Introduction
Pilomatricoma is a rare benign neoplasm with
differentiation towards hair matrix cells, most commonly observed
in the head-and-neck region and occurring usually during the first
two decades of life. It was first described by Malherbe and
Chenantais in 1880 (1), who used the
term ‘calcifying epithelioma’ to refer to a hard, calcified, benign
tumor arising from the sebaceous glands. Dubreuilh and Cazenave
reported the hallmark histopathological features of the tumor in
1922 (2), but it was not until 1961
that Forbis and Helwig defined the tumors origin from the outer
root sheath of the hair follicle, suggesting also the word
‘pilomatricoma’ as a more accurate alternative to Malherbe and
Chenantaiss term (3).
The locally aggressive malignant equivalent of
pilomatricoma, which was first identified by Lopansri and Mihm in
1980 (4), has been referred to as
‘pilomatrix carcinoma’, ‘malignant pilomatricoma’, ‘trichomatrical
carcinoma’ or ‘calcifying epitheliocarcinoma of Malherbe’. Over 12
cases of pilomatrix carcinoma have been reported (5). This extremely rare tumor has a strong
tendency to relapse locally. A high local recurrence rate following
simple excision has been reported, and local recurrence may occur
even after excision with tumor-free surgical margins (6,7).
Although, in the past, this tumor was considered to be a low-grade
malignant tumor and unlikely to metastasize, at present its
significant metastatic potential has been well documented. Several
cases of lymph node metastases have been reported, while in several
patients, systemic (mainly pulmonary) metastases occurred (7–11).
In the present report, a case of pilomatrix
carcinoma of the parotic region with early local recurrence
following complete excision is described. To the best of our
knowledge, this is the first report of a pilomatrix carcinoma
recurrence occurring only 2 months after resection with negative
surgical margins. Diagnostic and therapeutic considerations are
discussed.
Case report
The patient was informed that data regarding her
case would be submitted for publication, and she consented.
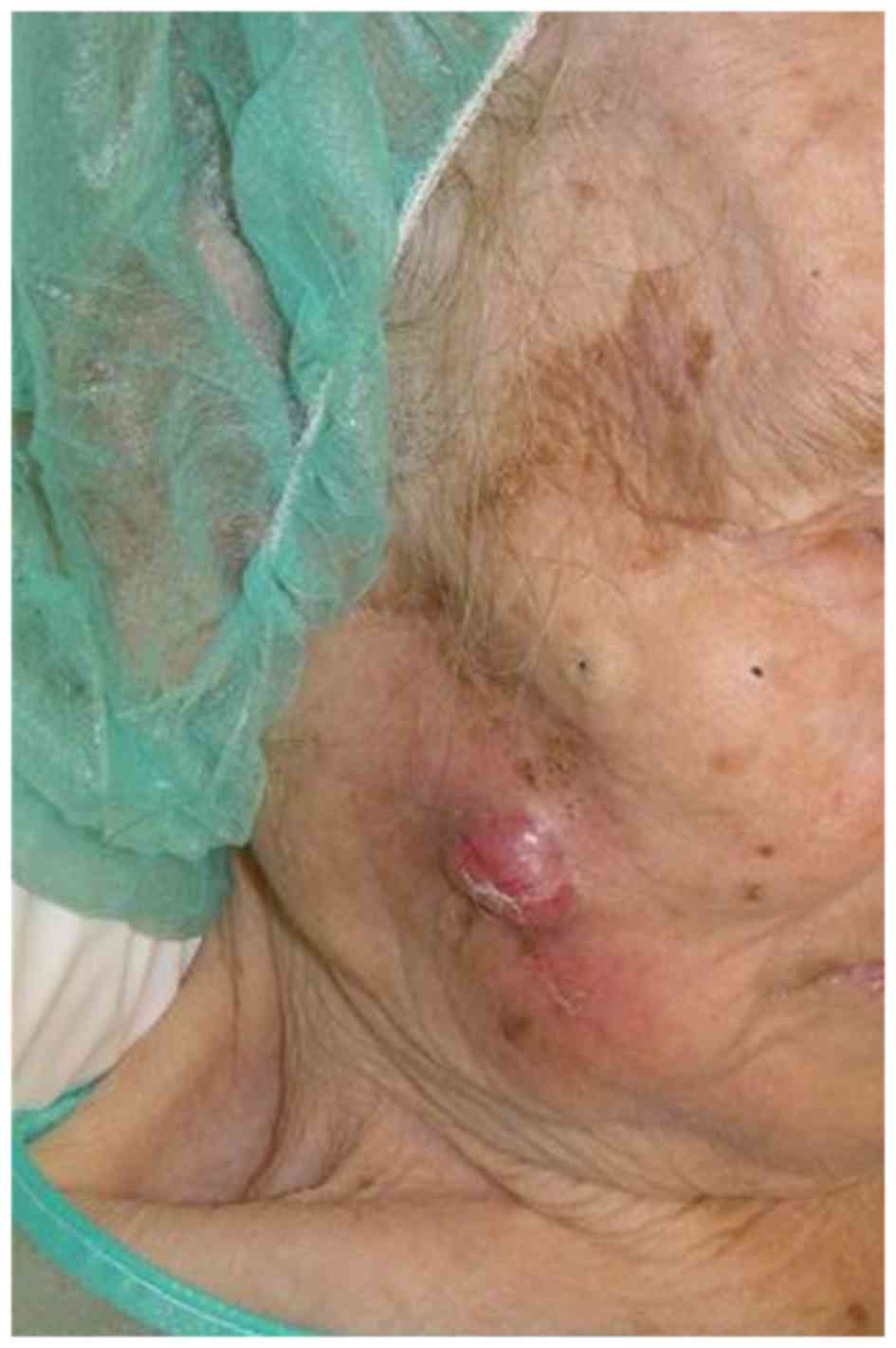
A 79-year-old woman presented with a hard, solitary,
subcutaneous, slow growing mass in the right parotid region
(Fig. 1). Clinical examination
demonstrated no evidence of lymph node involvement, and the lesion
was excised as a benign tumor with a 5 mm margin. The tumor was
intimately associated with the buccal branch of the facial nerve;
however, following excision, the function of the facial nerve
remained intact. The postoperative course was uneventful.
On pathological examination, a rather
well-circumscribed, dome-shaped, whitish solid tumor, measuring 4
cm in maximum diameter, was identified in the dermis and
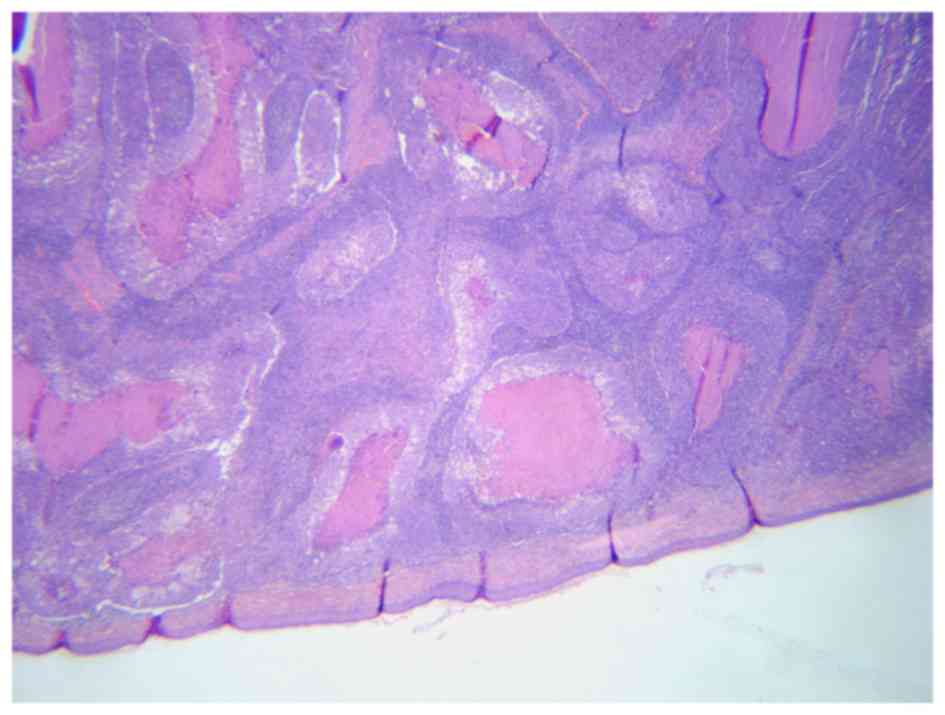
subcutaneous tissue of the excised skin. Microscopically, the tumor
exhibited the typical features of pilomatrix carcinoma. It was
composed of lobules of matrical cells with marked variation in size
and shape, and irregular foci of necrosis en masse in the center of
several of the lobules (Fig. 2). The
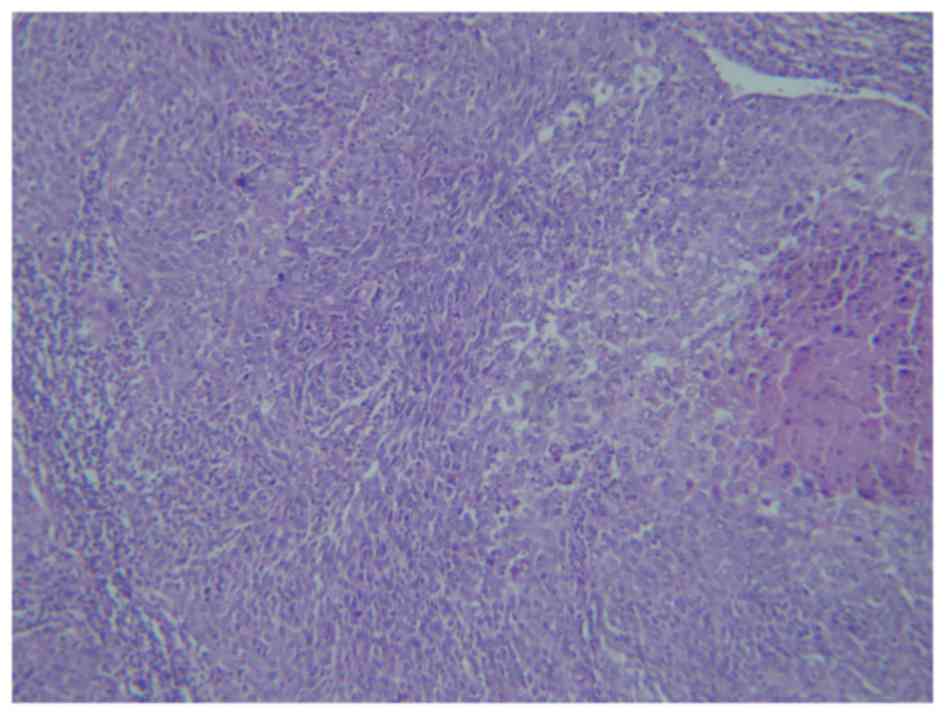
tumor cells were composed predominantly of basaloid cells with a
variable degree of anaplasia and a high mitotic rate, up to 12
mitotic events/high-power field, as well as atypical mitoses
(Fig. 3). In certain areas, the
basaloid cells had clear cytoplasm. In the center of several
lobules, there was pilar-type keratinization, with the presence of
‘ghost’ cells, whereas elsewhere, squamous cells were also
identified. Peripherally, the tumor cells had invaded the dense
fibrous desmoplastic stroma that surrounded the tumor nodules.
There was no definite vascular or lymphatic invasion. The epidermis
appeared thinner, but remained uninvolved. The tumor was close to
the surgical margins, but appeared to have been completely excised.
Immunohistochemically, the tumor cells featured cytoplasmic
staining with pan-cytokeratin CK14 markers and the monoclonal
antibody, Ber-Ep4, nuclear positivity with p63, and focal
cytoplasmic staining with carcinoembryonic antigen and CK14, while
being negative for CK20, S-100 and the neural cell adhesion
molecule, CD56.
Staging of the disease with computed tomography of
the neck, chest and abdomen regions was negative for metastatic
disease. Despite the tumor-free surgical margins, local recurrence
had already occurred 2 months following surgical excision. The
patient was reoperated, and subsequently underwent adjuvant
radiotherapy. At 4 years after surgical resection of the local
recurrence, no further local recurrence or distant metastasis has
been observed.
Discussion
Pilomatrix carcinoma is rare, and in contrast with
its benign counterpart, pilomatricoma, has a marked male
predominance (male-to-female ratio of 3:1), usually affecting
middle-aged adults (mean age of 46 years in a series of 63 cases)
(12). It typically presents as a
single, hard, firm, painless, asymptomatic, slow growing dermal or
subcutaneous mass, occasionally accompanied by bluish skin
discoloration or/and ulceration (13,14). A
mean size of 4 cm, as was identified in our case, among 60 patients
with pilomatrix carcinoma has been reported (12). The majority of the lesions are
located in the head-and-neck region, particularly in the
preauricular area, scalp, posterior neck and upper back (12,15,16). A
very small number of cases have been reported to arise from the
parotid region, as in our patient (17). Clinical differential diagnosis
includes not only benign pilomatricoma, epidermal cyst, basal cell
carcinoma and squamous cell carcinoma, but also malignant melanoma
and vascular lesions (12,18).
In pilomatrix carcinoma, histology typically reveals
irregularly shaped nests of large anaplastic, hyperchromatic
basaloid cells with prominent nucleoli, nuclear pleomorphism and
abundant mitotic figures, ranging from 8–62/high-power field.
Central areas with necrotic debris, shadow cells, clear cytoplasmic
cells, transition to squamous cells, invasion of blood and
lymphatic vessels, surface ulceration and infiltrative growth
pattern are often observed (13,14,19).
Follicular matrical differentiation with intratumoral melanocytic
hyperplasia has also been observed (‘melanocytic matricoma’). Thus,
not only cutaneous carcinomas, such as clear cell squamous
carcinoma, clear cell porocarcinoma, basal cell carcinoma with
matrical differentiation and undifferentiated sebaceous carcinoma,
but also malignant melanoma and Merkels carcinoma should be
considered as far as the histological differential diagnosis of
pigmented matrical tumors is concerned (18). Although there was no definite
vascular or lymphatic invasion, the diagnosis of pilomatrix
carcinoma in the present case study was made on the basis of the
significant pleomorphism and prominent, multiple nucleoli of the
basaloid cells, the number of mitoses, the presence of atypical
mitoses, the presence of sizeable areas of necrosis and the
infiltrating areas.
Whether pilomatrix carcinoma arises de novo
or represents malignant deformation of a pre-existing benign
pilomatricoma remains a controversial topic (5). Pilomatrix carcinoma and pilomatricoma
are the opposite extremities in the spectrum of hair matrix tumor
differentiation, with proliferating pilomatricoma and atypical
proliferating pilomatricoma occupying an intermediate place
(8,11). Kaddu et al (8) reported a higher probability for local
recurrence in incompletely excised proliferating pilomatricoma
compared with ordinary pilomatricomas. Benign and malignant
pilomatricomas have been associated with mutations in exon 3 of the
β-catenin gene (CTNNB1). Histologically, proliferating
pilomatricoma is characterized by lobular aggregations of basaloid
cells, slightly atypical nuclei, an expansive growth pattern and
lack of vascular or nerve invasion, whereas necrosis is rarely
observed (12). The differential
diagnosis between pilomatrix carcinoma and proliferating
pilomatricoma is often difficult (8). Consequently, the diagnostic accuracy in
certain cases reported as being malignant may be questioned
(11). Immunohistochemistry is not
able to reliably distinguish between benign and malignant hair
matrical tumors (20).
Initially, the majority of cases are surgically
treated as benign tumors. Local tumor recurrence, as in the present
case subject, is observed in the majority of cases in which simple
excision is the only treatment, even with the presence of clear
surgical margins during conventional histological examination
(6). Sau et al (6) reported a recurrence-free interval of
5–17 months following resection in the first large series published
(17 patients), whereas Bhasker et al (21) described a recurrence of scalp
pilomatrix carcinoma 6 months after resection. Herrmann et
al (7) reported, in 2014, three
recurrences in a series of 13 cases, 4–13 months following wide
local excision. Combining the existing literature with their
series, Hermann et al (7)
identified a recurrence rate of 23% in 43 widely excised tumors at
an average of 7 months later (median, 6 months) and a recurrence
rate of 83% in 42 simple excised tumors at an average of 11.9
months later (median, 8 months) (7).
This yields an overall recurrence rate of 53%, comparable with the
previously published recurrence rate of 60% from Sau et al
(6) in 1993. Due to the high local
recurrence rate, wide surgical (re-)excision appears to be
indicated. Wide re-excision was omitted, however, for the patient
in the present case report, and a conservative policy was advocated
due to the patient's wishes and the anatomic location of the
tumor.
The definition of adequate surgical margins remains
controversial. Several physicians consider a surgical margin of 2
cm as adequate (22), whereas
others, based on their recurrence-free experience, accept a closer
surgical margin of 5 mm (12). Mohs
micrographic surgery may provide the optimal treatment for this
tumor, given its ability to have complete histological margin
control (23). Lymph node metastases
should be treated with regional lymph node dissection, when
possible. In view of the high possibility of lymph node metastases
(9–11,24),
sentinel node biopsy could be considered. However, the option of
sentinel node biopsy is moderated by the fact that, in certain of
these cases, lymph node involvement is already clinically confirmed
at the initial presentation. Additionally, in over half of the
patients with nodal metastases, systemic metastases are
concurrently identified (9,10). Since systemic disease determines the
prognosis, the additional value of sentinel node biopsy and early
removal of non-palpable lymph node metastases is diminished.
Only rarely are systemic metastases present at
diagnosis (24). The presence of
local recurrence and tumor location on the extremities is
considered to increase the metastatic potential of malignant
pilomatricoma, although in 5 cases, metastasis occurred at the time
of initial presentation or in the absence of clinical recurrence
(7). In the majority of cases,
however, metastases are observed as recurrent disease, with a
median time from diagnosis to metastases of 18 months, and a median
survival following diagnosis of metastases of 16 months (13). Administration of various
chemotherapeutic agents has not proven to be effective (9,13).
The role of radiotherapy in the treatment of this
tumor has yet to be fully elucidated. However, adjuvant
radiotherapy following excision of primary and recurrent tumors, as
applied in the present case, has been reported to provide adequate
local tumor control (9).
Furthermore, radiotherapy has been considered to be a good
alternative in patients for whom adequate excision is not possible
(12). Radiotherapy may also be an
alternative to formal surgical dissection of involved lymph nodes,
and provide palliative treatment of systemic metastases (9,13).
In conclusion, in the present case study an early
recurrent pilomatrix carcinoma of the parotic region has been
reported. Pilomatrix carcinoma should always be considered in the
differential diagnosis of hard solitary tumors of the head-and-neck
region, as its recurrence rate following simple excision remains
considerably high. Lymph node and systemic metastases are also
observed in ~13% of the reported cases (7). Wide surgical excision of the primary
lesion is the principal modality of treatment, and should be
considered as the preferred option. Adjuvant radiotherapy may be
beneficial in local tumor control. Lymph node metastases may be
treated surgically, or with radiotherapy. Systemic disease is not
responsive to chemotherapy, and is hence associated with a poor
prognosis. Since the majority of nodal and systemic metastases
present following initial diagnosis and treatment, follow-up
examinations of these patients may be warranted, despite the
currently inadequate treatment options.
Acknowledgements
We would like to thank Mrs. Tzeni Bolbasis for
linguistically reviewing the manuscript.
References
|
1
|
Malherbe A and Chenantais J: Sur
l'epithelioma calcifie des glandes sebacees. Progr Med. 8:826–828.
1880.
|
|
2
|
Dubreuilh W and Cazenave E: De
l'epithelioma calcifie: Etude histologique. Ann Dermatol Syphilol.
3:257–268. 1922.
|
|
3
|
Forbis R Jr and Helwig EB: Pilomatrixoma
(calcifying epithelioma). Arch Dermatol. 83:606–618. 1961.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lopansri S and Mihm MC Jr: Pilomatrix
carcinoma or calcifying epitheliocarcinoma of Malherbe: A case
report and review of literature. Cancer. 45:2368–2373. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nishioka M, Tanemura A, Yamanaka T, Tani
M, Miura H, Asakura M, Tamai N and Katayama I: Pilomatrix carcinoma
arising from pilomatricoma after 10-year senescent period:
Immunohistochemical analysis. J Dermatol. 37:735–739. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sau P, Lupton GP and Graham JH: Pilomatrix
carcinoma. Cancer. 71:2491–2498. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herrmann JL, Allan A, Trapp KM and Morgan
MB: Pilomatrix carcinoma: 13 new cases and review of the literature
with emphasis on predictors of metastasis. J Am Acad Dermatol.
71:38–43.e2. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaddu S, Soyer HP, Wolf IH and Kerl H:
Proliferating pilomatricoma. A histopathologic simulator of
matrical carcinoma. J Cutan Pathol. 24:228–234. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tselis N, Heyd R, Vogt HG and Zamboglou N:
Pilomatrix carcinoma with lymph node and pulmonary metastases.
Strahlenther Onkol. 182:727–732. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bassarova A, Nesland JM, Sedloev T,
Danielsen H and Christova S: Pilomatrix carcinoma with lymph node
metastases. J Cutan Pathol. 31:330–335. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Autelitano L, Biglioli F, Migliori G and
Colletti G: Pilomatrix carcinoma with visceral metastases: Case
report and review of the literature. J Plast Reconstr Aesthet Surg.
62:e574–e577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hardisson D, Linares MD, Cuevas-Santos J
and Contreras F: Pilomatrix carcinoma: A clinicopathologic study of
six cases and review of the literature. Am J Dermatopathol.
23:394–401. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mikhaeel NG and Spittle MF: Malignant
pilomatrixoma with multiple local recurrences and distant
metastases: A case report and review of the literature. Clin Oncol
(R Coll Radiol). 13:386–389. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lan MY, Lan MC, Ho CY, Li WY and Lin CZ:
Pilomatricoma of the head and neck: A retrospective review of 179
cases. Arch Otolaryngol Head Neck Surg. 129:1327–1330. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Manivel C, Wick MR and Mukai K: Pilomatrix
carcinoma: An immunohistochemical comparison with benign
pilomatrixoma and other benign cutaneous lesions of pilar origin. J
Cutan Pathol. 13:22–29. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Waxtein L, Vega E, Alvarez L,
Cortes-Franco R, Hojyo T and Dominguez-Soto L: Malignant
pilomatricoma: A case report. Int J Dermatol. 37:538–540. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu JF, Li B, Fan ZX, Jiao T, Li C, Qin S,
Lang JY and Chen JX: Pilomatrix carcinoma on the left side of the
parotid region: A case report and review of the literature. Oncol
Lett. 10:313–316. 2015.PubMed/NCBI
|
|
18
|
Jani P, Chetty R and Ghazarian DM: An
unusual composite pilomatrix carcinoma with intralesional
melanocytes: Differential diagnosis, immunohistochemical
evaluation, and review of the literature. Am J Dermatopathol.
30:174–177. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scheinfeld N: Pilomatrical carcinoma: A
case in a patient with HIV and hepatitis C. Dermatol Online J.
14:42008.
|
|
20
|
Kondo T and Tanaka Y: Malignant
pilomatricoma in the parietal area. Pathol Oncol Res. 12:251–253.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bhasker S, Bajpai V, Bahl A and
Kalyanakuppam S: Recurrent pilomatrix carcinoma of scalp treated by
electron beam radiation therapy. Indian J Cancer. 47:217–219. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujiwara T, Yamamoto H and Hashiro M:
Malignant pilomatricoma. Scand J Plast Reconstr Surg Hand Surg.
36:119–121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sable D and Snow SN: Pilomatrix carcinoma
of the back treated by mohs micrographic surgery. Dermatol Surg.
30:1174–1176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tawfiq N, Lakhrib N, Mharech A,
Benchakroun N, Benider A, Benkirane A, Zamiati S, Mansouri I,
Roubal M, Fatmi ME Kadiri, et al: Malignant pilomatrixoma of head
and neck. A case report. Cancer Radiother. 14:198–201. 2010.(In
French). View Article : Google Scholar : PubMed/NCBI
|