Introduction
Colorectal cancer (CRC) is one of the most commonly
diagnosed cancers worldwide, with an overall incidence rate of
188.4/100,000 and a mortality rate of 120.1/100,000 in Eastern Asia
(1). Approximately one-third of all
CRCs are localized in the rectum (2). In China, CRC was ranked the fourth most
common type of cancer, which may be attributed to the changes in
lifestyle (3).
Despite encouraging advances in chemoradiotherapy
and targeted therapy, surgery remains the cornerstone of treatment
for rectal cancer. The surgical treatment for rectal cancer is
usually a challenge for a number of surgeons, as they must consider
the balance between cancer curability and functional preservation.
In the past, abdominoperineal resection (APR) with the construction
of a permanent stoma was the standard treatment for rectal cancer.
However, this surgical technique inevitably has an adverse impact
on patients' quality of life (QoL) (4). Subsequently, with the rapid technical
improvements, a sphincter-preserving procedure, referred to as low
anterior resection (LAR), has become the preferred method for
patients with rectal cancer and previous studies have suggested
that LAR has a similar oncological efficacy but a more favorable
outcome in terms of the patients' postoperative QoL compared with
APR (5–8). Laparoscopic surgery as an emerging
technique, has been recently proven to be a feasible alternative to
open surgery for rectal cancer in terms of oncological outcome.
However, it is suggested to be superior to traditional open
surgery, largely due to its favorable clinical outcomes, including
reduced blood loss, less pain and shorter recovery time (9). Therefore, laparoscopic LAR (LLAR) is
widely used for patients with rectal cancer. Numerous studies have
attempted to investigate its effect on patient outcome, in terms of
oncological results or QoL evaluation, but without taking the
significance of anorectal functional outcome into consideration
(10,11).
Anorectal function is a key factor affecting the
patients' QoL and current studies evaluating this function are
mostly based on subjective questionnaires, which may be easily
influenced by various social/individual factors, and may not
accurately reflect the postoperative anorectal function (12–14).
Therefore, a novel objective method combined with the traditional
questionnaire survey should be optimally applied to evaluate the
change in anorectal function following rectal surgery. Anorectal
manometry has been found to be an effective and objective test for
evaluating postoperative anorectal function (15,16). We
previously performed anorectal manometry combined with a
questionnaire survey to evaluate the anorectal function of Chinese
patients following partial intersphincteric resection and transanal
endoscopic microsurgery (17–19).
However, to the best of our knowledge, only a limited number of
studies employing manometry to evaluate anorectal function
following LLAR are available, and the number is even lower for
Chinese patients. Therefore, the aim of the present study was to
evaluate the anorectal function of Chinese patients following LLAR
using anorectal manometry and the Wexner questionnaire survey. To
further validate the postoperative effect of LLAR on patient QoL,
the acknowledged QLQ-C30 and QLQ-CR38 questionnaires were also
employed.
Materials and methods
Patient data
Between January, 2013 and December, 2014, a cohort
of 51 patients (22 men and 29 women), with a median age of 52 years
(range, 36–75 years) who had undergone LLAR at the Department of
General Surgery, Sixth People's Hospital Affiliated to Shanghai
Jiao Tong University (Shanghai, China) were included in our study.
All the patients were clinicopathologically diagnosed with
adenocarcinoma of the rectum (within 5–12 cm above the anal verge).
Tumor location was assessed by preoperative examination, including
digital rectal examination and colonoscopy. In addition, the
postoperative anastomotic height (measured from the anastomosis to
the anal verge) was assessed by colonoscopy and the patients were
allocated into two groups: The high anastomosis (HA) group,
comprising those with anastomosis >5 cm above the anal verge,
and the low anastomosis (LA) group, comprising those who had an
anastomosis within 5 cm of the anal verge. There was no significant
difference in general information between the two groups (all
P>0.05) as shown in Table I. The
exclusion criteria were applied as follows: i) Abdominal computed
tomography and magnetic resonance imaging showing distal metastases
or local invasion into the levator ani muscle and the external
sphincter; ii) laparoscopic surgery converted to open surgery due
to unexpected factors; and iii) patients with accompanying symptoms
including intestinal obstruction or perforation, dementia and
cognitive dysfunction. Furthermore, another cohort of 50 patients
(32 men and 18 women), with a median age of 51 years (range, 32–72
years) who had undergone other abdominal surgeries (not involving
the pelvis) were also included in our study as the control group to
test the consistency of the baseline and reduce bias. All the
patients received the same chemotherapy (12 courses of FOLFOX4)
postoperatively and patients who received radiotherapy were not
included in our study. The study was approved by the Ethics
Committee of The Sixth People's Hospital Affiliated to Shanghai
Jiao Tong University. Written informed consent was obtained from
all the patients prior to enrolment.
 | Table I.Clinicopathological characteristics of
rectal cancer patients following laparoscopic low anterior
resection. |
Table I.
Clinicopathological characteristics of
rectal cancer patients following laparoscopic low anterior
resection.
| Characteristics | HA group | LA group |
|---|
| Number of
patients | 31 | 19 |
| Age, years (mean ±
SD) | 50.6±13.3 | 52.7±14.7 |
| Gender
(male/female) | 14/17 | 7/12 |
| Body mass index,
kg/m2 (mean ± SD) | 21.8±4.4 | 21.6±4.9 |
| Number of patients
with comorbidities | 0 | 0 |
| Marital status
(married/divorced/widowed/single) | 29/2/0/0 | 18/1/0/0 |
| Education level
(underprimary/primary/secondary/tertiary or higher) | 8/7/10/6 | 5/4/7/3 |
| Number of patients
with complications | 0 | 0 |
| Reoperation | 0 | 0 |
| Tumor stage
(II/III) | 5/26 | 3/16 |
Surgical technique
All the surgical procedures were conducted by the
same group of surgeons experienced in laparoscopic colorectal
surgery; bowel preparation and perioperative intravenous antibiotic
prophylaxis were routinely performed. The laparoscopic process for
tumor resection has been previously described (20). In brief, after establishing a
pneumoperitoneum, a sharp dissection involving a total mesorectal
excision was performed by an ultrasound knife. The inferior
mesenteric vessels were ligated following identification of the
left ureter, and the distal rectum was successively separated. The
proximal end of the bowel was delivered through a small incision.
Finally, the bowel was resected with a distal mesorectal margin of
≥2 cm whenever possible, and the double-stapling technique was used
to perform an anastomosis as described by Kosmidis et al
(21). The presence of an
anastomotic leak was checked by transrectal insufflation of air.
For postoperative oncological follow-up, the patients underwent
laboratory tests every 3 months and radiological examination every
6 months to monitor local recurrence and/or distal metastasis.
Endoanal ultrasonography was also employed to investigate the
integrity and thickness of the internal anal sphincter (IAS) and
the external anal sphincter (EAS) postoperatively.
Functional assessment
For anorectal manometry, an 8-channel water-perfused
catheter with an external diameter of 5.5 mm and a computer system
(all from Medical Measurement Systems Corporation, Enschede,
Netherlands) were employed. Each patient assumed the left lateral
decubitus position and the stationary technique was used for
catheter insertion. Anorectal manometric parameters, including
mean/maximal anal resting pressure (mean/max ARP), maximal squeeze
pressure (MSP), initial/strong sensory volume (ISV/SSV), maximal
tolerable volume (MTV) and rectoanal inhibitory reflexes (RAIR)
were recorded. In a proportion of patients with low anastomosis,
postoperative manometry performed within a short period may result
in rupture of the anastomosis. Therefore, manometry was performed
at 1 week preoperatively and at 3, 6 and 9 months
postoperatively.
Questionnaire assessment for anal
function and life quality
The anal function was assessed based on Wexner
incontinence grading scale, while QoL was assessed by the QLQ-C30
and QLQ-CR38 questionnaires developed by the European Organisation
for Research and Treatment of Cancer. The Chinese versions of
QLQ-C30 and QLQ-CR38, the clinical validity and reliability of
which have been previously confirmed, were used for the
questionnaire survey (10,22). For this questionnaire survey, all the
patients were interviewed with standardized questionnaires at 1
week preoperatively and at 3, 6 and 9 months postoperatively.
Statistical analysis
The results are presented as mean ± standard
deviation (SD). The Student's t-test and the χ2 test
were performed by SPSS 17.0 statistical software (SPSS, Inc,
Chicago, IL, USA). A P-value of <0.05 was considered to indicate
statistically significant differences.
Results
General description
All the patients were followed up for at least 1
year and there were no reported deaths. Furthermore, radiological
examination indicated no local recurrence or distant metastasis.
Four patients had anastomotic leakage and received conservative
treatment. One patient of the LA group complained of severe stool
incontinence and his manometric parameters were by 50% lower
compared with those of other patients at 9 months postoperatively.
Therefore, this case was not included in our data.
Changes in anorectal pressure and
volume
First, as shown in Table
II, there was no significant difference in the preoperative
pressure between the control and trial groups (all P>0.05).
Generally, all the manometric parameters changed to different
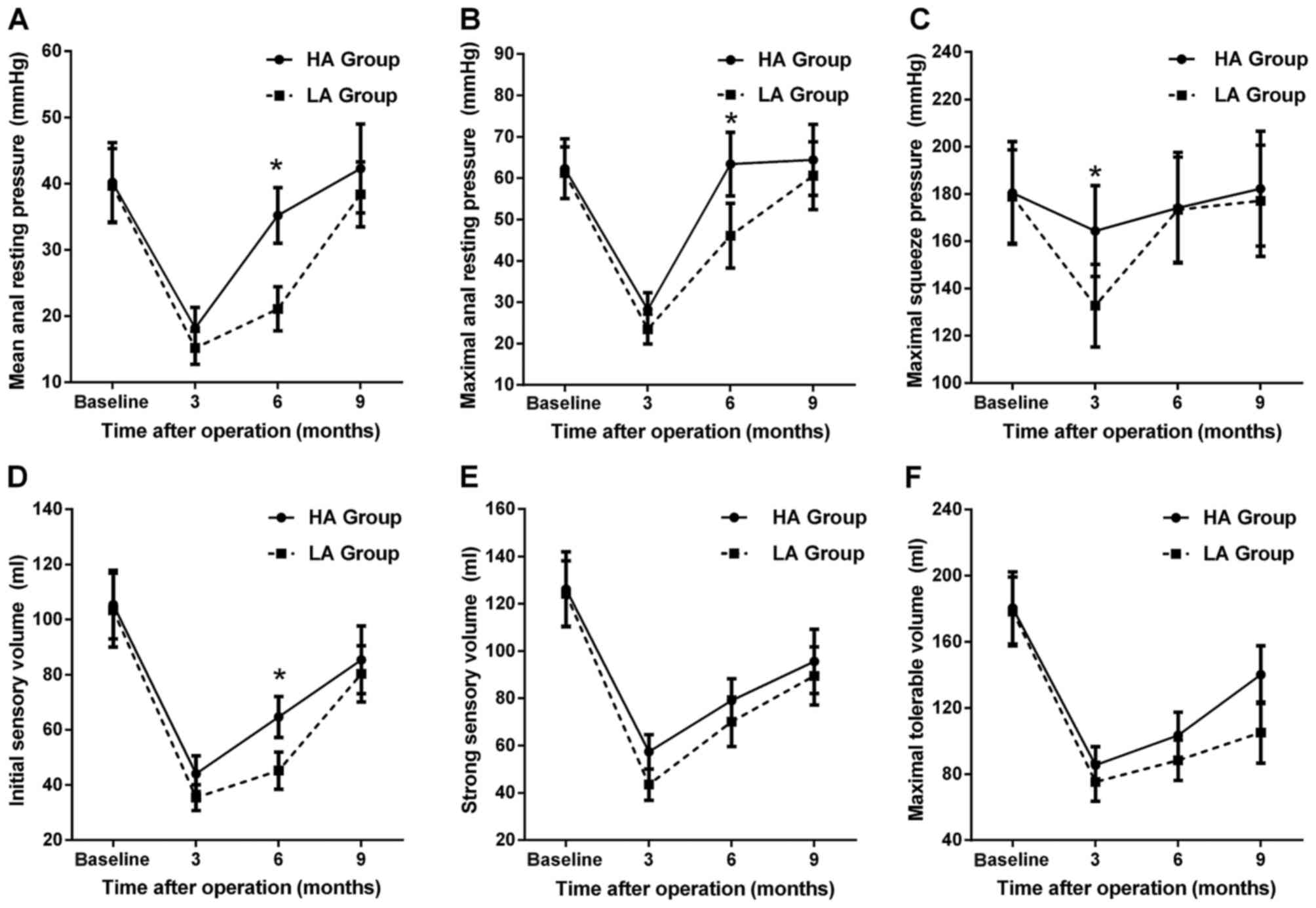
degrees following LLAR (Fig. 1A-C).
For the HA group, the mean/maximum ARP and MSP were decreased at 3
months postoperatively (all P<0.05), but had returned to
preoperative levels at 6 months postoperatively (all P>0.05).
For the LA group, the observations were similar at 3 months
postoperatively (all P<0.05), but the parameters gradually
returned to normal at 9 months postoperatively (P>0.05), except
for MSP, which had returned to normal level at 6 months
(P>0.05). With regard to intergroup comparison, the MSP of the
HA group was significantly higher compared with that of the LA
group at 3 months (P<0.05). In addition, the mean/maximal ARP of
the HA group was significantly higher compared with that of the LA
group at 6 months postoperatively (all P<0.05).
 | Table II.Baseline of functional parameters at
1 week preoperatively. |
Table II.
Baseline of functional parameters at
1 week preoperatively.
| Preoperative | Control group | Trial group |
|---|
| Mean ARP, mmHg | 45.1±5.4 | 40.2±6 |
| Maximum ARP,
mmHg | 67±7.5 | 62.3±7.2 |
| MSP, mmHg | 184.1±20.6 | 180.4±21.7 |
| ISV, ml | 110.3±12.1 | 105.4±12.4 |
| SSV, ml | 130.2±14.3 | 126.2±15.7 |
| MTV, ml | 190.7±20.1 | 180.4±21.8 |
| RAIR present | 50 (100.0) | 48 (96.0) |
| IAS, mm | 2.1±0.4 | 1.9±0.5 |
| EAS, mm | 4±0.5 | 3.8±0.7 |
| Gas/liquid/solid
incontinence | 0/0/0 | 0/0/0 |
| Wexner scores | 0 | 0 |
The ISV, SSV and MTV were significantly decreased in
both groups at 3 months postoperatively (all P<0.05), but had
gradually improved at 6 and 9 months postoperatively (Fig. 1D-F). However, all the volume
parameters remained significantly lower compared with the
preoperative level at 9 months postoperatively (all P<0.05).
Furthermore, the MTV of the HA group was significantly higher
compared with that of the LA group at 9 months postoperatively
(P<0.05).
Changes in rectoanal inhibitory reflex
(RAIR) and anal sphincter
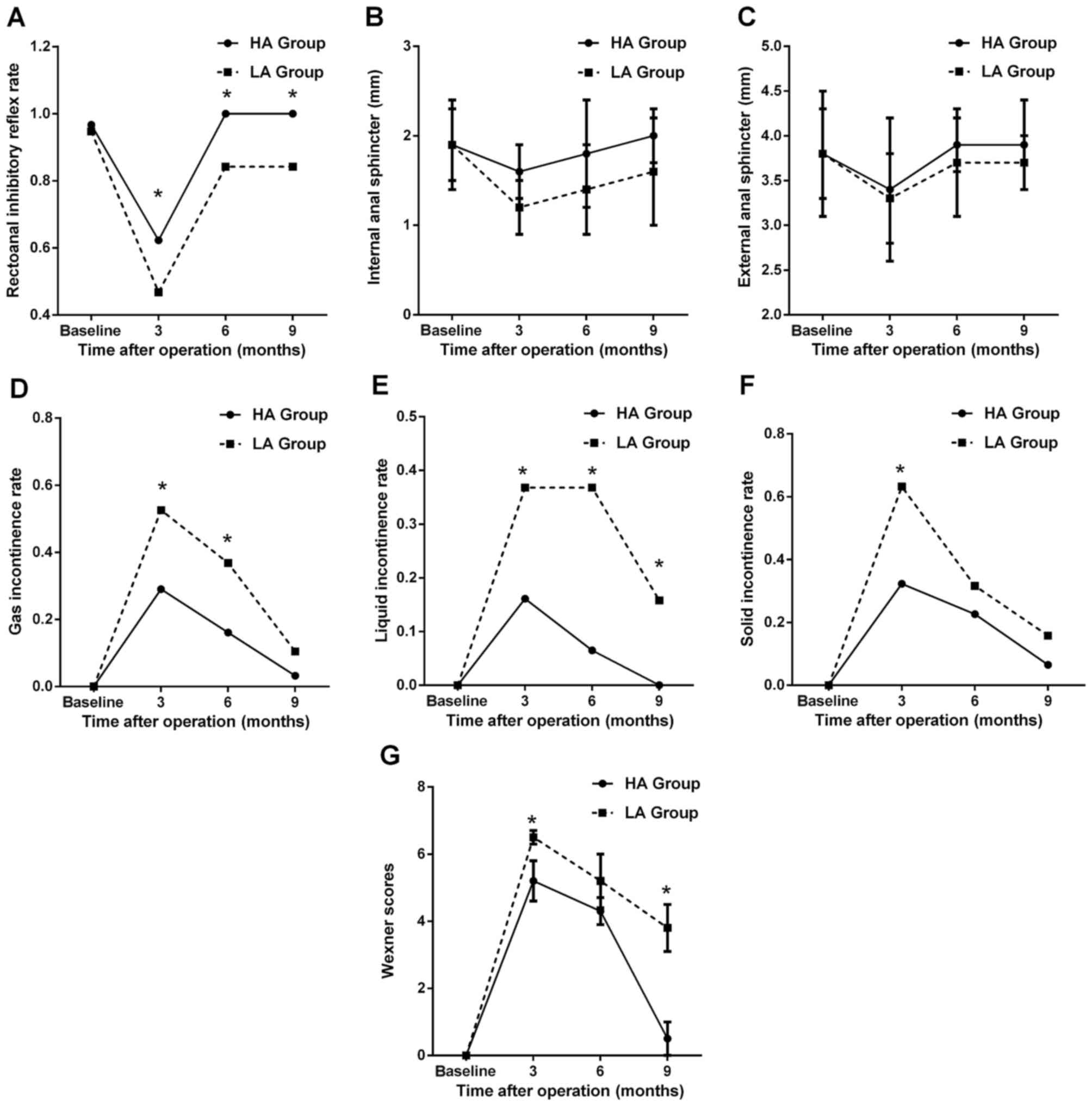
The changes in RAIR following LLAR are shown in
Fig. 2A. Generally, RAIR was absent
in 2 of the 50 patients at 1 week preoperatively. For the HA group,
RAIR was absent in 12 of the 31 patients at 3 months
postoperatively, whereas RAIR was absent in none of the patients at
6 and 9 months postoperatively. For the LA group, RAIR was absent
in 10 of the 19 patients at 3 months postoperatively. However, RAIR
was induced in 16 of the 19 patients at 6 and 9 months
postoperatively, with 3 patients remaining negative for RAIR
(including 2 patients who were negative for RAIR preoperatively).
In addition, the RAIR rate of the HA group was significantly higher
compared with that of the LA group between 3 and 9 months
postoperatively (all P<0.05). Endoanal ultrasonography
demonstrated rupture of IAS in 6 patients (1 in the HA and 5 in the
LA group), with full integrity of EAS in all the patients. The
changes in the thickness of IAS and EAS are shown in Fig. 2B and C. There was no difference in
the thickness of IAS/EAS between the control and trial groups (all
P>0.05, Table II). For the HA
group, although a decreasing tendency was observed in the thickness
of IAS and EAS at 3 months, the results were not statistically
significant (all P>0.05). Furthermore, at 6 and 9 months, the
thickness of both the IAS and EAS had increased to the preoperative
level (all P>0.05). For the LA group, similar changes were only
found in EAS; the thickness of the IAS was significantly reduced at
3 months postoperatively (P<0.05), with an insignificant
increase at 6 and 9 months postoperatively (all P>0.05).
Changes in incontinence and Wexner
score
The changes in gas, liquid and solid incontinence
rates are presented in Fig. 2D-F.
The patients of the control and trial groups reported no
incontinence prior to surgery. However, both subgroups experienced
incontinence to different degrees at 3 months after LLAR (as shown
in Fig. 2D-F). The liquid
incontinence rate of the LA group was significantly higher compared
with that of the HA group postoperatively (all P<0.05), but
gradually improved. The changes of the Wexner scores are
demonstrated in Fig. 2G. For the HA
group, the Wexner score exhibited an increase at 3 months
postoperatively, but was decreased at 6 and 9 months, which was
also observed in the LA group. Moreover, the Wexner scores of the
HA group were significantly lower compared with those of the LA
group at 3 and 9 months postoperatively (all P<0.05).
Changes in QoL
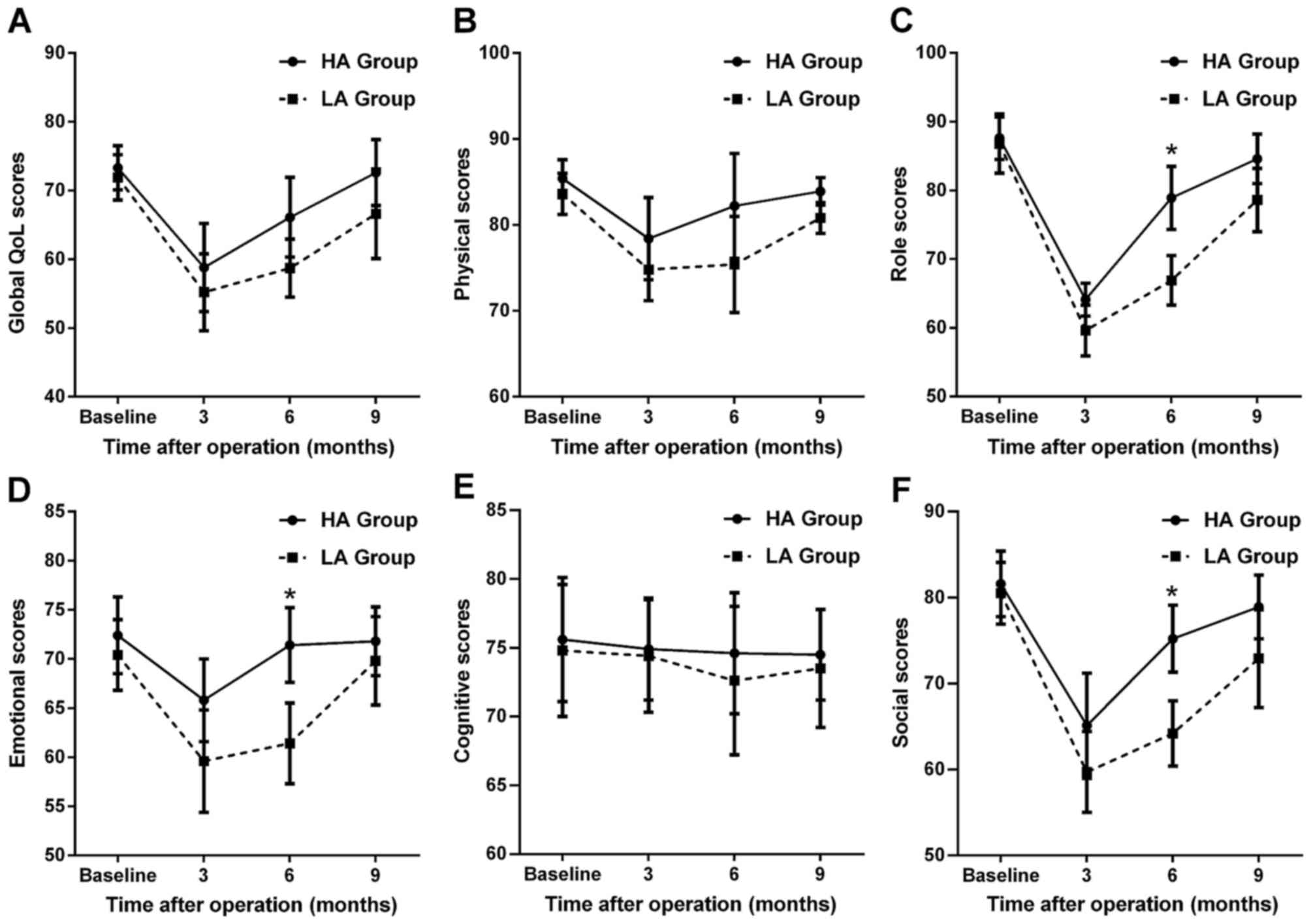
The functioning scales of QoL-C30 are shown in
Fig. 3. Compared with the
preoperative level, the function scores of both groups decreased at
3 months postoperatively, but gradually increased by 9 months
postoperatively, except for cognitive function (Fig. 3E). For the symptom scales of QoL-C30,
as shown in Fig. 4, the reverse
trends were observed, except for dyspnea (Fig. 4D) and constipation (Fig. 4G). On intergroup comparison, the HA
group had a significantly better role, emotional and social
function (Fig. 3C, D and F), and
fewer diarrhea problems (Fig. 4H) at
6 months postoperatively compared with the LA group (all
P<0.05).
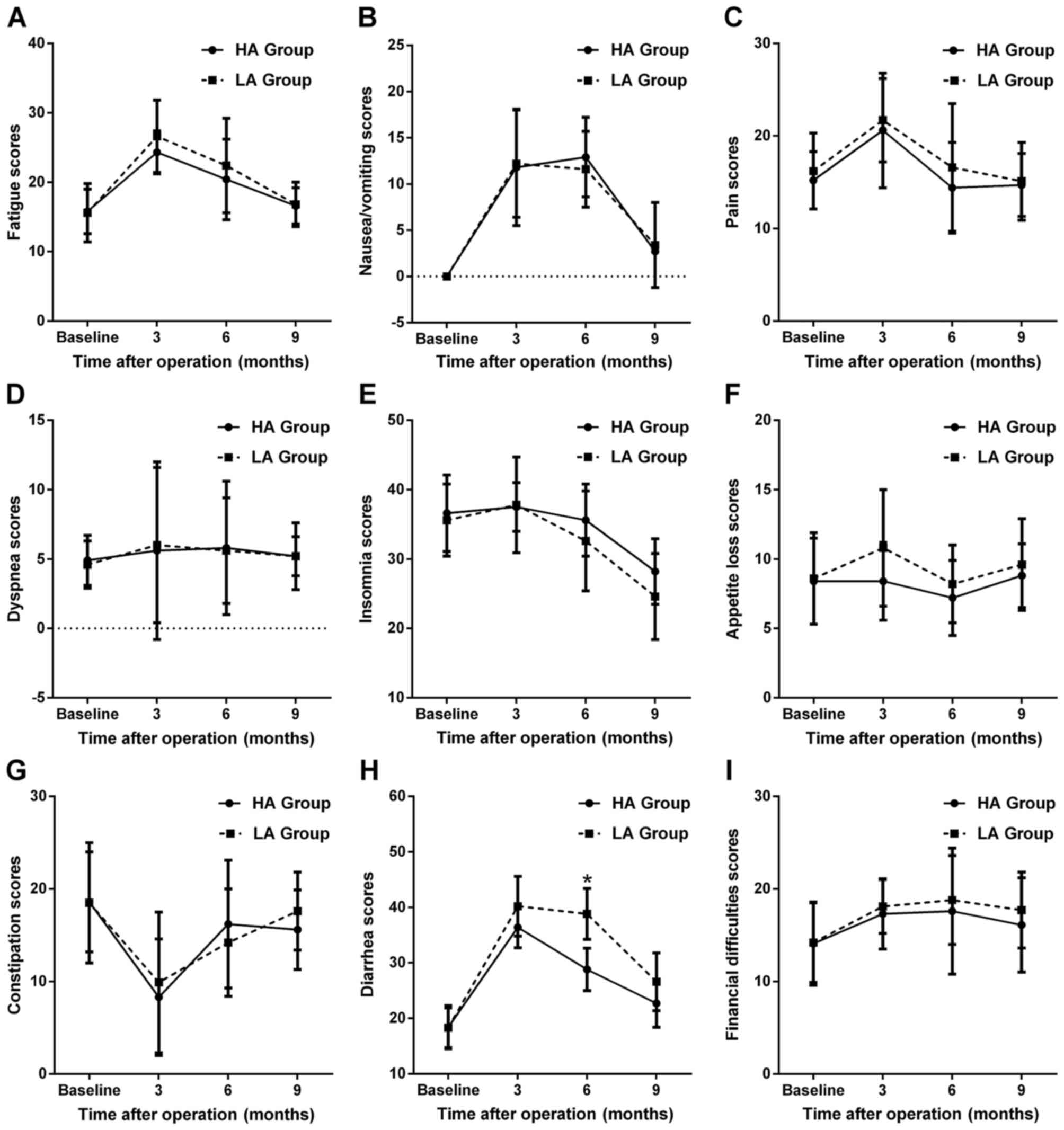
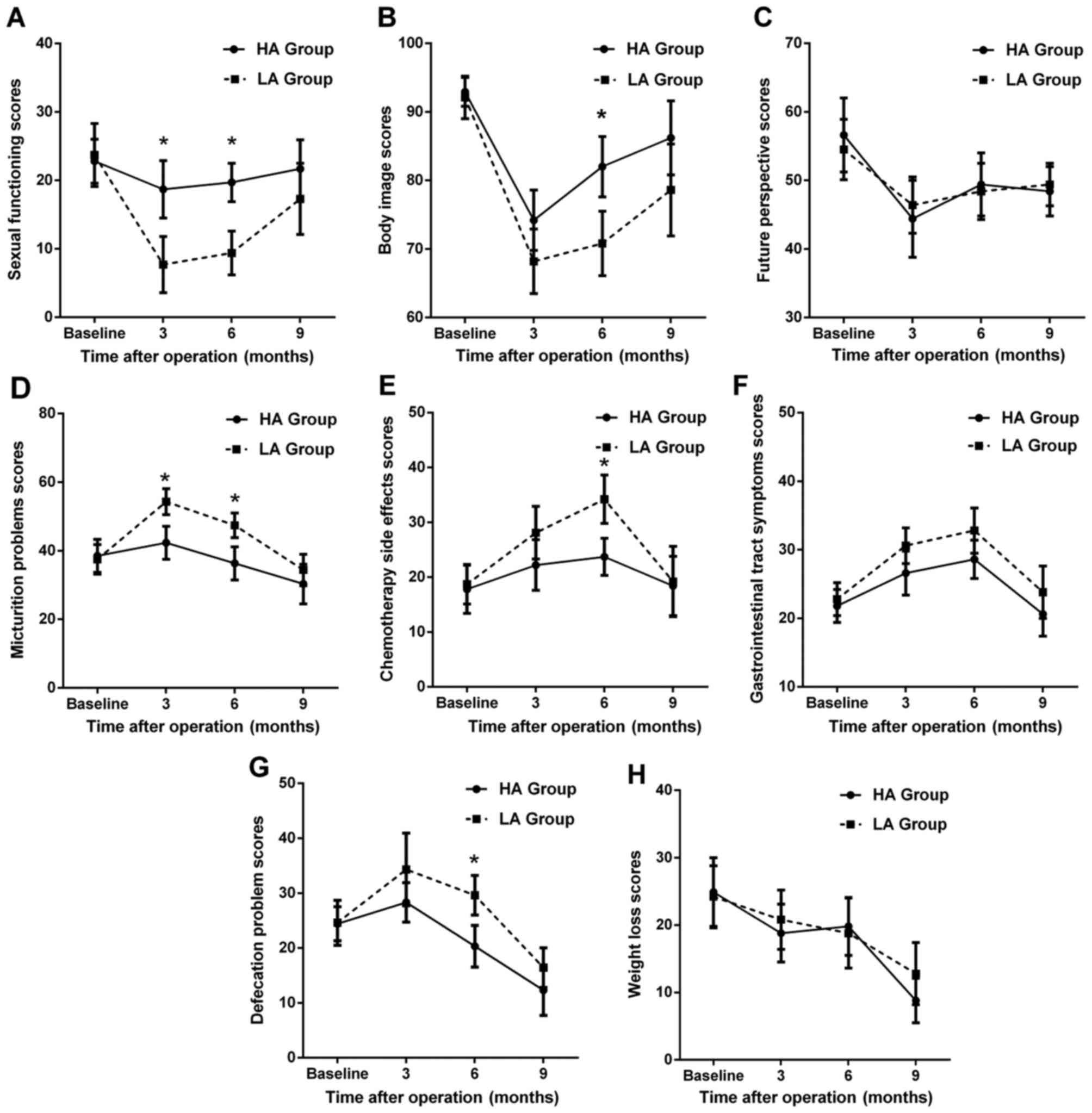
With regard to QoL-CR38 scores, as shown in Fig. 5, certain scales had changes similar
to QoL-C30, such as sexual function (Fig. 5A), body image (Fig. 5B) and micturition problems (Fig. 5D). Chemotherapy-related side effects
and gastrointestinal tract symptoms, which were increased prior to
6 months, had decreased to preoperative levels at 9 months
postoperatively (Fig. 5E and F).
Although the future perspective score was decreased at 3 months and
slowly increased by 9 months postoperatively, it did not return to
the preoperative level (Fig. 5C,
P<0.05). Moreover, there was a decreasing trend in the scores of
defecation problems (Fig. 5G) and
weight loss (Fig. 5H)
postoperatively. On intergroup comparison, the HA group appeared to
have a better sexual function (Fig.
5A) and fewer micturition problems (Fig. 5D) at 3 and 6 months postoperatively,
with a better body image (Fig. 5B)
and fewer chemotherapy-related side effects (Fig. 5E) and defecation problems (Fig. 5G) at 6 months compared with the LA
group (all P<0.05).
Discussion
In this study, routine anorectal manometry,
questionnaire survey and radiological examination were first
performed to investigate the functional outcome of patients
following LLAR. In anorectal manometry, ARP is mainly dependent on
IAS and associated with maintaining continence, while MSP is mainly
dependent on EAS and associated with maintaining continence under
stress. To the best of our knowledge, although no studies have
investigated the manometric parameters following LLAR, several
studies have demonstrated different manometric results after open
LAR. Bittorf et al found the mean/maximum ARP to be
significantly decreased, with unchanged MSP (23). Rasmussen et al reported that
anal manometry was normal postoperatively, although the majority of
the patients complained of poor functional results (24). A recent study indicated that both the
ARP and MSP were significantly decreased at 6 and 12 months
postoperatively (25). In our study,
both the ARP and MSP were significantly reduced at 3 months
postoperatively, but gradually improved to preoperative levels at 6
and 9 months. Since the anal sphincter plays a key role in
maintaining pressure, endoanal ultrasonography examination was
performed and the results displayed a similar trend in the
thickness of IAS and EAS. This temporary injury of the anal
sphincter was largely attributed to anal dilatation by stapling
instruments during surgery, which finally resulted in the decreased
pressures. With regard to the volume change of the rectum, a
similar trend was also observed. However, all the volume parameters
remained significantly lower compared with the preoperative values
at 9 months after LLAR, largely due to the reconstruction of the
rectum during the operation.
RAIR is defined as an important parasympathetic
reflex controlling anorectal continence and involving a number of
pelvic muscles, such as IAS and EAS. In this study, absent RAIR was
found in 22 of the 50 patients at 3 months after LLAR. However,
RAIR was gradually induced in the majority of the patients at 6
(47/50) and 9 (47/50) months after LLAR. This result was superior
to the one demonstrated by Kakodkar et al, who found RAIR to
be absent in all the patients at 6 months postoperatively, but
present in half of the patients at 12 months after LAR (25). With regard to postoperative
continence, a number of patients complained of gas, liquid or solid
incontinence at 3 months postoperatively, which had improved in the
majority of the patients at 6 and 9 months postoperatively. This
process was then confirmed by employing the Wexner score system,
suggesting that LLAR may have a favorable outcome in terms of
postoperative continence.
The patients were divided into subgroups to further
investigate the effect of anastomotic height on anorectal function
and QoL after LLAR. As a result, we found that the HA group had a
higher ARP and MSP compared with the LA group at 3 and 6 months
postoperatively. This difference was also observed in MTV, RAIR and
IAS thickness postoperatively. Moreover, the LA group appeared to
have a higher frequency of incontinence and higher Wexner scores
compared with the HA group. Therefore, we hypothesized that
anastomotic height may be a potential factor affecting functional
outcome and QoL in Chinese patients following LLAR. This hypothesis
has been indirectly supported by several studies regarding LAR.
Montesani et al found that patients with anastomosis at 4–6
cm from the anal verge had poorer functional results compared with
those with anastomosis at 6–8 cm, despite the fact that the
differences in most manometry parameters were statistically
insignificant (26). In a recent
questionnaire survey based on 381 cases, patients with low
anastomosis were more likely to present with an increased frequency
of defecation problems compared with those with a higher
anastomosis (27). Additionally, a
long-term study indicated that patients with anastomoses at <4
cm from the anal verge had poorer functional results compared with
those with anastomoses at 5–8 cm above the anal verge during the
first 5 postoperative years (28).
According to our surgical experience, this anastomotic effect on
anorectal function may be explained as follows: i) Patients with
low anastomosis usually undergo more extensive pelvic dissection
during surgery compared with those with higher anastomosis, which
may cause more injury to the IAS, finally resulting in poorer
anorectal function; and ii) the nerve plexuses in the rectal wall
play an important role in controlling the reflex of defecation and
may be more easily injured in patients with low anastomosis.
QoL questionnaires are a subjective method for
evaluating the impact of a disease and its related treatments on
the patient's physical, psychological and social functioning
(29). Previous studies have
indicated that patients appeared to have better health-related QoL
scores after LAR, when compared with those who underwent high
anterior resection (30,31). In the present study, we also observed
that the HA group had more favorable QoL scores compared with the
LA group postoperatively. These differences may be largely
explained by the hypothesis that the LA group patients are more
prone to develop what is referred to as the ‘LAR syndrome’, which
comprises frequent defecation, fecal urgency and stool
incontinence. Several previous studies have closely associated poor
QoL with low anastomosis (32,33).
However, no significant differences were observed in most
functional or symptom scales between the two groups at 9 months
postoperatively. This observation probably reflects the fact that
LLAR may allow better preservation of the pelvic autonomic nerves
through the magnified vision and less traumatic surgery (34). Although the future perspective score
increased slowly from 3 to 9 months postoperatively, it did not
return to the preoperative level, suggesting that high preoperative
expectations for LLAR do not appear to translate into patient
satisfaction within 9 months postoperatively, and long-term
follow-up is required for further evaluation.
In summary, anorectal function following LLAR was
found to be impaired, but improved over time. Patients with low
anastomosis have poorer functional results and QoL compared with
those with high anastomosis. Therefore, in terms of functional
preservation, LLAR is generally acceptable for Chinese patients
with rectal cancer, particularly for those with middle or high
rectal cancer. A multicentric, long-term follow-up is required to
further investigate the functional effect of LLAR in Chinese
patients with rectal cancer.
Acknowledgements
The present study was supported by a grant from the
Science and Technology Commission of Shanghai Municipality (no.
124119a720). The authors would like to thank Drs Jia-Yuan Peng and
Qing-Chao Zhu (Department of Surgery, Sixth People's Hospital
Affiliated to Shanghai Jiao Tong University) for their crucial
assistance with our follow-up study.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bonjer HJ, Deijen CL, Abis GA, Cuesta MA,
van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA,
Andersson J, Angenete E, et al: A randomized trial of laparoscopic
versus open surgery for rectal cancer. N Engl J Med. 372:1324–1332.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gu J and Chen N: Current status of rectal
cancer treatment in China. Colorectal Dis. 15:1345–1350. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoerske C, Weber K, Goehl J, Hohenberger W
and Merkel S: Long-term outcomes and quality of life after rectal
carcinoma surgery. Br J Surg. 97:1295–1303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pachler J and Wille-Jørgensen P: Quality
of life after rectal resection for cancer, with or without
permanent colostomy. Cochrane Database Syst Rev.
12:CD0043232012.PubMed/NCBI
|
|
6
|
How P, Stelzner S, Branagan G, Bundy K,
Chandrakumaran K, Heald RJ and Moran B: Comparative quality of life
in patients following abdominoperineal excision and low anterior
resection for low rectal cancer. Dis Colon Rectum. 55:400–406.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kasparek MS, Hassan I, Cima RR, Larson DR,
Gullerud RE and Wolff BG: Quality of life after coloanal
anastomosis and abdominoperineal resection for distal rectal
cancers: Sphincter preservation vs quality of life. Colorectal Dis.
13:872–877. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Leersum N, Martijnse I, den Dulk M,
Kolfschoten N, Le Cessie S, van de Velde C, Tollenaar R, Wouters M
and Rutten HJ: Differences in circumferential resection margin
involvement after abdominoperineal excision and low anterior
resection no longer significant. Ann Surg. 259:1150–1155. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Veldkamp R, Kuhry E, Hop WC, Jeekel J,
Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S, et
al: Laparoscopic surgery versus open surgery for colon cancer:
Short-term outcomes of a randomised trial. Lancet Oncol. 6:477–484.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ng SS, Leung WW, Wong CY, Hon SS, Mak TW,
Ngo DK and Lee JF: Quality of life after laparoscopic vs open
sphincter-preserving resection for rectal cancer. World J
Gastroenterol. 19:4764–4773. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chi P, Huang SH, Lin HM, Lu XR, Huang Y,
Jiang WZ, Xu ZB, Chen ZF, Sun YW and Ye DX: Laparoscopic
transabdominal approach partial intersphincteric resection for low
rectal cancer: Surgical feasibility and intermediate-term outcome.
Ann Surg Oncol. 22:944–951. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kneist W, Kauff DW, Juhre V, Hoffmann KP
and Lang H: Is intraoperative neuromonitoring associated with
better functional outcome in patients undergoing open TME? results
of a case-control study. Eur J Surg Oncol. 39:994–999. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kauff DW, Koch KP, Somerlik KH, Hoffmann
KP, Lang H and Kneist W: Evaluation of two-dimensional
intraoperative neuromonitoring for predicting urinary and anorectal
function after rectal cancer surgery. Int J Colorectal Dis.
28:659–664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maris A, Penninckx F, Devreese AM, Staes
F, Moons P, Van Cutsem E, Haustermans K and D'Hoore A: Persisting
anorectal dysfunction after rectal cancer surgery. Colorectal Dis.
15:e672–e679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kye BH, Kim HJ, Kim JG, Kim SH, Shim BY,
Lee NS and Cho HM: Short-term effects of neoadjuvant chemoradiation
therapy on anorectal function in rectal cancer patients: A pilot
study. Radiat Oncol. 8:2032013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pietsch AP, Fietkau R, Klautke G, Foitzik
T and Klar E: Effect of neoadjuvant chemoradiation on postoperative
fecal continence and anal sphincter function in rectal cancer
patients. Int J Colorectal Dis. 22:1311–1317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gong X, Jin Z and Zheng Q: Anorectal
function after partial intersphincteric resection in ultra-low
rectal cancer. Colorectal Dis. 14:e802–e806. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin Z, Yin L, Xue L, Lin M and Zheng Q:
Anorectal functional results after transanal endoscopic
microsurgery in benign and early malignant tumors. World J Surg.
34:1128–1132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang HW, Han XD, Wang Y, Zhang P and Jin
ZM: Anorectal functional outcome after repeated transanal
endoscopic microsurgery. World J Gastroenterol. 18:5807–5811. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu QL, Feng B, Lu AG, Wang ML, Hu WG, Li
JW, Mao ZH and Zheng MH: Laparoscopic low anterior resection for
rectal carcinoma: Complications and management in 132 consecutive
patients. World J Gastroenterol. 16:4605–4610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kosmidis C, Efthimiadis C, Anthimidis G,
Grigoriou M, Fotiadis P, Vasiliadou K, Mekras D, Ioannidou G, Baka
S and Basdanis G: Laparoscopic low anterior resection for early
rectal cancer. Tech Coloproctol. 15 Suppl 1:S75–S77. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Konanz J, Herrle F, Weiss C, Post S and
Kienle P: Quality of life of patients after low anterior,
intersphincteric and abdominoperineal resection for rectal cancer-a
matched-pair analysis. Int J Colorectal Dis. 28:679–688. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bittorf B, Stadelmaier U, Göhl J,
Hohenberger W and Matzel KE: Functional outcome after
intersphincteric resection of the rectum with coloanal anastomosis
in low rectal cancer. Eur J Surg Oncol. 30:260–265. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rasmussen OO, Petersen IK and Christiansen
J: Anorectal function following low anterior resection. Colorectal
Dis. 5:258–261. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kakodkar R, Gupta S and Nundy S: Low
anterior resection with total mesorectal excision for rectal
cancer: Functional assessment and factors affecting outcome.
Colorectal Dis. 8:650–656. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Montesani C, Pronio A, Santella S,
Boschetto A, Aguzzi D, Pirozzi R, D'Amato A and Vestri A: Rectal
cancer surgery with sphincter preservation: Functional results
related to the level of anastomosis. clinical and instrumental
study. Hepatogastroenterology. 51:718–721. 2004.PubMed/NCBI
|
|
27
|
Knowles G, Haigh R, McLean C, Phillips HA,
Dunlop MG and Din FV: Long term effect of surgery and radiotherapy
for colorectal cancer on defecatory function and quality of life.
Eur J Oncol Nurs. 17:570–577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hida J, Yoshifuji T, Matsuzaki T, Hattori
T, Ueda K, Ishimaru E, Tokoro T, Yasutomi M, Shiozaki H and Okuno
K: Long-term functional changes after low anterior resection for
rectal cancer compared between a colonic J-pouch and a straight
anastomosis. Hepatogastroenterology. 54:407–413. 2007.PubMed/NCBI
|
|
29
|
Outcomes of cancer treatment for
technology assessment and cancer treatment guidelines. American
society of clinical oncology. J Clin Oncol. 14:671–679. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grumann MM, Noack EM, Hoffmann IA and
Schlag PM: Comparison of quality of life in patients undergoing
abdominoperineal extirpation or anterior resection for rectal
cancer. Ann Surg. 233:149–156. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Engel J, Kerr J, Schlesinger-Raab A, Eckel
R, Sauer H and Hölzel D: Quality of life in rectal cancer patients:
A four-year prospective study. Ann Surg. 238:203–213. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hassan I, Larson DW, Cima RR, Gaw JU, Chua
HK, Hahnloser D, Stulak JM, O'Byrne MM, Larson DR, Wolff BG and
Pemberton JH: Long-term functional and quality of life outcomes
after coloanal anastomosis for distal rectal cancer. Dis Colon
Rectum. 49:1266–1274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ziv Y, Zbar A, Bar-Shavit Y and Igov I:
Low anterior resection syndrome (LARS): Cause and effect and
reconstructive considerations. Tech Coloproctol. 17:151–162. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Scheele J, Lemke J, Meier M, Sander S,
Henne-Bruns D and Kornmann M: Quality of life after
sphincter-preserving rectal cancer resection. Clin Colorectal
Cancer. 14:e33–e40. 2015. View Article : Google Scholar : PubMed/NCBI
|