Introduction
Breast cancer is the second most common cancer in
the world, and the most frequent cancer in women, with 1.67 million
newly diagnosed cases in 2012 (1).
Furthermore, it is estimated that 20–30% of all breast cancer cases
will become metastatic (2). Although
survival outcomes for patients with metastatic breast cancer have
improved owing to modern chemotherapy developments, a method for
predicting lifetime expectancy has not been established.
The neutrophil-to-lymphocyte ratio (NLR) has been
widely used as an evaluation tool for inflammatory and immune
responses. Since increased levels of inflammation, or of an immune
reaction, reflect tumor aggressiveness or the host condition, NLR
may also be used as a predictor for survival in patients with solid
malignancies (3–5). A high value for the NLR is linked with
cancer-associated inflammation. Neutrophilia resulting from
inflammation inhibits the immune system by suppressing the
cytolytic activity of immune cells, including lymphocytes, T cells
and natural killer cells, thereby promoting cancer progression
(6).
Several studies have demonstrated that NLR is useful
in terms of predicting survival prognoses in breast cancer patients
(5,7,8).
Cross-sectional studies revealed that patients with a higher NLR
had poorer survival outcomes. However, the majority of studies
targeted patients with early breast cancer, and no studies, thus
far, have focused on metastatic breast cancer (5,7–9).
The aim of present study was to evaluate the
usefulness of NLR as a prediction tool for patients with recurrent
breast cancer.
Patients and methods
Inclusion criteria
A total of 89 patients who had breast cancer
recurrence during the follow-up period after surgery between
January 2005 and December 2014 at Chiba Graduate School of Medicine
were enrolled in the present study. Ethical approval was obtained
for this study, and informed consent was obtained from each of the
patients prior to their inclusion in the present study. Recurrent
sites included local and distant sites: The remnant breast,
regional lymph nodes, bone, brain, and internal organs. Patients
who already had a metastatic site at the time of initial diagnosis
were excluded from the present study to avoid selection and timing
biases.
Neutrophil-to-lymphocyte ratio
NLR calculations were made by dividing the serum
neutrophil count by the lymphocyte count. According to previous
reports, the NLR value may range widely, from 2.5 to 4.0, and a
standard cut-off value for a high NLR has not been established
(6). A value of 3.0 was selected for
the present study on the basis of a previous report that focused on
the association between breast cancer subtypes and NLR (9). To compare differences in NLR from the
time of initial treatment to recurrence, NLR was calculated at each
of these time points. Specimens harvested within one month
following chemotherapy were avoided, so as to eliminate any effects
due to drugs on NLR.
Treatments for metastatic breast
cancer
Tumor staging upon initial treatment was summarized
using the American Joint Committee on Cancer/Union for
International Cancer Control tumor-lymph node-metastasis (TNM)
classification system (10). The
current treatment algorism established by Hortobagyi (11), based on tumor subtype, was followed.
Bone-modifying agents were added when patients presented with bone
metastasis.
Covariates
The following variables were selected as covariates
in multivariate analysis, as obtained from medical records:
Menopausal status, body mass index (BMI; categorized by the World
Health Organization), lactate dehydrogenase (LDH) and subtype.
Statistical analysis
Univariate analyses were performed to compare
clinical and pathological characteristics between two groups
categorized by the NLR value (NLR <3 vs. NLR ≥3). Statistical
tests were chosen on the basis of a variable distribution.
Student's t-tests were used to compare normally distributed
variables; Mann-Whitney U tests were used for non-normally
distributed variables. One-way analysis of variance (ANOVA) and
one-way factorial ANOVA were used to compare NLR values across
tumor subtypes between initial treatment and recurrence. The
relative NLR change from initial treatment to the time of
recurrence was categorized into two groups according to the third
quartile of NLR change (third quartile, 1.66).
Overall survival following recurrence (OSrec) was
defined as the time between recurrence and death due to breast
cancer. Survival curves were obtained using Kaplan-Meier survival
analysis, and the curves were subsequently compared using a
log-rank test. Multivariate analysis was also performed to identify
factors that independently influenced survival. All tests were
two-tailed, and P<0.05 was considered to indicate a
statistically significant difference. SPSS® software
version 23 (IBM, Inc., Tokyo, Japan) was used for the statistical
analysis, and GraphPad Prism 6® (GraphPad Software,
Inc., La Jolla, CA, USA) was used to generate the graphs.
Results
Demographic and clinical
characteristics
A total of 73 patients (82%) had tumors ≥2 cm in
size, and 48 patients (54%) had axillar metastasis. In examining
the tumor subtypes based on immunohistochemical staining, estrogen
receptor (ER)-positive/human epidermal growth factor 2 receptor
(HER2)-negative (ER+/HER−),
ER+/HER+, HER2 type and triple negative (TN)
type were present in 31 (35%), 20 (22%), 14 (16%) and 24 (27%)
cases, respectively. A total of 83 patients (93%) received
chemotherapy [47 (53%) neoadjuvant and 36 (40%) adjuvant
chemotherapy cases]. Thirty-eight cases (43%) demonstrated local
recurrence, and 51 (57%) had distant metastasis (Table I).
 | Table I.Demographics of the patients. |
Table I.
Demographics of the patients.
| Variable | No. of patients
(n=89) | % |
|---|
| Age (year, average ±
SD) | 50.9±11.3 |
|
| BMI
(kg/m2, average ± SD) | 22.3±3.6 |
|
| WHO BMI
classification |
|
|
|
Underweight (BMI <18.5
kg/m2) | 9 | 10 |
| Normal
range (18.5≤ BMI <25 kg/m2) | 59 | 66 |
|
Overweight (25≤ BMI <30
kg/m2) | 19 | 21 |
| Obese
(BMI ≥30 kg/m2) | 2 | 3 |
| Menopausal
status |
|
|
|
Premenopausal | 40 | 45 |
|
Postmenopausal | 49 | 55 |
| T stage |
|
|
| 1 | 16 | 18 |
| 2 | 53 | 60 |
| 3 | 8 | 9 |
| 4 | 12 | 13 |
| N stage |
|
|
| 0 | 41 | 46 |
| 1 | 36 | 40 |
| 2 | 2 | 2 |
| 3 | 10 | 12 |
| Subtypes |
|
|
|
ER+,
HER− | 31 | 35 |
|
ER+,
HER+ | 20 | 22 |
| HER2
type | 14 | 16 |
| Triple
negative type | 24 | 27 |
| Neoadjuvant
chemotherapy |
|
|
| Yes | 47 | 53 |
| No | 42 | 47 |
| Adjuvant
chemotherapy |
|
|
| Yes | 36 | 40 |
| No | 53 | 60 |
| Recurrent site |
|
|
|
Local | 21 | 24 |
| Lymph
node | 17 | 19 |
| Lung | 15 | 17 |
|
Liver | 11 | 12 |
| Bone | 12 | 13 |
|
Brain | 6 | 7 |
|
Other | 7 | 8 |
Changes in NLR
The TN type demonstrated an NLR of 4.59, which was
highest among the four tumor subtypes at the time of recurrence
(P<0.05). On the other hand, the initial NLR was not
significantly different (P=0.58; Table
II).
 | Table II.Longitudinal change in the
neutrophil-to-lymphocyte ratio from the time of initial treatment
to recurrence of the cancer. |
Table II.
Longitudinal change in the
neutrophil-to-lymphocyte ratio from the time of initial treatment
to recurrence of the cancer.
|
| Initial | Recurrent | Change | Relative change
(%) |
|---|
|
|
|
|
|
|
|---|
| Variable | Mean | SD | Mean | SD | Mean | 95% CI | P-value | Mean | 95% CI |
|---|
| NLR | 2.24 | 1.01 | 2.83 | 2.18 | 0.59 | 0.15–1.04 | <0.05 | 138.4 | 118.1–158.7 |
|
ER+/HER− | 2.00 | 0.92 | 2.67 | 2.17 | 0.66 | −0.11–1.43 |
0.09 | 154.5 | 122.8–186.1 |
|
ER+/HER+ | 2.44 | 1.24 | 2.46 | 1.47 | 0.03 | −0.63–0.68 |
0.94 | 128.9 | 77.6–180.3 |
| TN type | 2.59 | 0.77 | 4.59 | 3.1 | 2.00 | 0.23–3.77 | <0.05 |
92.1 | 56.9–127.2 |
| HER2 type | 2.17 | 1.00 | 2.33 | 1.57 | 0.16 | −0.53–0.85 |
0.63 | 152.6 | 106.6–198.6 |
Overall, NLR increased by 0.59 at the time of
recurrence, as compared with the initial treatment [95% confidence
interval (CI), 0.15–1.04; P<0.05]. The highest change in NLR was
observed in the TN type, which increased by 2.0 (95% CI, 0.23–3.77;
P<0.05; Table II). Other
subtypes also demonstrated an increased NLR between the two time
points, although these changes did not reach the level of
significance (Table II).
Survival analysis
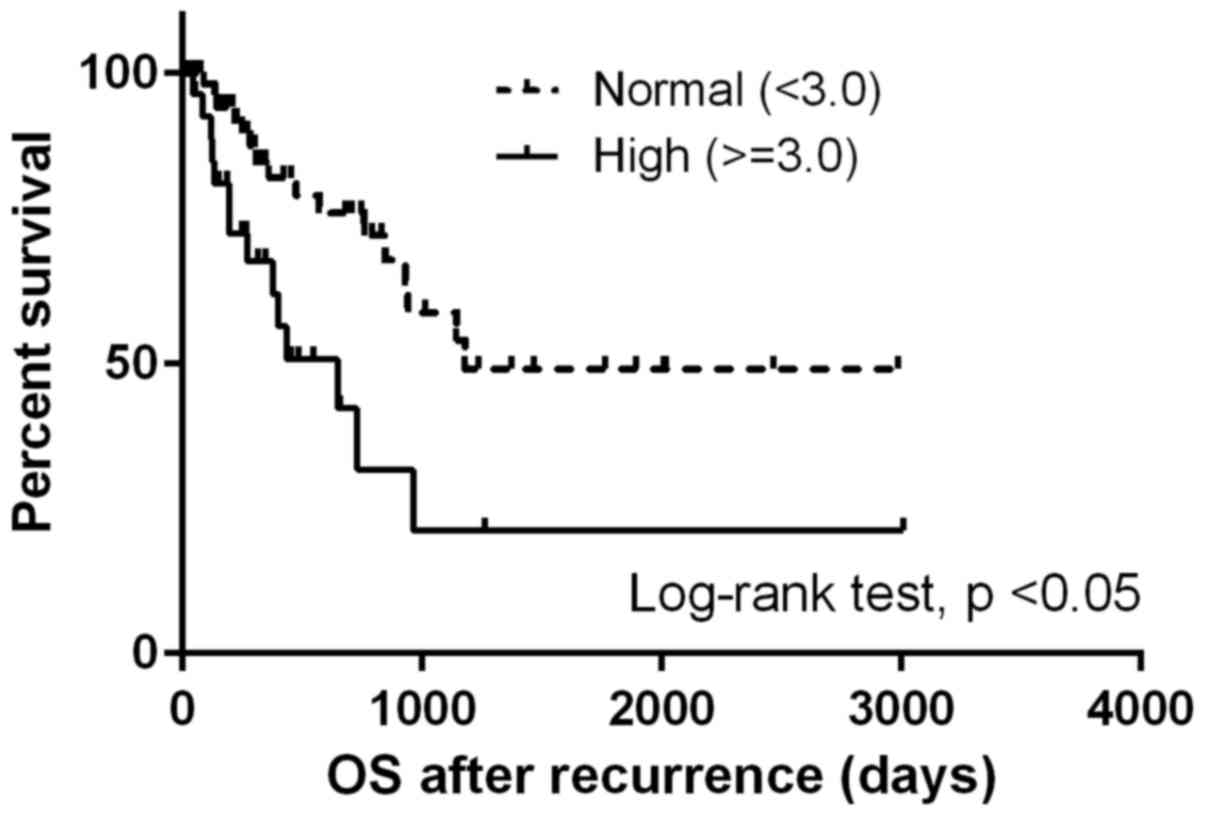
The median OSrec was 964 days. Patients with a high
NLR at the time of recurrence demonstrated significantly shorter
OSrec times [Hazard ratio (HR), 2.68; 95% CI, 1.29–5.57; P<0.05)
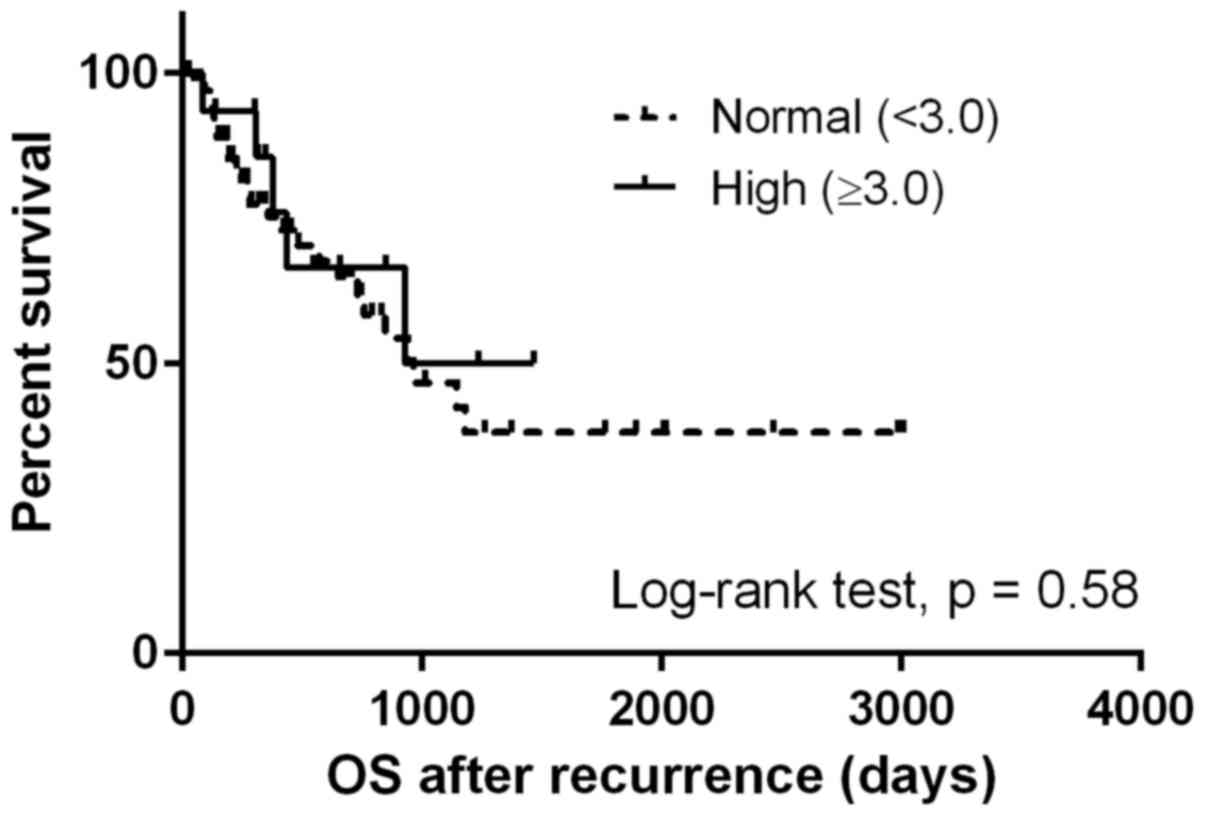
(Fig. 1), whereas no difference was
observed in the OSrec stratified by NLR at initial treatment
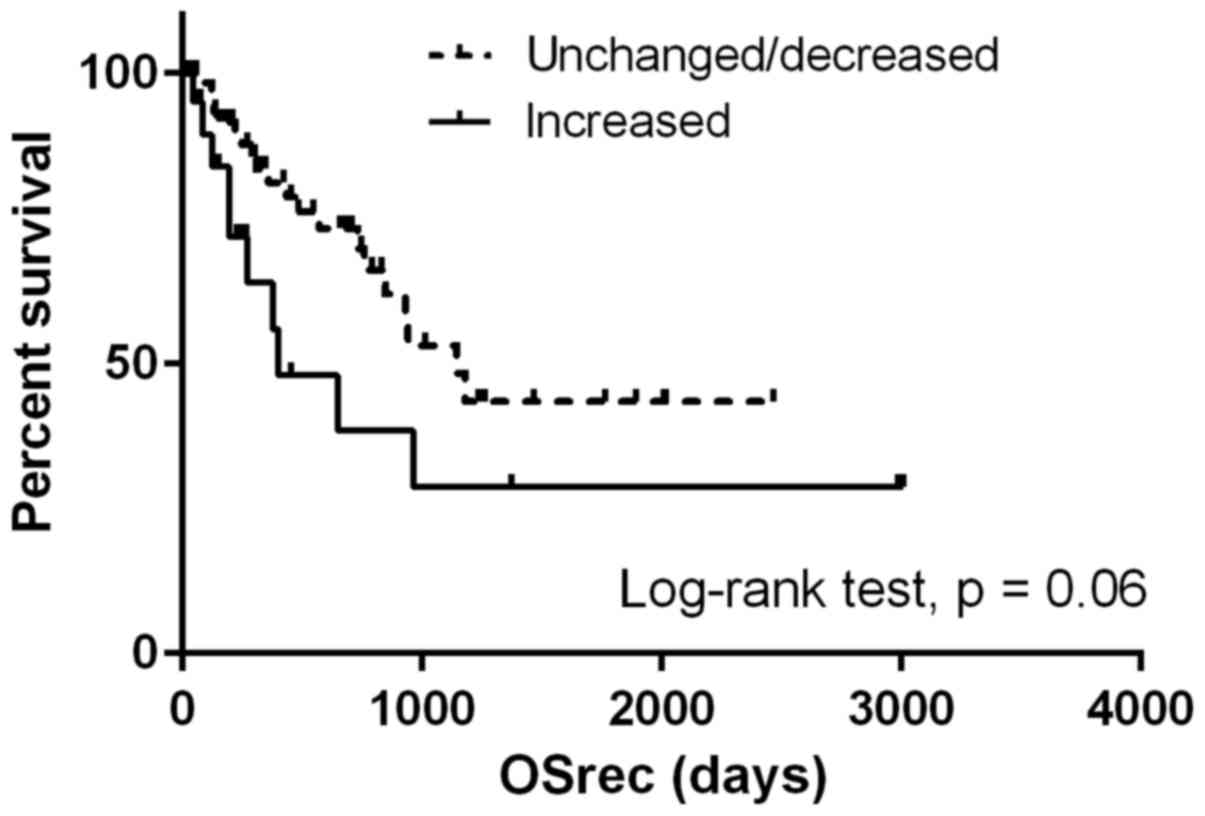
(P=0.58; Fig. 2). Patients with NLR
values increased by more than a third quartile demonstrated shorter
OSrec times (P=0.06; Fig. 3). The TN
type demonstrated significantly shorter OSrec times among the four
subtypes, with a median survival of 308 days (P<0.05).
When adjusted by covariates (menopausal status, BMI,
LDH and subtypes), NLR and tumor subtype were significantly
associated with OSrec (HR, 3.31; 95% CI, 1.01–10.86 for the TN
type, and HR, 2.93; 95% CI, 1.29–6.64 for NLR>3.0; Table III).
 | Table III.The result of Cox proportional
hazards model. |
Table III.
The result of Cox proportional
hazards model.
| Variable | Hazard ratio | 95% CI | P-value |
|---|
| NLR |
|
| >0.05 |
| Normal
(<3.0) | Ref |
|
|
| High
(≥3.0) | 2.93 | 1.29–6.64 |
|
| BMI |
|
| 0.09 |
|
Underweight (<18.5) | Ref |
|
|
| Normal range (18.5≤
BMI <25) | 0.38 | 0.09–1.44 |
|
| Overweight/obese
(BMI ≥25) | 0.88 | 0.21–3.72 |
|
| Menopause |
|
| 0.28 |
|
Premenopausal | Ref |
|
|
|
Postmenopausal | 1.56 | 0.69–3.47 |
|
| LDH | 1.00 | 1.00–1.00 | >0.05 |
| Subtypes |
|
| >0.05 |
|
ER+,
HER− | Ref |
|
|
|
ER+,
HER+ | 0.28 | 0.05–1.43 |
|
| Triple
negative type | 3.31 | 1.01–10.86 |
|
| HER2
type | 0.77 | 0.29–2.08 |
Discussion
To the best of our knowledge, the present study is
the first one to evaluate the predictive value of NLR changes in
the recurrent breast cancer setting. It was determined that a
higher NLR at the time of recurrence predicted poorer OSrec times
in patients with breast cancer. Furthermore, TN breast cancer had
significantly higher NLRs at the time of recurrence, as compared
with other subtypes.
The predictive value of the NLR for survival outcome
has been evaluated in several solid malignancies, and a high NLR
has been confirmed to be associated with significantly shorter
overall survival by meta-analysis (3). Several studies have demonstrated that
the pre-treatment NLR predicts survival outcomes for patients with
early breast cancer (7–9). In addition, a meta-analysis that
included eight studies revealed that an increased NLR was a strong
predictor of poor survival (6).
However, in five of those studies, the inclusion criteria were
inconsistent, and patients with or without metastasis were
included. The present study has demonstrated that NLR may be used
as a predictive marker for patients not only with pre-treatment
status, but also in a metastatic setting.
The present study also analyzed the longitudinal NLR
change from pre-treatment to the time of recurrence. The average
NLR was increased at the time of recurrence as compared with
pre-treatment. Although the difference was not significant, the
group with an NLR more than a third quartile higher tended to have
shorter OSrec times. To the best of our knowledge, only found three
previous studies have analyzed longitudinal NLR changes in colon
and hepatocellular carcinoma (12–14); no
study has been focused on breast cancer. Those studies focused on
the change of NLR from the time of pretreatment to post-treatment,
demonstrating that patients with a significantly increased NLR
during the treatment term have shorter survival.
The present study also revealed that the
inflammatory status upon initial treatment is associated not only
with a high recurrence rate, but also with the survival outcome
following recurrence. The importance of inflammation for prognosis
was also confirmed by another inflammation-based prognostic score,
the modified Glasgow prognostic score (mGPS), which was calculated
for serum C-reactive protein and albumin, surrogating the degree of
the nutrition and inflammatory status in patients with solid
malignancies (15–17). Furthermore, the mGPS system was
improved by the addition of neutrophil counts (18). Based on this result, it may be
postulated that the inflammatory status during the follow-up period
after initial treatment exerts a key role in survival following
recurrence. Monitoring the NLR during the follow-up period may
contribute towards early detection for patients who potentially
have a higher risk of recurrence.
TN breast cancer and NLR
The present study demonstrated that TN breast cancer
had a significantly higher NLR at the time of recurrence, and the
highest degree of NLR change compared with the other subtypes.
Previous studies demonstrated that TN breast cancer patients with a
high pre-treatment NLR had significantly shorter disease-free and
overall survival rates (9). TN
breast cancer is aggressive, and demonstrates a high rate of
recurrence at an earlier time point following initial treatment
(19). That aggressive behavior is
promoted by inflammation. For example, the expression of
interleukin (IL)-6 and IL-8 inhibits colony formation, cell
survival and predicts patient survival (20). A further study also demonstrated that
inflammatory cytokines, including tumor necrosis factor-α, induce
the endothelial-mesenchymal transition in TN breast cancer cells
(21). Additionally, a recent
clinical trial confirmed that the inflammatory reaction at local
tumor sites in TN breast cancer could be indicative of a
chemotherapeutic effect, by analyzing the number of
tumor-infiltrating lymphocytes (22,23).
Taken together, these previously published studies demonstrated
that inflammation serves a key role in TN breast cancer
progression.
To date, numerous surrogate inflammation markers
have been evaluated either in vivo or in vitro for TN
breast cancer, but none have entered common use in a clinical
setting due to lack of availability, and their expensiveness. By
using NLR as a simple surrogate marker for the degree of
inflammation, it is possible to predict survival outcomes, even in
patients with metastases. Furthermore, it is possible to determine
the current status of inflammation in high-risk patients by
monitoring the NLR during the follow-up period. The data associated
with longitudinal NLR changes require further refinement in order
to confirm the role of inflammation for survival outcome,
particularly for the inflammation-based tumor type.
The present study had several limitations. Due to
the limited number of patients, the statistical power of the
analysis may have been reduced. Secondly, a historical matching
group was not used to compare the NLR between recurrent and
non-recurrent groups; thus, the difference in the relative NLR
change between these two groups was not included in the present
study.
In conclusion, it has been demonstrated that an
increased NLR predicts survival outcome, even in patients with
recurrent breast cancer. In addition, the present study has
demonstrated the potential usefulness of NLR as an inflammation
marker for TN breast cancer.
Acknowledgements
The authors would like to express their thanks to
all patients who participated in this study. This study was
presented at the 3rd Advanced Breast Cancer Third International
Consensus Conference, 2–4 November 2015, in Lisbon, Portugal.
References
|
1
|
Estimated cancer incidence, mortality and
prevalence worldwide in 2012. World Health Organization. Available
from. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
|
|
2
|
O'Shaughnessy J: Extending survival with
chemotherapy in metastatic breast cancer. Oncologist. 10:(Suppl 3).
S20–S29. 2005. View Article : Google Scholar
|
|
3
|
Templeton AJ, McNamara MG, Šeruga B,
Vera-Badillo FE, Aneja P, Ocana A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: A systematic review and meta-analysis. J
Natl Cancer Inst. 106:dju124. 2014. View Article : Google Scholar
|
|
4
|
Sharaiha RZ, Halazun KJ, Mirza F, Port JL,
Lee PC, Neugut AI, Altorki NK and Abrams JA: Elevated preoperative
neutrophil: Lymphocyte ratio as a predictor of postoperative
disease recurrence in esophageal cancer. Ann Surg Oncol.
18:3362–3369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Azab B, Bhatt VR, Phookan J, Murukutla S,
Kohn N, Terjanian T and Widmann WD: Usefulness of the
neutrophil-to-lymphocyte ratio in predicting short- and long-term
mortality in breast cancer patients. Ann Surg Oncol. 19:217–224.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen J, Deng Q, Pan Y, He B, Ying H, Sun
H, Liu X and Wang S: Prognostic value of neutrophil-to-lymphocyte
ratio in breast cancer. FEBS Open Bio. 5:502–507. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Noh H, Eomm M and Han A: Usefulness of
pretreatment neutrophil to lymphocyte ratio in predicting
disease-specific survival in breast cancer patients. J Breast
Cancer. 16:55–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Azab B, Shah N, Radbel J, Tan P, Bhatt V,
Vonfrolio S, Habeshy A, Picon A and Bloom S: Pretreatment
neutrophil/lymphocyte ratio is superior to platelet/lymphocyte
ratio as a predictor of long-term mortality in breast cancer
patients. Med Oncol. 30:4322013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pistelli M, De Lisa M, Ballatore Z,
Caramanti M, Pagliacci A, Battelli N, Ridolfi F, Santoni M,
Maccaroni E, Bracci R, et al: Pre-treatment neutrophil to
lymphocyte ratio may be a useful tool in predicting survival in
early triple negative breast cancer patients. BMC Cancer.
15:1952015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Edge SBBD, Compton CC, Fritz AG, Greene FL
and Trotti A: Ajcc cancer staging manual. 7th. Springer; New York:
2010
|
|
11
|
Hortobagyi GN: Treatment of breast cancer.
N Engl J Med. 339:974–984. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dan J, Zhang Y, Peng Z, Huang J, Gao H, Xu
L and Chen M: Postoperative neutrophil-to-lymphocyte ratio change
predicts survival of patients with small hepatocellular carcinoma
undergoing radiofrequency ablation. PloS One. 8:e581842013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guthrie GJ, Roxburgh CS, Farhan-Alanie OM,
Horgan PG and McMillan DC: Comparison of the prognostic value of
longitudinal measurements of systemic inflammation in patients
undergoing curative resection of colorectal cancer. Br J Cancer.
109:24–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng W, Li C, Wen TF, Yan LN, Li B, Wang
WT, Yang JY and Xu MQ: Neutrophil to lymphocyte ratio changes
predict small hepatocellular carcinoma survival. J Surg Res.
192:402–408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li MX, Bi XY, Li ZY, Huang Z, Han Y, Zhou
JG, Zhao JJ, Zhang YF, Zhao H and Cai JQ: Prognostic role of
glasgow prognostic score in patients with hepatocellular carcinoma:
A systematic review and meta-analysis. Medicine (Baltimore).
94:e21332015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roxburgh CS, Salmond JM, Horgan PG, Oien
KA and McMillan DC: Comparison of the prognostic value of
inflammation-based pathologic and biochemical criteria in patients
undergoing potentially curative resection for colorectal cancer.
Ann Surg. 249:788–793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sheng T, Wang B, Wang SY, Deng B, Qu L, Qi
XS, Wang XL, Deng GL and Sun X: The relationship between serum
interleukin-6 and the recurrence of hepatitis b virus related
hepatocellular carcinoma after curative resection. Medicine
(Baltimore). 94:e9412015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Proctor MJ, Horgan PG, Talwar D, Fletcher
CD, Morrison DS and McMillan DC: Optimization of the systemic
inflammation-based glasgow prognostic score: A glasgow inflammation
outcome study. Cancer. 119:2325–2332. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hartman ZC, Poage GM, den Hollander P,
Tsimelzon A, Hill J, Panupinthu N, Zhang Y, Mazumdar A, Hilsenbeck
SG, Mills GB and Brown PH: Growth of triple-negative breast cancer
cells relies upon coordinate autocrine expression of the
proinflammatory cytokines il-6 and il-8. Cancer Res. 73:3470–3480.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qiao Y, Shiue CN, Zhu J, Zhuang T, Jonsson
P, Wright AP, Zhao C and Dahlman-Wright K: Ap-1-mediated chromatin
looping regulates ZEB2 transcription: New insights into
tnfα-induced epithelial-mesenchymal transition in triple-negative
breast cancer. Oncotarget. 6:7804–7814. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Adams S, Gray RJ, Demaria S, Goldstein L,
Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, et
al: Prognostic value of tumor-infiltrating lymphocytes in
triple-negative breast cancers from two phase III randomized
adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin
Oncol. 32:2959–2966. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsumoto H, Koo SL, Dent R, Tan PH and
Iqbal J: Role of inflammatory infiltrates in triple negative breast
cancer. J Clin Pathol. 68:506–510. 2015. View Article : Google Scholar : PubMed/NCBI
|