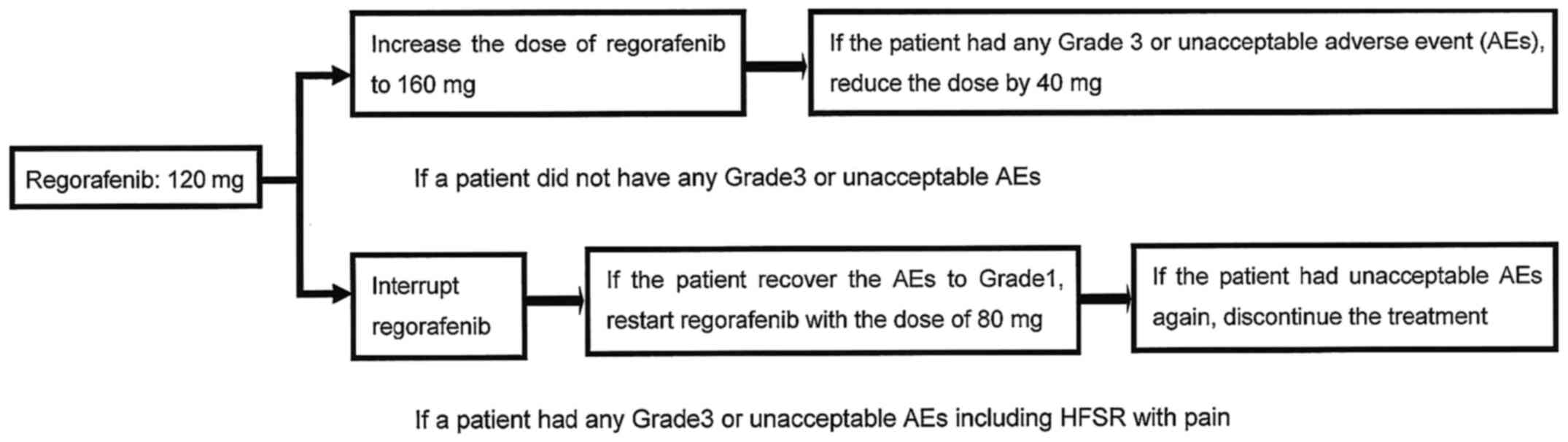
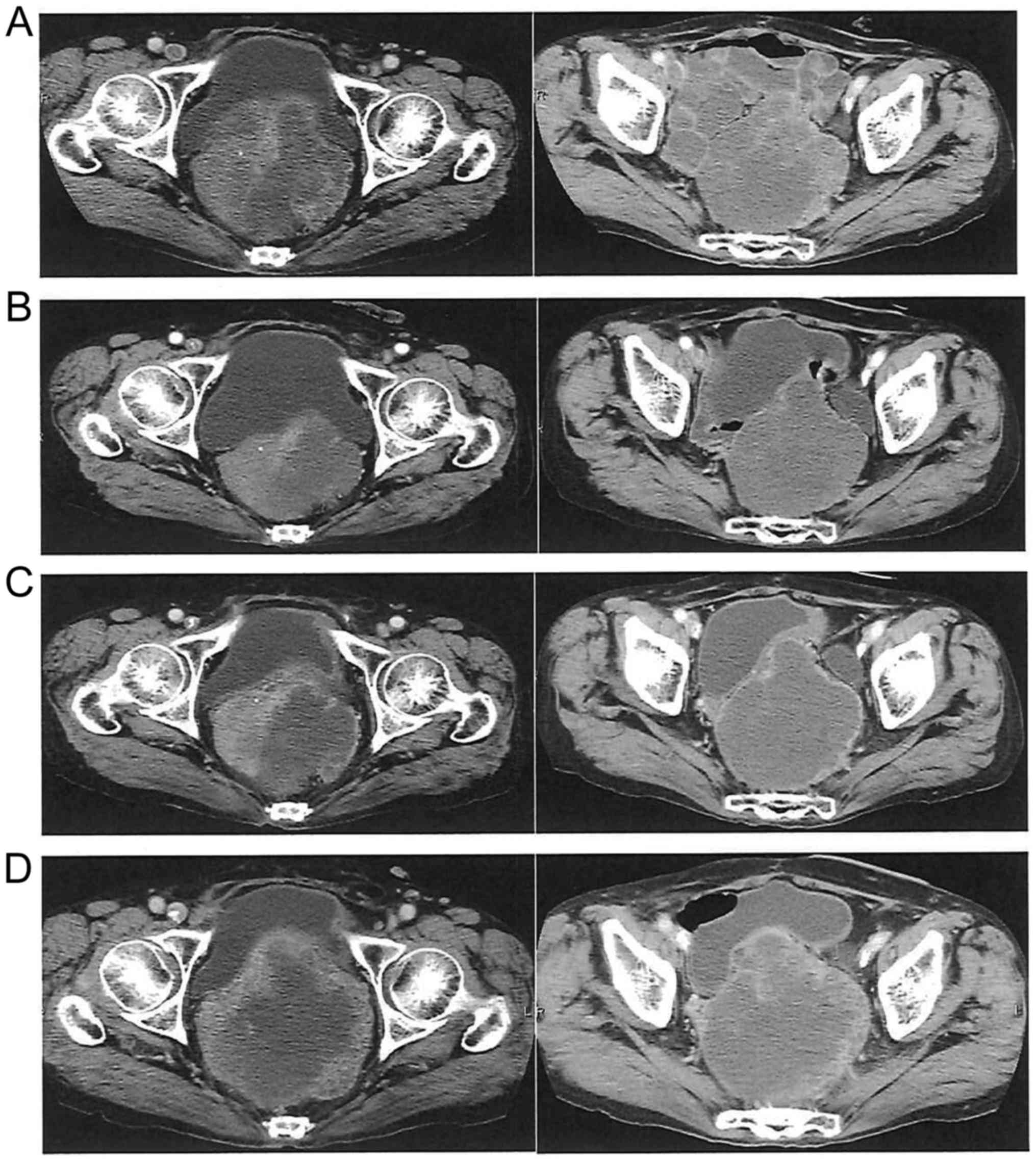
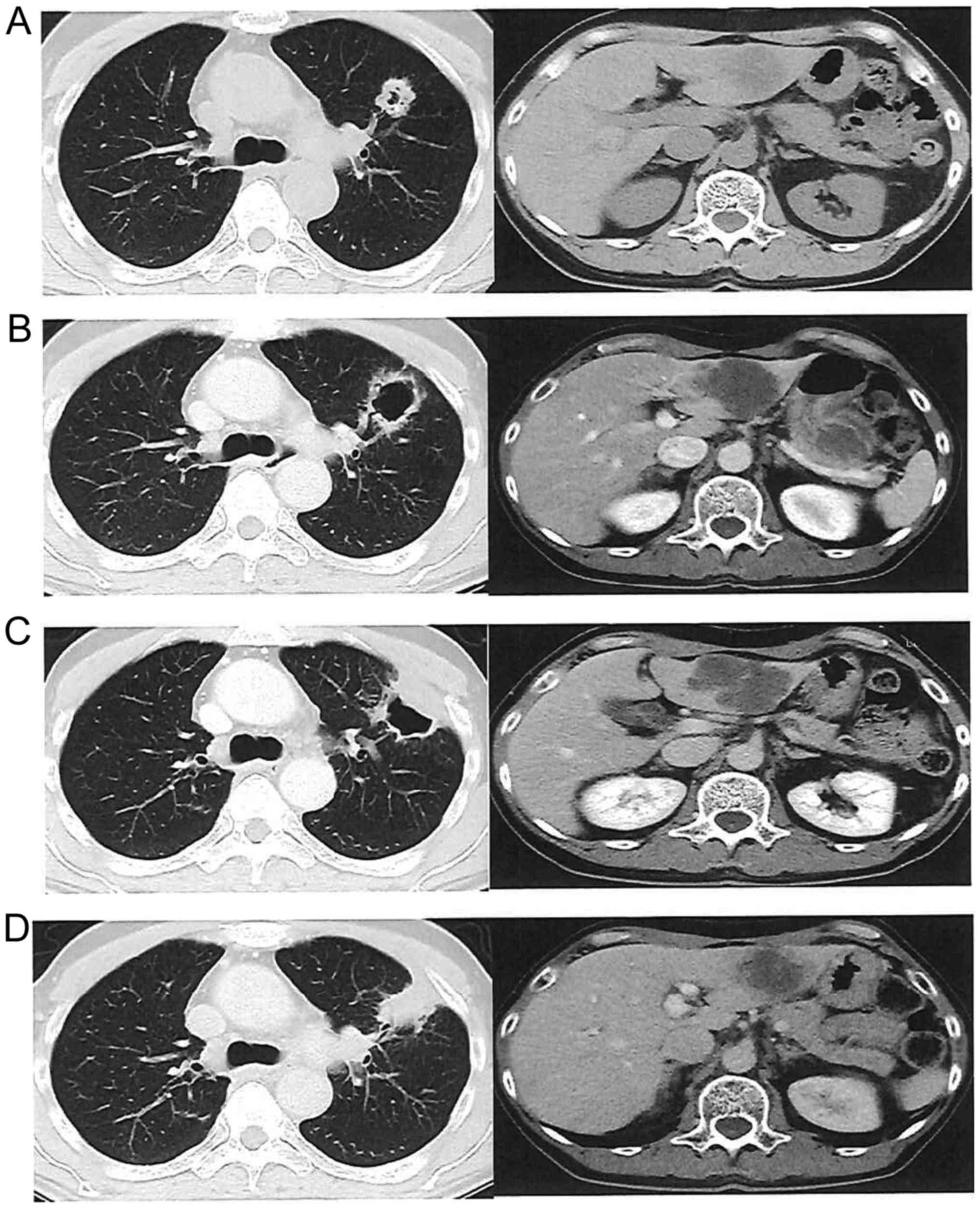
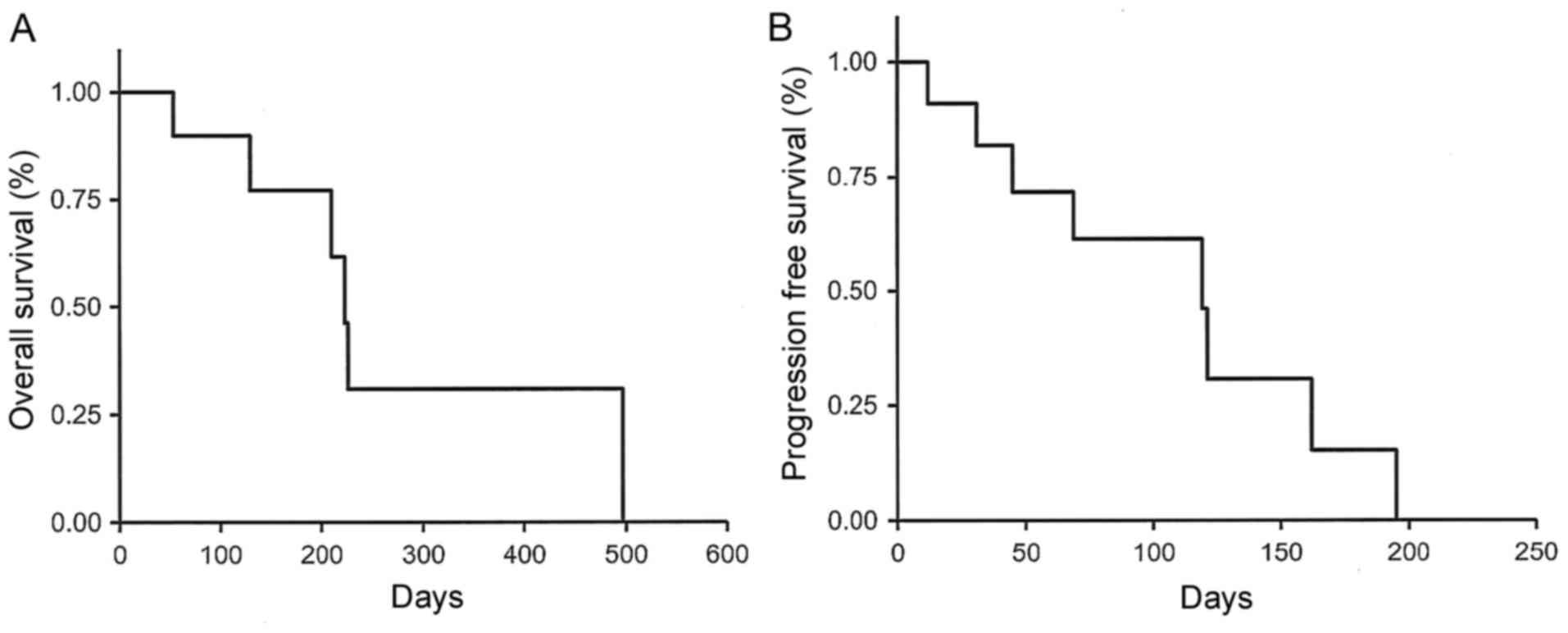
|
1
|
Matsuda A, Matsuda T, Shibata A, Katanoda
K, Sobue T and Nishimoto H and Nishimoto H: Japan Cancer
Surveillance Research Grou: Cancer incidence and incidence rates in
Japan in 2008: A study of 25 population-based cancer registries for
the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J
Clin Oncol. 44:388–396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haggar FA and Boushey RP: Colorectal
cancer epidemiology: Incidence, mortality, survival, and risk
factors. Clin Colon Rectal Surg. 22:191–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arnold D and Stein A: New developments in
the second-line treatment of metastatic colorectal cancer:
Potential place in therapy. Drugs. 73:883–891. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Cutsem E, Cervantes A, Nordlinger B
and Arnold D: ESMO Guidelines Working Group: Metastatic colorectal
cancer: ESMO clinical practice guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 25:(Suppl 3). iii1–iii9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bokemeyer C, Bondarenko I, Hartmann JT, de
Braud F, Schuch G, Zubel A, Celik I, Schlichting M and Koralewski
P: Efficacy according to biomarker status of cetuximab plus
FOLFOX-4 as first-line treatment for metastatic colorectal cancer:
The OPUS study. Ann Oncol. 22:1535–1546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Van Cutsem E, Köhne CH, Láng I, Folprecht
G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D,
Tejper S, et al: Cetuximab plus irinotecan, fluorouracil, and
leucovorin as first-line treatment for metastatic colorectal
cancer: Updated analysis of overall survival according to tumor
KRAS and BRAF mutation status. J Clin Oncol. 29:2011–2019. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Panitumumab-FOLFOX4 treatment and RAS mutations
in colorectal cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Modest DP, Stintzing S, von Weikersthal
LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T,
Lerchenmuller C, Kahl C, et al: Impact of subsequent therapies on
outcome of the FIRE-3/AIO KRK0306 trial: First-line therapy with
FOLFIRI plus cetuximab or bevacizumab in patients with KRAS
wild-type tumors in metastatic colorectal cancer. J Clin Oncol.
33:3718–3726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Venook AP, Niedzwiecki D, Lenz HJ,
Innocenti F, Mahoney MR, O'Neil BH, Shaw JM, Polite BN, Hochster
HS, Atkins JN, et al: CALGB/SWOG 80405: Phase III trial of
irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin
(mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients
(pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma
of the colon or rectum (MCRC). J Clin Oncol. 32:(Suppl; abstr
LBA3). 5s2014.
|
|
12
|
Yamazaki K, Nagase M, Tamagawa H, Ueda S,
Murata K Tamura, Tsuda T, Baba E, Tsuda M, Moriwaki T, Esaki T, et
al: A randomized phase III trial of mFOLFOX6 plus bevacizumab
versus FOLFIRI plus bevacizumab as first-line treatment for
metastatic colorectal cancer: West Japan Oncology Group study 4407G
(WJOG4407G). J Clin Oncol. 32:(Suppl; abstr 3534). 5s2014.
|
|
13
|
Schmieder R, Hoffmann J, Becker M,
Bhargava A, Müller T, Kahmann N, Ellinghaus P, Adams R, Rosenthal
A, Thierauch KH, et al: Regorafenib (BAY 73–4506): Antitumor and
antimetastatic activities in preclinical models of colorectal
cancer. Int J Cancer. 15:1487–1496. 2014. View Article : Google Scholar
|
|
14
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: Regorafenib monotherapy for previously treated metastatic
colorectal cancer (CORRECT): An international, multicentre,
randomised, placebo-controlled, phase 3 trial. Lancet. 381:303–312.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoshino T, Komatsu Y, Yamada Y, Yamazaki
K, Tsuji A, Ura T, Grothey A, Van Cutsem E, Wagner A, Cihon F, et
al: Randomized phase III trial of regorafenib in metastatic
colorectal cancer: Analysis of the CORRECT Japanese and
non-Japanese subpopulations. Invest New Drugs. 33:740–750. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu
J, Bai Y, Chi Y, Wang L, et al: Regorafenib plus best supportive
care versus placebo plus best supportive care in Asian patients
with previously treated metastatic colorectal cancer (CONCUR): A
randomised, double-blind, placebo-controlled, phase 3 trial. Lancet
Oncol. 16:619–629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grothey A, Sobrero AF, Siena S, Falcone A,
Ychou M, Humblet Y, Bouche O, Mineur L, Barone C, Adenis A, et al:
Time profile of adverse events (AEs) from regorafenib (REG)
treatment for metastatic colorectal cancer (mCRC) in the phase III
CORRECT study. J Clin Oncol. 31:(Suppl; abstr 3637). 2013.
|
|
18
|
McLellan B, Ciardiello F, Lacouture ME,
Segaert S and Van Cutsem E: Regorafenib-associated hand-foot skin
reaction: Practical advice on diagnosis, prevention, and
management. Ann Oncol. 26:2017–2026. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mross K, Frost A, Steinbild S, Hedbom S,
Büchert M, Fasol U, Unger C, Krätzschmar J, Heinig R, Boix O, et
al: A phase I dose-escalation study of regorafenib (BAY 73–4506),
an inhibitor of oncogenic, angiogenic, and stromal kinases, in
patients with advanced solid tumors. Clin Cancer Res. 18:2658–2667.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ricotta R, Sartore-Bianchi A, Verrioli A,
Vanzulli A and Siena S: Regorafenib for metastatic colorectal
cancer. Lancet. 381:15372013. View Article : Google Scholar : PubMed/NCBI
|