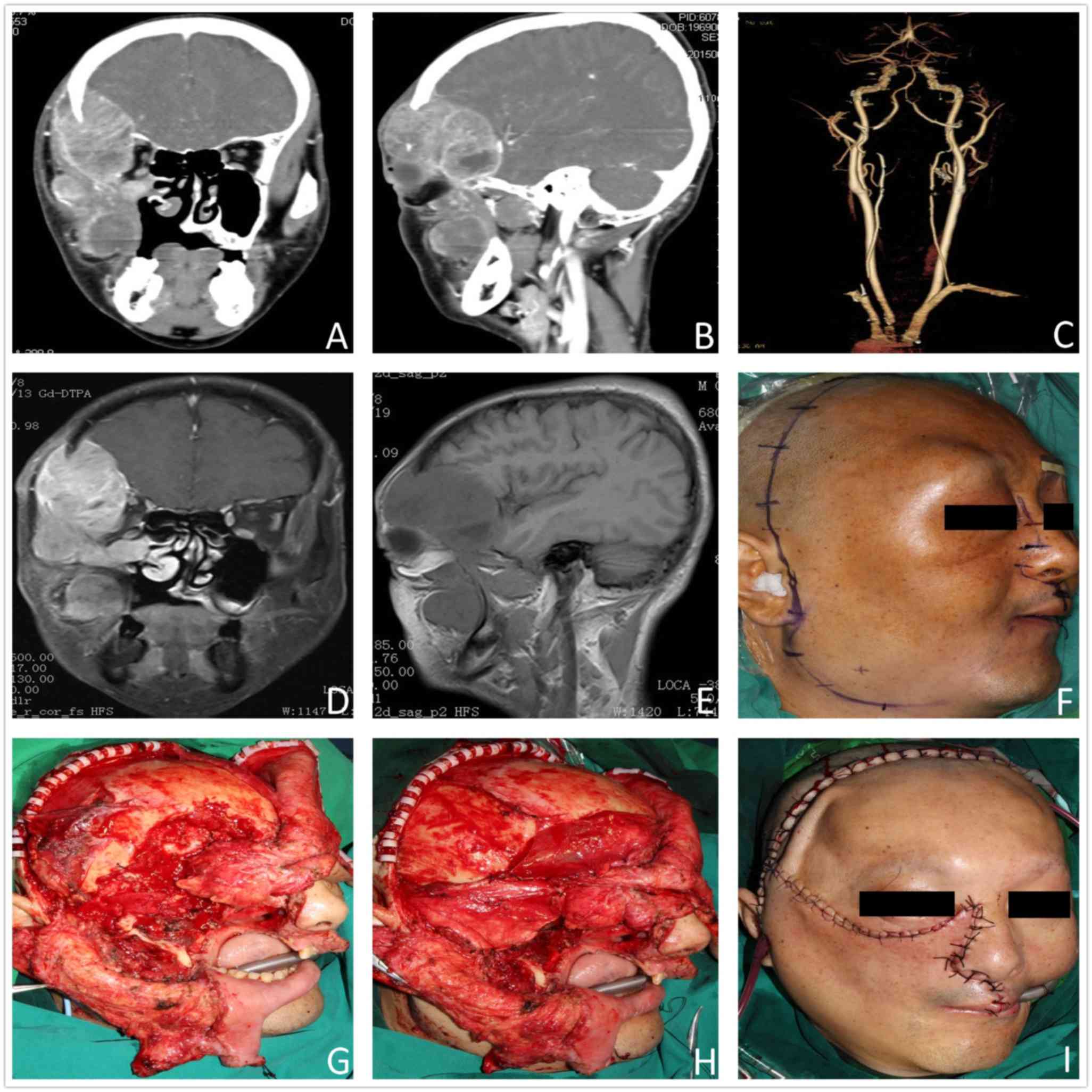
high-resolution images are always essential for LSBTs (Fig. 1A-E).
Surgical techniques
Completing surgical excision and minimizing
morbidity are the main purposes of treatment of LSBTs. However,
patients should be well informed about the potential for
complications during the perioperation. Varieties of surgical
approaches for the resection of LSBTs were used: The
transmandibular, transmaxillary, transparotid-transmandibular,
transcervical and the combined approaches. All approaches have
their own specifications, advantages and disadvantages. The most
common criterion for choosing an appropriate approach is maximizing
the exposure for completing tumor excisions and the conservation of
anatomic structures, while minimizing the functional and esthetic
defects of patients. In addition, the plastic reconstruction, which
consists of repair of the craniofacial tissue defects,
osteosynthesis, and elimination of the operative cavity, is
required for the patients.
The transmandibular approach
The transmandibular approach is adopted in cases
where patients suffer from extremely large tumors, especially
tumors with vascular lesions or tumors with lesions invading the
skull base. They need better exposure to allow a safe excision. A
cutaneous incision extending from the mastoid to the submandibular
region in the midline is performed. For fear of damage to the
marginal mandibular branch of the facial nerve, we identify 1.0–1.5
cm margins anteriorly and inferiorly to the mandibular angle to
protect this nerve. Once the mandible is split, we call it
‘mandibulotomy’. In the case of mandibulotomy, nasotracheal
intubation is used in order to permit evaluation of the occlusion.
Through this method, surgeons can get a better view. Moreover,
prior to completing the osteotomy, using two titanium miniplates is
performed for pre-plating. This method allows surgeons to open the
superior portion of the osteotomy without completely separating the
inferior bone, thus holding the condylar position and removing the
tumor under direct visualization of the surrounding tissues.
Finally, the mandible is repositioned to the initial position after
tumor resection.
The transmaxillary approach
The transmaxillary approach is made by a
Weber-Ferguson incision, and a fronto-temporal incision is marked
for preparation if the transmaxillary approach alone is not
sufficient to have the adequate operation field. A cheek flap,
including half of the upper lip, is turned laterally to expose the
anterior portion of the maxilla. Osteotomy of the maxilla comprises
inferior and lateral orbital walls, malar process, maxillary
process and palatal suture. Afterwards, resection of the partial
orbital floor and lateral wall is performed; thereby surgeons can
gain a wide access to the lesion region. If necessary, the
posterior maxillary and the pterygoid plate are removed to create
more posterior space. When the lesion excision is completed, the
relatively large operative cavity is packed with the vascularized
flap, although secondary plastic reconstruction may still be
required.
The transparotid-transmandibular
approach
In the transparotid-transmandibular approach, a
Blair incision is performed in the preauricular skin, made around
the earlobe and then extended to the neck. Usually, this approach
is suitable for LSBTs that are derived from the deep lobe of the
parotid gland. A parotidectomy is performed after identification of
the facial nerve and all of its branches. Tumors of the deep lobe
of the parotid gland often stay underneath the branches of the
facial nerve. In such cases, the branches of the facial nerve are
separated from the capsule of the tumor. Then, the tumor is taken
out from this space with a combination of blunt and sharp
separation under direct visibility. Furthermore, a
transparotid-transcervical approach can be performed through an
extended incision.
The transcervical approach
In the transcervical approach, surgeons will make a
curved transverse skin incision at ~2–3 cm underneath the lower
border of the mandible. This approach is indicated for a minority
of the dissection of LSBTs that originate from the inferior portion
of the parapharyngeal space. However, through the transcervical
approach alone, it is always difficult to obtain enough visibility
and to remove LSBTs completely. Therefore, surgeons will consider
adopting a transparotide-transcervical approach in order to
increase exposure and facilitate tumor removal.
The combined approach
The combined approaches can be classified as the
transcervical-transoral, transparotid-transcervical,
transmandibular-transcervical, transmastoid-transcervical,
transmaxillary-transparotid-transmandibular approaches (Fig. 1F, I), etc. In certain complex cases,
surgeons may find it hard to perform a tumor resection with one
single approach. Accordingly, single approaches are modified into
these combined approaches for complete mass removal. The real
difficulty of the combined approaches is to finish preoperative
surgical planning and to adjust intraoperative surgical planning.
As a consequence, on account of the characteristics of multiplicity
and individuation, the combined approaches require that surgeons
must be very experienced and have excellent resourcefulness.
Otherwise, the operations still will be have the desired
effects.
Mandibulectomy
A mandibulotomy is necessary when large, recurrent
or malignant tumors require better exposure to facilitate removal.
On account of its postoperative complications (such as facial scar
and malocclusion), an important consideration is to try to avoid
dividing the mandible as far as possible. It is our opinion that
this radical approach may serve an important role, where
appropriate.
The plastic reconstruction
When the tumor resection is finished, the dura
defects are reconstructed with autogenous fascia or artificial
materials (Fig. 1G and H). Facial
tissue defects are recovered with regional flaps or free flaps.
Large mucosal oroparapharyngeal defects need a radial-forearm
fasciocutaneous flap. Combined skin and mucosal defects require
free flaps, which may be folded over themselves with the
epithelialized surface inside. Craniofacial skeletons moved for
exposure are replaced and stabilized with titanium plates and
screws.
Postoperative measures
Patients, following the relevant surgery, should
continue to be checked regularly. They should be re-examined every
3 months within 3 years, and then the rechecking schedule should be
changed to every 6 months. If necessary, postoperative
chemoradiotherapy may be performed 1 week after the operation,
while postoperative radiotherapy may be carried out 1 week after
taking out the suture.
Results
This was a retrospective analysis of LSBTs in 21
patients (13 males; 8 females) treated primarily by surgery, whose
mean age was 46 years (range 25–70 years). Patients who had
undergone surgery for LSBTs in other institutions and patients with
other tumors were excluded from the study (Table I).
 | Table I.Clinical data. |
Table I.
Clinical data.
| No. | Sex | Age (yrs) | Benign/malignant | Surgical
approach | Complication | Follow-up
(months) | Outcome |
|---|
| 1 | F | 33 | Benign | TMY | NC | 26 | NED |
| 2 | F | 42 | Benign | TMR | TPOFN | 75 | NED |
| 3 | M | 51 | Benign | TMY | TPOFN | 65 | NED |
| 4 | M | 40 | Malignant | TPTM | NC | 61 | LRBT |
| 5 | M | 25 | Benign | TMR | NC | 64 | NED |
| 6 | F | 61 | Benign | TMR | TPOFN | 50 | NED |
| 7 | F | 48 | Malignant | TPTM | NC | 32 | NED |
| 8 | M | 70 | Benign | TPTM | TPOFN | 62 | NED |
| 9 | M | 64 | Malignant | TPTM | NC | 43 | NED |
| 10 | M | 46 | Benign | TMY | NC |
101 | NED |
| 11 | F | 35 | Benign | TPTM | NC | 49 | NED |
| 12 | F | 30 | Malignant | TC | UPOTVC | 14 | LRMT, DOD |
| 13 | F | 62 | Benign | TPTM | Bleeding | 30 | NED |
| 14 | F | 48 | Benign | TPTM | Frey's
syndrome | 56 | NED |
| 15 | M | 43 | Malignant | TMR | Dysphagia | 5 | NED |
| 16 | M | 57 | Benign | TC | UPOTVC | 64 | NED |
| 17 | M | 36 | Benign | TPTM | NC | 13 | NED |
| 18 | M | 40 | Malignant | TMR | Trismus | 11 | NED |
| 19 | M | 54 | Benign | TMY | NC | 45 | NED |
| 20 | M | 33 | Benign | TMY | TPOFN | 78 | NED |
| 21 | M | 55 | Malignant | Combined | UPOTVC | 19 | LRMT, DOD |
The incidence and frequency of symptoms were
analyzed (Table II). The common
symptoms were maxillofacial pain and dysphagia, which presented in
7 patients (33%). Other, less frequent symptoms included facial
paralysis (14%), foreign body sensation (10%), trismus (5%) and
dysphonia (5%). From Table II, the
most frequent clinical signs were facial mass (29%) and parotid
mass (24%). It is noteworthy that the incidence of asymptomatic
patients was significant. A total of >50% of the patients (11
patients; 52%) had an asymptomatic mass. It was usually identified
during a conventional checkup, or accidentally detected after an
imaging study for other diseases was performed.
 | Table II.Clinical features of LSBTs. |
Table II.
Clinical features of LSBTs.
|
| Total |
|---|
|
|
|
|---|
|
| N | % |
|---|
| Symptom |
|
|
|
Maxillofacial pain | 7 | 33 |
|
Dysphagia | 7 | 33 |
| Facial
paralysis | 3 | 14 |
| Foreign
body sensation | 2 | 10 |
|
Trismus | 1 | 5 |
|
Dysphonia | 1 | 5 |
|
Hoarseness | 0 | 0 |
| Visual
change | 0 | 0 |
|
Rhinocleisis | 0 | 0 |
| Sign |
|
|
| Facial
mass | 6 | 29 |
| Parotid
mass | 5 | 24 |
|
Oropharyngeal mass | 2 | 10 |
| Neck
mass | 1 | 5 |
| No
symptoms or signs | 11 | 52 |
All patients underwent surgical operation of their
tumors. The approach to the tumors is given in Table III. The most frequent operative
technique was the transparotid-transmandibular approach in 8 cases
(38%). In 5 (24%) patients, a transmandibular approach was used. A
transmaxillary approach was applied in 5 (24%) patients as well.
The number of patients for whom a transcervical approach was
applied was 2 (10%). Finally, 1 (5%) patient was subjected to a
combined approach treatment. Moreover, mandibulotomy was required
in 6 (29%) cases.
 | Table III.Surgical approach to LSBTs. |
Table III.
Surgical approach to LSBTs.
|
| Total |
|---|
|
|
|
|---|
| Surgical
approach | N | % |
|---|
| 1.
Transparotid-transmandibular |
8 | 38 |
| 2.
Transmandibular |
5 | 24 |
| 3.
Transmaxillary |
5 | 24 |
| 4.
Transcervical |
2 | 10 |
| 5. Combined |
1 | 4 |
| Total | 21 | 100 |
Complications of our surgical treatments were noted
in 12 patients (Table IV).
Temporary paralysis of the facial nerve (mainly the marginal
branch) was observed in 5 patients. Frey's syndrome was seen in 2
patients, but over a period of time, this gradually faded.
Unilateral paralysis of the vocal cords was observed in 2 patients,
followed by bleeding in 1 patient (24 h following surgery),
dysphagia in 1 patient and trismus in 1 patient.
 | Table IV.Complications of surgical
treatments. |
Table IV.
Complications of surgical
treatments.
|
| Total |
|---|
|
|
|
|---|
| Complication | N | % |
|---|
| Temporary paralysis
of facial nerve | 5 | 24 |
| Frey's
syndrome | 2 | 10 |
| Unilateral
paralysis of the vocal cords | 2 | 10 |
| Bleeding | 1 | 5 |
| Dysphagia | 1 | 5 |
| Ttrismus | 1 | 5 |
| Total | 12 | 57 |
The results of histopathological analysis are
presented in Table V. In our series,
there is a distinct predominance of benign tumors [in 14 patients
(67%), compared with malignant tumors in 7 patients (33%)]. The
most frequent one was pleomorphic adenoma (9 patients) among the
benign tumors. The rest of the benign tumors may be grouped under
the heading ‘sundry’. In addition, the histopathological
distribution of the 7 malignant neoplasms was mixed. We found that
there was no clear tendency about the types of malignant neoplasms
in our series from Table V.
 | Table V.Results of histopathological
analysis. |
Table V.
Results of histopathological
analysis.
|
| Total |
|---|
|
|
|
|---|
| Histopathology | N | % |
|---|
| Benign tumors | 14 | 67 |
| Pleomorphic
adenoma | 9 | 43 |
| Schwannoma | 3 | 14 |
| Inflammatory
myofibroblastic tumor | 1 | 5 |
| Carotid body
paraganglioma | 1 | 5 |
| Malignant
tumors | 7 | 33 |
|
Chondroblastoma | 1 | 5 |
| Maxillary sinus
carcinoma | 1 | 5 |
| Osteosarcoma | 1 | 5 |
| Gingival squamous
cell carcinomas | 1 | 5 |
|
Myxofibrosarcoma | 1 | 5 |
| Malignant fibrous
histiocytoma | 1 | 5 |
| Mucoepidermoid
carcinoma | 1 | 5 |
There were no identified cases of mortality in the
perioperative period and the mean follow-up was 46 months.
Regarding postoperative recidivation, of the 14 (66.7%) patients
with benign tumors, only 1 case of pleomorphic adenoma was recorded
5 years after the initial surgery. Beyond that, malignant tumors
occupied 33.3% of the patients (7 patients), and the follow-up
revealed 2 patients (9.5%) died due to having a local recurrent
malignancy. Postoperative radiotherapy was used in 5 patients, and
2 patients were treated with combined chemoradiotherapy. The
patients with malignant tumors had ordered controls that included
clinical examination, lung X-ray and CT (1 month after completing
radiotherapy; if patients do receive postoperative
chemoradiotherapy, they should have a CT scan after finishing every
third cycle of the chemotherapy). In this group of patients, 3 were
disease-free, 2 were alive although they still had the disease, and
2 died of recurrence or metastasis in the follow-up period (14 and
19 months following surgery).
Discussion
The LSB is the lateral part of the skull base that
includes the deep areas of the temporal and zygomatic bone. There
are many vital structures in this area, which contains the parotid
gland and minor salivary glands, the VII, IX, X and XI cranial
nerves, the cervical sympathetic nerve, lymph nodes, the internal
jugular vein, the external carotid artery and its branches, and
especially, the internal carotid artery (10,11). The
internal carotid artery is a terminal branch of the common carotid
artery. It arises from the common carotid artery, which bifurcates
into the internal and external carotid artery. The internal carotid
artery is vitally important as it supplies the brain. When
encountering patients with LSBTs, the approach of resection of
LSBTs always requires careful protection of the internal carotid
artery. However, how to protect the internal carotid artery
effectively remains a key problem to be solved. A series of methods
can be adopted as follows. i) Be quite familiar with the anatomic
structure, sign and localization prior to the operation; ii) the
sufficient preoperative imaging assessment can improve the
protective effect of the internal carotid artery; iii) be careful
while opening the carotid sheath that contains the common carotid
artery, the internal carotid artery, the internal jugular vein and
the vagus nerve; iv) if opening the carotid sheath and exposing its
contents is necessary, it is essential to mark the contents for
recognition and protection; v) sufficient attention has to be paid
to avoid the vital vessels and nerves when using postoperative
drainage. Whichever method is adopted, care, patience and caution
are absolutely required.
From our cases, the benign tumors (66.7%) hold a
significant predominance, which is the same as that in the majority
of articles we reviewed (6,12,13).
Additionally, pleomorphic adenoma is the most common histological
type. Beyond that, however, a group of rare tumors, such as
chondroblastoma, fibrohistiocytoma and inflammatory myofibroblastic
tumors, existed in the LSB area on account of the high complexity
of body tissues and structures (5,12,14,15).
The majority of LSBTs derive from the parotid gland, whereas
neurogenic tumors occurred less frequently in our patient
series.
Consequently, preoperative imaging, especially CT
and MRI, is of vital importance in terms of in choosing the best
diagnostic and the most proper surgical approach for masses of the
LSB. Usually, the CT and MRI images can both be used as the first
option. MRI supplies us with more information, by and large.
However, CT images are able to gain a better demarcation between
normal and lesion tissues (16,17).
Therefore, it was the most widely used method in our cases on
account of its greater level of acceptance and lower cost. In
addition to these, angiography was indicated for tumors that
originated in vessels, and in certain patients where embolization
needed to be performed, the angiography had to be carried out 2 or
3 days before surgery (18–20). All of these radiological studies
supplied us with information about the vital vessels, the location
of masses and the relationships among the various tissues nearby.
With the help of CT, MRI and angiography, decisions can be made by
analyzing the location, size and character of masses in the LSB
area.
Nowadays, it remains unclear whether the effect of
fine needle aspiration biopsy (FNAB) can be taken as part of the
conventional preoperative assessment of patients with LSBTs. Some
researchers have reported that they took an FNAB during an
evaluation of patients when the possibility of an open biopsy was
precluded, and concluded that FNAB might be a useful tool in this
situation (21–23). However, there remain several
researchers who do not hold with this opinion. They consider that
FNAB results are not accurate enough (24–26).
Hence, we did not use FNAB in our cases. Furthermore, we considered
that, when the diagnosis of vascular diseases is ruled out by
imaging results, FNAB is more appropriate for patients with masses
located in relatively superficial areas. Moreover, the preoperative
diagnosis may be much more meaningful for metastatic diseases,
lymphomas and other malignancies (27).
Due to the anatomical complexity and low morbidity
of LSBTs, diagnosis and treatment is quite difficult for surgeons.
Patients with LSBTs are willing to accept operations to remove the
lesion. Although different surgical approaches have been suggested
for the complete resection of lesions of the LSB, access to this
complex and variable region remains somewhat difficult due to the
proximity to vital neurovascular structures and the obstruction of
bones. In our cases, the reason that we chose the transmandibular,
transmaxillary, transparotid-transmandibular and transcervical
approaches as our surgical approaches of choice depended on the
tumor location, the relationship of the crucial nerves and vessels,
and the dubiety of malignancy. The transmandibular approach with or
without osteotomy was the most used treatment in our cases. Owing
to the limited visual field of the deep part of the intratemporal
fossa area and its relatively large operating distance to the LSB
area, in our opinion it is suitable for LSBTs that are mainly
derived from the parapharyngeal space. As a classic means of access
described by Fisch and Pillsbury (28,29), the
transmaxillary approach provides surgeons with a great exposure for
the operation. However, this approach may result in vital nerve
injury by translocation and dysfunction of the he temporal
mandibular joint (TMJ). In addition, the risk of facial deformity
resulting from facial incision or maxilla osteotomy is another
potential drawback. The transparotid-transmandibular approach,
which could be considered as a variant of the transmandibular
approach, was proposed by Sekhar et al (30). This approach comprises improvements
on both the visual field and the operating distance, so that a
wider and more direct exposure for lesions that are derived from
the deep parotid lobe may be obtained. However, the restriction of
the primary lesion location remains its main shortcoming. The
transcervical approach exposes the LSB to an inferior access
without mandible and maxillary osteotomy. As a consequence, it is
able to provide a good protection of crucial neurovascular
structures of facial and deep tissues. However, its limitation is
that the exposure of the LSB is relatively insufficient. For
instance, it may be hard to reach the LSB region in some large
masses via this access. Outside of these four surgical approaches,
the transmandibular-zygomatic (31),
transoral (32) and combined
approach (33) have been described
to cope with LSB lesions, and these methods have achieved their
objectives as well. We do not propose to go further into the
details about them here.
The postoperative complications are listed in
Table IV. However, these did not
occur randomly. In our cases, temporary paralysis of the facial
nerve easily occurred when patients were subjected to the
transparotid-transmandibular, the transmandibular and the
transmaxillary approaches. Frey's syndrome mostly occurred when
surgeons adopted the transparotid-transmandibular and the
transmaxillary approaches. Unilateral paralysis of the vocal cords
and dysphagia occurred after operations with the transcervical
approach. Bleeding and trismus occurred following surgery with the
patient for whom the combined approach was applied. Especially
bleeding, irrespective of what kind of approach is applied by the
surgeon, following surgery is a possibility, and this is the most
dangerous problem. By reason of the complexity of LSB surgeries,
the postoperative complications are not rare. Hence, there is a
risk of intricate problems occurring when care is not taken to
reduce damage to the neurovascular structures as much as possible,
and a good hemostasis intraoperatively is not achieved, also taking
into consideration the corresponding postoperative treatments
(4,27).
All of our cases were followed up to check for
recurrence after surgical operations. We found the recidivation of
malignant tumors was significantly higher than benign tumors, and
malignant ones always had a worse prognosis. These findings were in
accordance with those of other studies (4,6).
In our cases, the follow-up of our patients with
malignancies of LSB revealed that postoperative radiotherapy or
chemotherapy did not reach an ideal treatment outcome. There was an
apparent selective bias in our small group of patients, and thus we
could not conclude that radiotherapy or chemotherapy had no effect
on improving the survival of patients with malignancies of LSB.
Although LSBTs have a low incidence rate and various
pathologies, the majority of these tumors are treatable. Owing to
the unique anatomical structure of the LSB, masses tend to be
symptomless at the early stages. Therefore, clinicians should be
aware of the possibility of the occurrence of LSBTs when patients
present with a facial mass, medial displacement or enlargement of
the pharyngeal wall. In general, radiology, CT and MRI are the
major methods of diagnosis. However, in certain cases, conventional
angiography or FNAB are required to confirm the diagnosis.
The majority of patients receive surgery for the
removal of the lesions. Successful surgery should achieve total
tumor resection with minimum sequelae, although this depends on the
location of the tumor, as well as the structures involved. By
contrast, the transparotid-transmandibular approach is suitable for
the majority of LSBTs derived from the deep parotid lobe, or it
requires a vast surgical field to ensure clean margins. The
transcervical approach is more appropriate for LSBTs that stem from
the poststyloid parapharyngeal space subdivision with benign
characteristics, while it is crucial to identify and protect the
vital vessels and nerves of the neck. Complex cases for which a
single approach would not be sufficient may require the combined
approach.
References
|
1
|
Hazarika P, Sahota JS, George S and Raja
A: Surgical treament of lateral skull base tumours our experience.
Indian J Otolaryngol Head Neck Surg. 2:19–22. 1993.
|
|
2
|
Pai PS, Moiyadi A and Nair D: Management
of lateral skull base tumours. Indian J Surg Oncol. 1:125–132.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuet ML, Kasbekar AV, Masterson L and Jani
P: Management of tumors arising from the parapharyngeal space: A
systematic review of 1,293 cases reported over 25 years.
Laryngoscope. 125:1372–1381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Riffat F, Dwivedi RC, Palme C, Fish B and
Jani P: A systematic review of 1143 parapharyngeal space tumors
reported over 20 years. Oral Oncol. 50:421–430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spinazzi EF, Desai SV, Fang CH, Jyung RW,
Liu JK, Baredes S and Eloy JA: Lateral skull base Inflammatory
pseudotumor: A systematic review. Laryngoscope. 125:2593–2600.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khafif A, Segev Y, Kaplan DM, Gil Z and
Fliss DM: Surgical management of parapharyngeal space tumors: A
10-year review. Otolaryngol Head Neck Surg. 132:401–406. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jian-feng L, Qiu-hang Z, Da-zhang Y and
Qiu-yi Q: Transcervical approach for resection of lateral skull
base tumors. J Otology. 2:102–108. 2007. View Article : Google Scholar
|
|
8
|
Prasad SC, Piccirillo E, Chovanec M, La
Melia C, De Donato G and Sanna M: Lateral skull base approaches in
the management of benign parapharyngeal space tumors. Auris Nasus
Larynx. 42:189–198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dimitrijevic MV, Jesic SD, Mikic AA,
Arsovic NA and Tomanovic NR: Parapharyngeal space tumors: 61 case
reviews. Int J Oral Maxillofac Surg. 39:983–989. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jacquesson T, Simon E, Berhouma M and
Jouanneau E: Anatomic comparison of anterior petrosectomy versus
the expanded endoscopic endonasal approach: Interest in petroclival
tumors surgery. Surg Radiol Anat. 37:1199–1207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kirsch CF: Advances in magnetic resonance
imaging of the skull base. Int Arch Otorhinolaryngol. 18:(Suppl 2).
S127–S135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rong BG, Chen WL, Ding YP, Xie G, Chen Y
and Wang TD: Surgical approaches to the skull base neoplasms.
Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 40:291–294. 2005.(In
Chinese). PubMed/NCBI
|
|
13
|
Fernández Ferro M, Fernández Sanromán J,
Costas López A, Sandoval Gutiérrez J and López de Sánchez A:
Surgical treatment of benign parapharyngeal space tumours.
Presentation of two clinical cases and revision of the literature.
Med Oral Patol Oral Cir Bucal. 13:E61–E64. 2008.PubMed/NCBI
|
|
14
|
Reid LB, Wong DS and Lyons B:
Chondroblastoma of the temporal bone: A case series, review, and
suggested management strategy. Skull Base Rep. 1:71–82. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maire JP, Eimer S, San Galli F,
Franco-Vidal V, Galland-Girodet S, Huchet A and Darrouzet V:
Inflammatory myofibroblastic tumour of the skull base. Case Rep
Otolaryngol. 2013:1036462013.PubMed/NCBI
|
|
16
|
Carrau RL, Weissman JL, Janecka IP,
Snyderman CH, Curtin HD, Sekhar L and Lee HS: Computerized
tomography and magnetic resonance imaging following cranial base
surgery. Laryngoscope. 101:951–959. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang ZY, Allen K, Kutz JW Jr and Isaacson
B: Clinical impact of early CT scans after lateral skull-base
surgery. Otolaryngol Head Neck Surg. 149:786–788. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Durden DD and Williams DW III: Radiology
of skull base neoplasms. Otolaryngol Clin North Am. 341043–1064.
(vii)2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meli GA, Chiaramonte R, Cavallaro T,
Puglisi C and Pero G: Carotid body paraganglioma. Diagnosis and
treatment by angiography. Neuroradiol J. 19:645–648. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tong Y: Role of duplex ultrasound in the
diagnosis and assessment of carotid body tumour: A literature
review. Intractable Rare Dis Res. 1:129–133. 2012.PubMed/NCBI
|
|
21
|
Farrag TY, Lin FR, Koch WM, Califano JA,
Cummings CW, Farinola MA and Tufano RP: The role of pre-operative
CT-guided FNAB for parapharyngeal space tumors. Otolaryngol Head
Neck Surg. 136:411–414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Papadogeorgakis N, Petsinis V, Goutzanis
L, Kostakis G and Alexandridis C: Parapharyngeal space tumors:
Surgical approaches in a series of 13 cases. Int J Oral Maxillofac
Surg. 39:243–250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cramer H, Lampe H and Downing P: Intraoral
and transoral fine needle aspiration. A review of 25 cases. Acta
Cytol. 39:683–688. 1995.PubMed/NCBI
|
|
24
|
Suárez-Fente V, Llorente-Pendás JL,
Gómez-Martínez J, García-González LA, López-Álvarez F and
Suárez-Nieto C: Primary tumours of the parapharyngeal space. Our
experience in 51 patients. Acta Otorrinolaringol Esp. 60:19–24.
2009.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luna-Ortiz K, Navarrete-Alemán JE,
Granados-Garcia M and Herrera-Gómez A: Primary parapharyngeal space
tumors in a Mexican cancer center. Otolaryngol Head Neck Surg.
132:587–591. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hughes KV III, Olsen KD and McCaffrey TV:
Parapharyngeal space neoplasms. Head Neck. 17:124–130. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cassoni A, Terenzi V, Della Monaca M,
Bartoli D, Battisti A, Rajabtork Zadeh O and Valentini V:
Parapharyngeal space benign tumours: Our experience. J
Craniomaxillofac Surg. 42:101–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fisch U: Infratemporal fossa approach to
tumours of the temporal bone and base of the skull. J Laryngol
Otol. 92:949–967. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fisch U and Pillsbury HC: Infratemporal
fossa approach to lesions in the temporal bone and base of the
skull. Arch Otolaryngol. 105:99–107. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sekhar LN, Schramm VL Jr and Jones NF:
Subtemporal-preauricular infratemporal fossa approach to large
lateral and posterior cranial base neoplasms. J Neurosurg.
67:488–499. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Donovan MG, Ondra SL, Illig JJ and
Dickerson NC: Combined transmandibular-zygomatic approach and
infratemporal craniotomy for intracranial skull base tumors. J Oral
Maxillofac Surg. 51:754–758. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carrau RL, Myers EN and Johnson JT:
Management of tumors arising in the parapharyngeal space.
Laryngoscope. 100:583–589. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Betka J, Chovanec M, Klozar J, Taudy M,
Plzák J, Kodetová D and Lisý J: Transoral and combined
transoral-transcervical approach in the surgery of parapharyngeal
tumors. Eur Arch Otorhinolaryngol. 267:765–772. 2010. View Article : Google Scholar : PubMed/NCBI
|