Introduction
Adenoid cystic carcinoma (ACC) is a common salivary
gland malignancy. Although its clinical and pathological features
are well known, several controversial issues regarding its
behaviour and management remain to this day. In the World Health
Organization (WHO) classification (2005) (1), ACC was classified as a malignant
epithelial tumour within the group of carcinomas. Histologically,
this neoplasm is composed of two types of cells: Duct-lining and
myoepithelial cells, arranged in two subtypes designated as
glandular (cribriform) and solid patterns. Perzin et al
(2) subsequently described a more
differentiated ACC type: The tubular form. At present, there is no
consensus on the association between the histological pattern and
prognosis of ACC, although the solid pattern appears to be
associated with a worse prognosis compared with other histological
types (3,4).
ACC accounts for 5–10% of all salivary gland tumours
(5). It is considered to be one of
the most common malignant tumours that occur in minor salivary
glands (6), representing 10–15% of
all these neoplasms (4,7). Intraorally, the palate is the most
common site, comprising ~50% of all malignant palatal tumours
(3,8–14). Less
common sites of involvement include the lower lip,
retromolar-tonsillar pillar region, sublingual gland, buccal
mucosa, and floor of the mouth (15–18).
Waldron et al (4) reported
that 5% of incidences of ACC of the minor salivary glands occurred
in the upper lip.
Classically, the clinical behaviour of ACC is
considered somewhat paradoxical. It is usually a slow-growing
neoplasm with insidious evolution, although occasionally it may be
very aggressive from the beginning. It tends to spread along
perineural sheaths, causing neuropathic pain. High rates of local
recurrences and distant hematogenous metastasis are frequent in
this neoplasm, with lungs being the most common site (19). The ACC standard treatment is based on
a complete surgical resection with a 1 cm clear margin. When
located on the upper lip, tumour resection results in
medium-to-large defects that require an immediate reconstruction to
restore functional competence with optimal aesthetic outcome.
Depending on the size and location of the lip defect, the patient's
age and gender and the surgeon's experience, several techniques may
be used to ensure the proper restoration of lip form and function.
Established methods of reconstruction, including the Estlander
(20), Karapanzic (21), or Gillies (22) flaps, yield good results, although
multiple steps are often required. In 2010, the present authors
reported, to the best of our knowledge for the first time, use of
the reverse Yu flap for reconstruction of full-thickness defects up
to half of the upper lip, involving the commissure, nasolabial fold
and philtrum (23). This technique
combines a buccal and mental rotation flap, an upper lip
advancement flap, and a buccal mucosal flap. This approach produced
a good functional and cosmetic outcome in a single-stage
procedure.
The purpose of the present study was to
retrospectively analyze three patients with ACC of the minor
salivary gland located in the upper lip operated on at the
Department of Oral and Maxillofacial Surgery, Virgen Del Rocio
University Hospital, Seville, Spain, and to present our experience
with the surgical excision and immediate reconstruction of labial
defect using a reverse Yu flap.
Patients and methods
Between September 2012 and March 2014, three
patients diagnosed with upper lip ACC underwent excision and
primary reconstruction with a reverse Yu flap in the Virgen del
Rocio University Hospital (Seville, Spain). The patients comprised
two men and one woman aged from 66–80 years (mean, 73 years).
Surgical technique
Under general anesthesia, an inverted, heart-shaped,
full-thickness upper lip excision was first performed, including
the tumour with a macroscopically clear margin of >1 cm.
Subsequently, the reverse Yu flap was raised to perform a primary
lip reconstruction. Skin and subcutaneous tissue were horizontally
incised from the labial commissure. This incision was slightly
longer than the defect width when the flap was unilateral, and more
than half of the defect width when bilateral. At this point, the
orbicularis oris muscle on its medial half was partially cut, while
the lateral half of the muscle was kept intact. Subsequently, a
curved incision parallel to the nasolabial fold was made, and at
the end, it continued almost perpendicularly to the horizontal
line, which was approximately half of the distance between them. At
this point in the operation, two flaps were designed: The superior
advancement flap of the upper lip, which was pulled to the defect
site, and the inferior rotating flap of the buccal and mental
regions, which was moved to repair the donor site defect that was
created by the advancement flap. The defect of the rotation flap
was closed directly. Finally, a buccal mucosal flap was turned and
pulled outwards to reconstruct the new vermilion of the upper lip,
and the flap was sutured layer to layer. A non-absorbable
monofilament 4/0 suture was used for orbicularis oris repair,
subcutaneous sutures with 4/0 vycril were used to relieve tension
on suture lines, and skin was closed using nylon 4/0 and 5/0. No
nasogastric tube was routinely required, although the patient was
provided with a soft diet for approximately one week following
surgery.
Patient 1
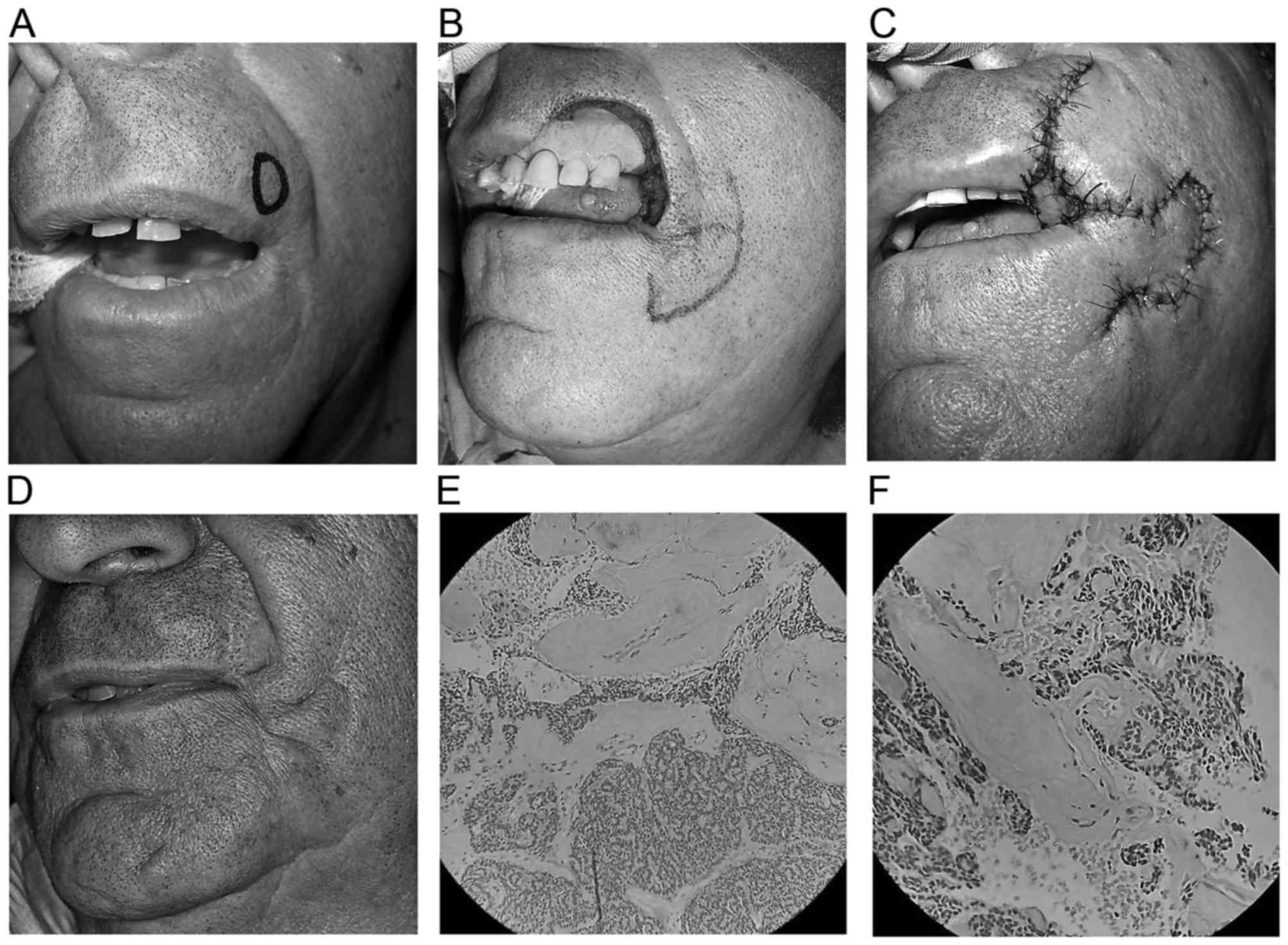
A 66-year-old man presented with a painless
submucosal nodule in the upper lip of 1.5 cm in diameter. Wide
surgical excision followed by immediate reconstruction of a defect
measuring 3×2 cm with a reverse Yu flap was performed.
Histopathological examination revealed an ACC with a predominant
cribriform pattern without evidence of perineural spread (Fig. 1).
Patient 2
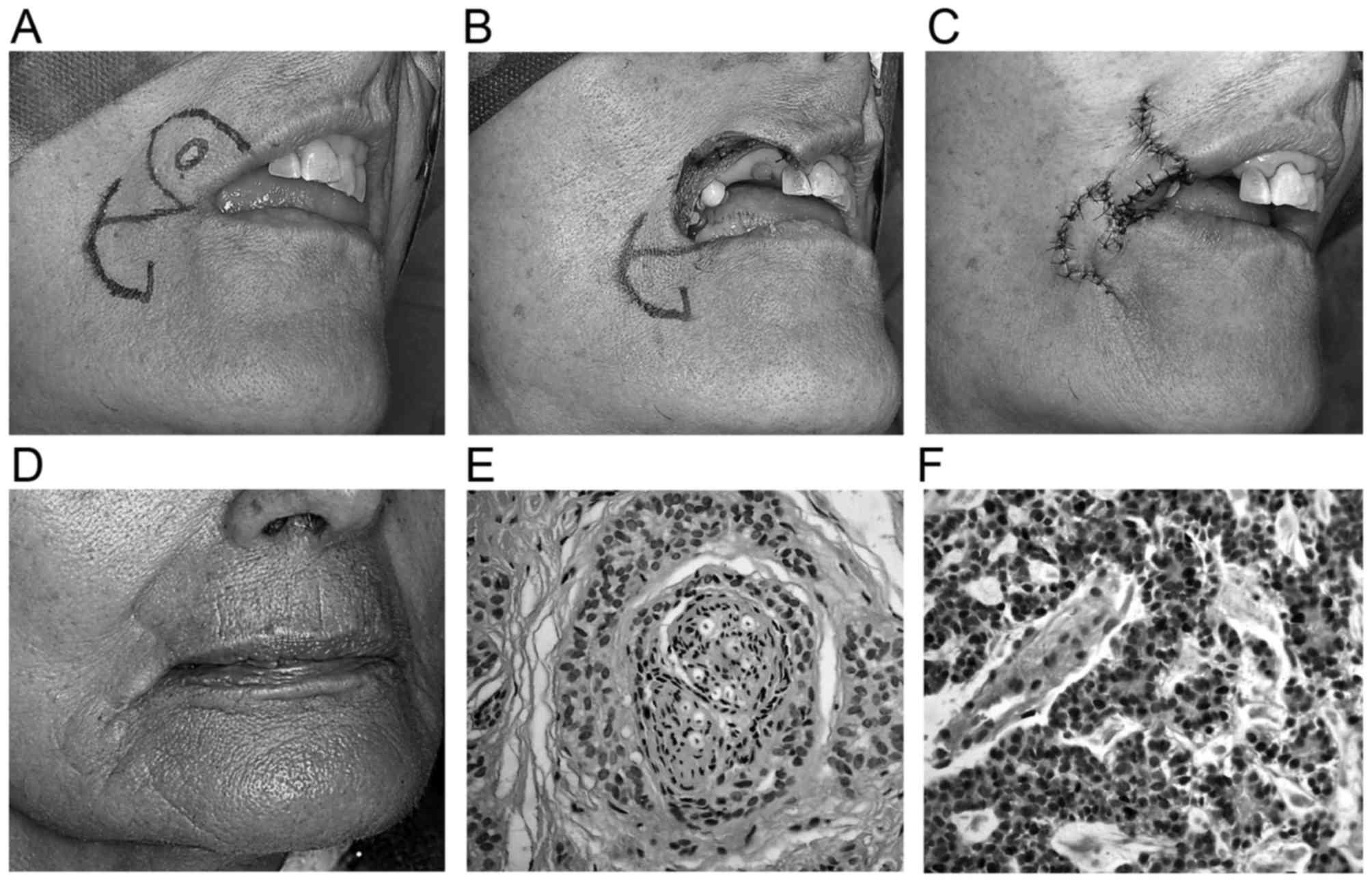
An 80-year-old woman presented with a nodule that
had invaded the labial commissure. There was no evidence of
regional nodal involvement or metastatic disease. Following tumour
resection, the defect was 3.7×3.5 cm in size, and a reverse Yu flap
was used for reconstruction. Histologically, the lesion revealed a
predominant solid pattern (Fig.
2).
Patient 3
A 74-year-old man with a history of partial
gastrectomy for complicated duodenal ulcer, acute biliary
pancreatitis with cholecystectomy, and a right lateral upper lip
ACC resected 2 years earlier in a different hospital, presented
with a solitary pulmonary nodule identified as an incidental
finding. Positron emission tomography/computed tomography (PET/CT)
suggested high suspicion of a malignancy. Pathology from the
segmental resection revealed a lung metastasis from ACC of 3.3 cm,
and no systemic therapy was required. At 2 years follow-up, the
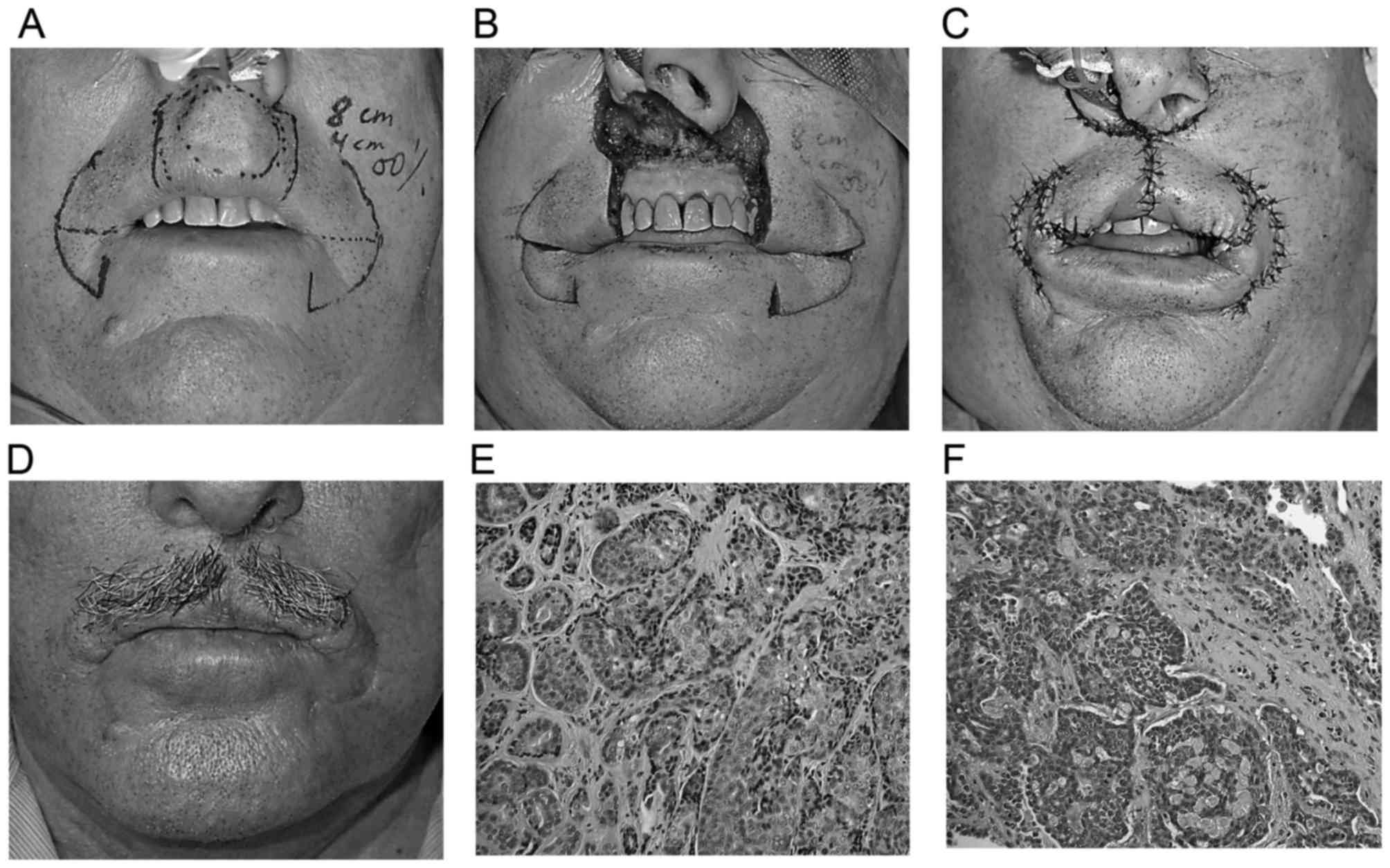
patient presented with a swelling in the midline on the upper lip
of ~2 months' duration. Intraoral examination of the labial mucosa
revealed an oval submucosal nodule measuring ~2 cm, without
ulceration and tenderness on palpation. The histopathological
examination revealed a local recurrence of ACC, with a
predominantly solid growth pattern, which required wide excision,
creating a defect of 4×3.5 cm and reconstruction with bilateral
reverse Yu flap (Fig. 3).
Results
Two cases were located in the upper lip lateral
side, and one in the midline (Table
I). The main complaint in all patients was the appearance of an
enlarging nodule on the upper lip. No patient reported spontaneous
pain. The time elapsed from the onset of symptoms to diagnosis
varied between 3–26 months. Patient 3 presented following a local
recurrence of ACC, and evidence of distant lung metastasis.
According to the predominant histological pattern, two cases
revealed a solid pattern, and the other case, a cribriform one. In
all patients, complete surgical excision with clear margins of at
least 1 cm was achieved. The resulting defect ranged from one-third
to two-thirds of the upper lip (mean, 35.7×30 mm). Two patients had
unilateral flaps, and one patient had a bilateral one. No flap
failed, and the aesthetic and functional outcome was very
satisfactory. Postoperative radiotherapy was not administered in
any case. Follow-up ranged from 12–30 months, and all patients
remain free of disease. Oral competence was good, and there was no
sign of microstomia. The residual scars remained camouflaged along
the nasolabial fold and commissure, and the upper lip was
symmetrical.
 | Table I.Demographic characteristics of the
patients (n=3). |
Table I.
Demographic characteristics of the
patients (n=3).
| Pat. no. | Age
(year)/gender | Location | Size of the
defect | Initial
diagnosis | Treatment | Follow-up | Observations | Functional and
aesthetic outcome |
|---|
| 1 | 66/M | Left upper lip | 30×20 mm | ACC
(cribriform) | Surgery alone | 2.5 years | No
complications | Excellent |
| 2 | 80/F | Right upper
lip | 37×35 mm | ACC (solid) | Surgery alone | 1.5 years | No
complications | Excellent |
| 3 | 74/M | Midline upper
lip | 40×35 mm | Local recurrence of
ACC (solid) | Surgery alone | 1 year | Previous lung
metastasis | Excellent |
Discussion
ACC tumours located in the upper lip rarely occur,
having been reported only in a very few cases to date in the
literature. The first case was reported by Pizer and Dubois
(24) in 1985, who underlined its
rarity; it is also worth mentioning the study by Gorlin et
al (25), who pointed out that
the incidence of ACC in the upper lip was ~3%. Subsequently,
several studies have referred to ACC at this location, although
very few of them have focused on its clinical behaviour and
management. Waldron et al (4)
reported 426 cases of intraoral minor salivary gland neoplasms, and
identified two cases of ACC on the upper lip; Yih et al
(26) identified one case out of
213; Pires et al (27)
identified two cases out of 546; and Wang et al (28) identified six cases out of 397.
Luna-Ortiz et al (29)
presented a series of 59 cases of upper lip malignant neoplasms,
and only one of these was ACC. Alfaro-Rubio et al (30) reported a case of ACC on the upper lip
with a cervical lymph node metastasis 10 years after treatment of
the primary tumour.
Upper lip ACC presents as a slow-growing nodule that
commonly does not exhibit early significant symptoms, so it may
pass unnoticed. The most frequent symptom in the series in the
present study was the finding of a painless submucosal nodule on
the upper lip. Due to its apparently benign slow evolution, patient
diagnosis is often delayed (12). In
patient 3, the period of time that elapsed from the first symptom
to treatment was over 2 years following the initial removal of the
primary lesion.
The biological behaviour of ACC has yet to be fully
elucidated (31). Several aspects
have been associated with a poor prognosis, including the clinical
stage, tumour location, presence of metastatic nodules,
symptoms-to-diagnosis interval, recurrence following treatment,
histological subtype, nerve invasion, and positive surgical margins
(32,33). Histological features have also been
correlated with the prognosis with inconsistent results. Several
authors have suggested that the solid pattern appears to be more
aggressive, and that it may be associated with an adverse clinical
course and poor prognosis (32).
From a histological point of view, the predominant patterns in the
present case series were cribriform and solid. In case 1, a
predominant cribriform pattern was reported without neural
invasion. However, in patients 2 and 3, the subtype was less
favourable, as a solid-predominant pattern was identified with
evidence of neural invasion. On the other hand, other studies have
not identified any correlation between histological subtype and
clinical behaviour (34), or been
able to link survival rates with the location or the clinical
stage.
The infiltrative growth pattern and perineural
invasion are other characteristics associated with the prognosis
(19). This would explain the high
risk of recurrence of this neoplasm, which could be interpreted as
an incomplete surgical removal. Nevertheless, other authors have
not identified any correlation between perineural invasion and
prognosis (32). Similarly, a
positive microscopic surgical margin is associated with a worse
prognosis (8,35). In these cases, postoperative
radiotherapy is recommended. In the present case series, surgical
resection was complete, and clear margins were achieved in all
patients. Considering that lip tumours have a relatively easier
surgical management compared with cancer in other oral cavity
locations, the findings of the present study suggest that the
prognosis of upper-lip ACC may be improved compared with other
salivary glands.
The preferred treatment for upper-lip ACC is radical
excision with immediate reconstruction (36). Resection must be complete, with a
clear margin of at least 1 cm including the nearby nerves, to avoid
recurrence, due to its propensity for perineural invasion (37). Although these tumours are highly
radiosensitive and are thus responsive to radiotherapy, they are
not radiocurable. For this reason, radiation therapy may be
indicated in unresectable tumours (38). For tumours confined to the lip,
excision with clear surgical margins and no evidence of nerve
invasion can achieve good results. In cases 1 and 2 in the present
study, the tumour was located in an accessible lip area, and
surgical resection was complete with clear margins. Patient 3
revealed a more aggressive behaviour, with lung metastasis and
local recurrence observed following a previous non-curative
surgical resection. Despite wide excision, a very close follow-up
of patients is required for at least 10 years in order to detect
local recurrences and distant metastases (5). Postoperative radiotherapy may be
indicated with positive surgical margins, or when perineural
invasion or bone infiltration occur (3,10,37,39–41).
Following a radical resection of a lip tumour,
immediate reconstruction is essential to restore functional
competence and to achieve optimal aesthetic results. In the
literature, several methods have been described for reconstructing
upper lip defects, indicating that there is no procedure that may
be applied unilaterally. Reverse Yu flap is a rotation-advancement
flap described in 2010 by Belmonte-Caro et al (23) as a modification of the original Yu
flap designed in 1989 for repair of full-thickness defects of the
lower lip (42). It may be either
unilateral or bilateral, and represents a highly reliable technique
that combines the advantages of both rotation and advancement flaps
with good functional and aesthetic results in the upper lip
reconstruction. This procedure was first described for the
reconstruction of a defect that involved nearly half of the upper
lip (35×35 mm) in a 66-year-old man following resection of a
squamous cell carcinoma. There were no postoperative complications,
and the results were very satisfactory in terms of function and
aesthetics (23). In all three cases
of the present study, the result was a functional lip, able to
control saliva, mastication and speech. Furthermore, the stoma size
was maintained, and labial sphincter function was preserved. Good
aesthetic results were obtained, since scars were placed in the
nasolabial fold and the grooves of the commissure following the
direction of the wrinkles of the face, and color and thickness of
the reconstructed skin, were similar of those of the original lip
skin. A symmetrical upper lip was achieved with minimal donor site
deformity, and produced less microstomia than other flaps. The main
disadvantages of the method are that it is a relatively more
complicated procedure to perform, and it may be more time-consuming
compared with other techniques.
To date, there have been only 12 well-documented
cases of reverse Yu flap reported in the English literature. Lee
et al (43) in 2012 reported
two cases of reconstruction of upper-lip defects, following a case
of squamous cell carcinoma and a dog bite, respectively. These
cases demonstrated that this technique may also be used in young
patients with little or no skin redundancy, and not only for an
oncological purpose. Li et al (44) reported the largest case series to
date, using a reverse Yu flap in eight patients with various types
of tumour (squamous cell carcinoma, basal cell carcinoma and
malignant melanoma). These authors recommended this procedure to
close lateral or central defects up to two-thirds the length of the
upper lip. García de Marcos et al (45) in 2014 described the first case of a
bilateral reverse Yu flap to repair almost two-thirds of the upper
paramedian lip following excision of a Merkel cell carcinoma,
obtaining very good results. The present study adds three more
cases, as well as lending support to the reliability of this
single-stage procedure for reconstructing full-thickness lateral
defects from one-third to two-thirds of the total upper lip length
in patients with ACC.
In conclusion, reverse Yu flap has been
predominantly used for the reconstruction of full-thickness defects
involving up to two-thirds of the upper lip. The findings of the
present study indicate that it may be a particularly valuable
method for the simultaneous reconstruction of medium-sized defects
following radical excision of ACC. It is easy to raise and has a
high success rate, with good aesthetic and functional results and
minimal donor site morbidity. When the indications and
contraindications are respected and used with knowledge of its
clinical utility and limitations, a well-planned reverse Yu flap
constitutes an elegant surgical option that should be taken into
consideration when reconstructing the upper lip.
References
|
1
|
El-Naggar AK and Huvos AG: Adenoid cystic
carcinomaBarnes L, Eveson JW, Reichart P and Sidransky D: Pathology
and Genetics of Head and Neck Tumours. IARC Press; Lyon: World
Health Organization (WHO); pp. 221–222. 2005
|
|
2
|
Perzin KH, Gullane P and Clairmont AC:
Adenoid cystic carcinoma arising in salivary glands: A correlation
of histologic features and clinical course. Cancer. 42:265–282.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Darling MR, Schneider JW and Phillips VM:
Polymorphous low-grade adenocarcinoma and adenoid cystic carcinoma:
A review and comparison of immunohistochemical markers. Oral Oncol.
38:641–645. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Waldron CA, el-Mofty SK and Gnepp DR:
Tumors of the intraoral minor salivary glands: A demographic and
histologic study of 426 cases. Oral Surg Oral Med Oral Pathol.
66:323–333. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Triantafillidou K, Dimitrakopoulos J,
Iordanidis F and Koufogiannis D: Management of adenoid cystic
carcinoma of minor salivary glands. J Oral Maxillofac Surg.
64:1114–1120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bjørndal K, Krogdahl A, Therkildsen MH,
Overgaard J, Johansen J, Kristensen CA, Homøe P, Sørensen CH,
Andersen E, Bundgaard T, et al: Salivary gland carcinoma in Denmark
1990–2005: A national study of incidence, site and histology.
Results of the Danish Head and Neck Cancer Group (DAHANCA). Oral
Oncol. 47:677–682. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bradley PJ: Adenoid cystic carcinoma of
the head and neck: A review. Curr Opin Otolaryngol Head Neck Surg.
12:127–132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tomich CE: Adenoid cystic
carcinomaSurgical Pathology of the Salivary Glands. Ellis GL,
Auclair PL and Gnepp DR: Saunders; Philadelphia: pp. 333–349.
1991
|
|
9
|
Avery CM, Moody AB, McKinna FE, Taylor J,
Henk JM and Langdon JD: Combined treatment of adenoid cystic
carcinoma of the salivary glands. Int J Oral Maxillofac Surg.
29:277–279. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fordice J, Kershaw C, El-Naggar A and
Goepfert H: Adenoid cystic carcinoma of the head and neck:
Predictors of morbidity and mortality. Arch Otolaryngol Head Neck
Surg. 125:149–152. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spiro RH and Huvos AG: Stage means more
than grade in adenoid cystic carcinoma. Am J Surg. 164:623–628.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang M, Ma D, Sun K, Yu G, Guo C and Gao
F: Factors influencing survival rate in adenoid cystic carcinoma of
the salivary glands. Int J Oral Maxillofac Surg. 26:435–439. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Norberg-Spaak L, Dardick I and Ledin T:
Adenoid cystic carcinoma: Use of cell proliferation, BCL-2
expression, histologic grade, and clinical stage as predictors of
clinical outcome. Head Neck. 22:489–497. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bianchi B, Copelli C, Cocchi R, Ferrari S,
Pederneschi N and Sesenna E: Adenoid cystic carcinoma of intraoral
minor salivary glands. Oral Oncol. 44:1026–1031. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weber RS, Palmer JM, el-Naggar A, McNeese
MD, Guillamondegui OM and Byers RM: Minor salivary gland tumours of
the lip and buccal mucosa. Laryngoscope. 99:6–9. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seaver PR Jr and Kuehn PG: Adenoid cystic
carcinoma of the salivary glands: A study of ninety-three cases. Am
J Surg. 137:449–455. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giannini PJ, Shetty KV, Horan SL, Reid WD
and Litchmore LL: Adenoid cystic carcinoma of the buccal vestibule:
A case report and review of the literature. Oral Oncol.
42:1029–1032. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Marcos JA Garcia, Calderón-Polanco J,
Poblet E, del Castillo-Pardo de Vera JL, Arroyo-Rodríguez S,
Galdeano-Arenas M and Dean-Ferrer A: Primary adenoid cystic
carcinoma of the mandible: Case report and review of the
literature. J Oral Maxillofac Surg. 66:2609–2615. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hemprich A and Schmidseder R: The adenoid
cystic carcinoma. Special aspects of its growth and therapy. J
Craniomaxillofac Surg. 16:136–139. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abbe R: A new plastic operation for the
relief of deformity due to double harelip. Plast Reconstr Surg.
42:481–483. 1968.PubMed/NCBI
|
|
21
|
Karapandzic M: Reconstruction of lip
defects by local arterial flaps. Br J Plast Surg. 27:93–97. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gillies HD and Millard DR: The principles
and art of plastic surgery. Butterworth; London: 1957
|
|
23
|
Belmonte-Caro R, Infante-Cossio P,
Garcia-Perla-Garcia A and Torres-Carranza E: Reverse Yús flap for
upper lip reconstruction. J Plast Reconstr Aesthet Surg.
63:148–150. 2010. View Article : Google Scholar
|
|
24
|
Pizer ME and Dubois DD: Adenoid cystic
carcinoma of the upper lip. Oral Surg Oral Med Oral Pathol.
59:70–73. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gorlin RJ, Goldman HM and Hurt W: Thoma's
oral pathology. J Periodontol. 43:575–577. 1972.PubMed/NCBI
|
|
26
|
Yih WY, Kratochvil FJ and Stewart JC:
Intraoral minor salivary gland neoplasms: Review of 213 cases. J
Oral Maxillofac Surg. 63:805–810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pires FR, Pringle GA, de Almeida OP and
Chen SY: Intra-oral minor salivary gland tumors: A
clinicopathological study of 546 cases. Oral Oncol. 43:463–470.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang D, Li Y, He H, Liu L, Wu L and He Z:
Intraoral minor salivary gland tumors in a chinese population: A
retrospective study on 737 cases. Oral Surg Oral Med Oral Pathol
Oral Radiol Endod. 104:94–100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luna-Ortiz K, Güemes-Meza A,
Villavicencio-Valencia V and Mosqueda-Taylor A: Upper lip malignant
neoplasms. A study of 59 cases. Med Oral Patol Oral Cir Bucal.
17:e371–e376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alfaro-Rubio A, Jiménez O Sanmartin,
Serra-Guillén C, Caballero C Requena, Gabriel L Hueso,
Botella-Estrada R, Enguídanos E Nagore, Cussac B Llombart and
Guillén Barona C: Adenoid cystic carcinoma. Actas Dermosifiliogr.
97:578–580. 2006.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuhel W, Goepfert H, Luna M, Wendt C and
Wolf P: Adenoid cystic carcinoma of the palate. Arch Otolaryngol
Head Neck Surg. 118:243–247. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nascimento AG, Amaral AL, Prado LA,
Kligerman J and Silveira TR: Adenoid cystic carcinoma of salivary
glands. A study of 61 cases with clinicopathologic correlation.
Cancer. 57:312–319. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shingaki S, Saito R, Kawasaki T and
Nakajima T: Adenoid cystic carcinoma of the major and minor
salivary glands. A clinicopathological study of 17 cases. J
Maxillofac Surg. 14:53–56. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Spiro RH, Huvos AG and Strong EW: Adenoid
cystic carcinoma: Factors influencing survival. Am J Surg.
138:579–583. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brown JS: Prognostic factors in oral,
oropharyngeal and salivary gland cancerMaxillofacial Surgery. Booth
PW, Schendel SA and Hausamen J-E: 1. Churchill Livingstone;
Edinburgh London, New York: pp. 291–308. 1999
|
|
36
|
Kokemueller H, Eckardt A, Brachvogel P and
Hausamen JE: Adenoid cystic carcinoma of the head and neck-a 20
years experience. Int J Oral Maxillofac Surg. 33:25–31. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Garden AS, Weber RS, Morrison WH, Ang KK
and Peters LJ: The influence of positive margins and nerve invasion
in adenoid cystic carcinoma of the head and neck treated with
surgery and radiation. Int J Radiat Oncol Biol Phys. 32:619–26.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hirota J and Osaki T: Primary central
adenoid cystic carcinoma of the mandible. J Oral Maxillofac Surg.
47:176–179. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cohen AN, Damrose EJ, Huang RY, Nelson SD,
Blackwell KE and Calcaterra TC: Adenoid cystic carcinoma of the
sub-mandibular gland: A 35-year review. J Otolaryngol Head Neck
Surg. 131:994–1000. 2004. View Article : Google Scholar
|
|
40
|
Huber PE, Debus J, Latz D, Zierhut D,
Bischof M, Wannenmacher M and Engenhart-Cabillic R: Radiotherapy
for advanced adenoid cystic carcinoma: Neutrons, photons or mixed
beam? Radiother Oncol. 59:161–167. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Maciejewski A, Szymczyk C and Wierzgoń J:
Outcome of surgery for adenoid cystic carcinoma of head and neck
region. J Craniomaxillofac Surg. 30:59–61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu JM: A new method for reconstruction of
the lower lip after tumor resection. Eur J Plast Surg. 12:155–159.
1989. View Article : Google Scholar
|
|
43
|
Lee J, Oh SJ, Jung SW and Koh SH: Combined
rotation and advancement flap reconstruction for a defect of the
upper lip: 2 cases. Arch Plast Surg. 39:244–248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li ZN, Li RW, Tan XX, Xu ZF, Liu FY, Duan
WY, Fang QG, Zhang X and Sun C: Yu's flap for lower lip and reverse
Yu's flap for upper lip reconstruction: 20 years experience. Br J
Oral Maxillofac Surg. 51:767–772. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
García de Marcos JA, Rincón I Heras,
González Córcoles C, Sebastián Alfaro M, Poblet Martínez E and
Arroyo Rodríguez S: Bilateral reverse Yu flap for upper lip
reconstruction after oncologic resection. Dermatol Surg.
40:193–196. 2014. View Article : Google Scholar : PubMed/NCBI
|