Introduction
According to the World Health Organization (WHO),
intraductal tubulopapillary neoplasm (ITPN) is a type of
intraductal epithelial tumor of the pancreas (1,2). ITPNs
are located in pancreatic ducts and arise from the pancreatic
ductal epithelium. However, the lesions that originate in
pancreatic ducts may be quite complex. Based on the intraductal
papillary mucinous neoplasm (IPMN), the new term or classification
for this intraductal epithelial tumor of the pancreas was ITPN.
ITPNs displaying highly heteromorphic hyperplasia may be
distinguished from IPMNs by gross morphology, clinicopathological
characteristics and immunophenotype. Due to the rarity and lower
incidence of ITPNs, which only represent 0.4% of all pancreatic
tumors, the available data on the clinicopathological and molecular
characteristics of this type of tumor are limited (1). The aim of the present study was to
analyze the clinicopathological, immunophenotypic and molecular
genetic characteristics of ITPNs to gain a better insight into this
disease entity.
Case report
In October, 2015, a 38-year-old man presented to the
Zhongnan Hospital (Wuhan, China) without any prior medical history
of tiredness, anorexia, flatulence or abnormal findings on
urinalysis. The physical examination was unremarkable, with the
exception of mild jaundice of the sclerae. The results of the
laboratory analysis were as follows: Total bilirubin, 125.9 µmol/l
(normal range, 3.4–17.1 µmol/l); conjugated bilirubin, 77.8 µmol/l
(normal range, 0–6 µmol/l); unconjugated bilirubin (normal range,
1.7–10.2 µmol/l), 48.1 µmol/l; alanine aminotransferase, 261 U/l
(normal range, 0–40 U/l); aspartate aminotransferase, 93 U/l
(normal range, 0–45 U/l); γ-glutamyl transpetidase, 1,486 U/l
(normal range, 0–50 U/l); alkaline phosphatase, 180 U/l (normal
range, 40–160 U/l); and total bile acid, 299.6 µmol/l (normal
range, 0–10 µmol/l). These abnormal biochemical indicators
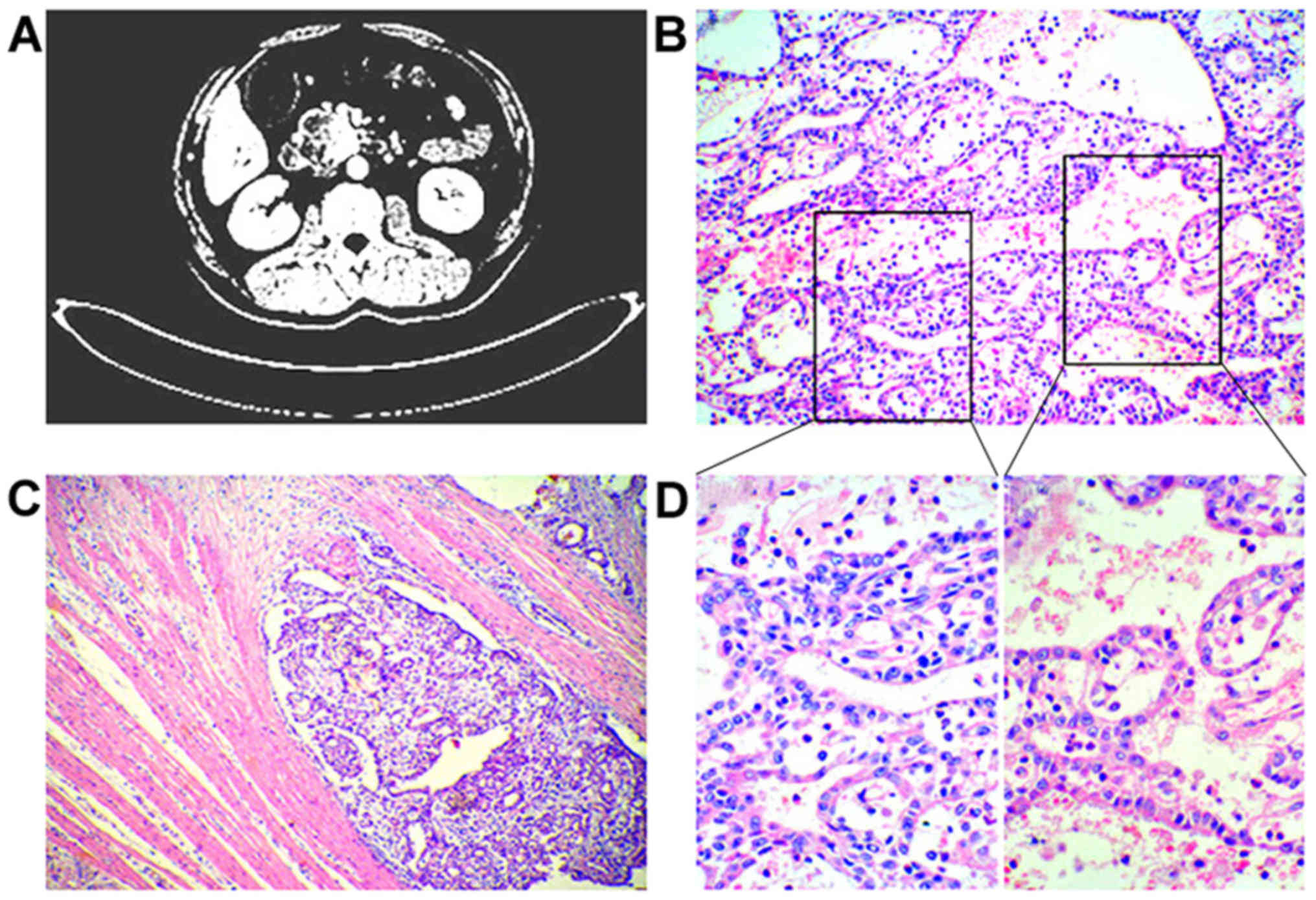
suggested obstructive jaundice. Computed tomography (CT) revealed a
low-attenuation mass, sized 42×40 mm, located in the head of the
pancreas. Patchy and heterogeneous shadowing of the portal artery
and vein was accompanied by partial capsule-like rim enhancement of
the surrounding tissue (Fig. 1A).
There was no evidence of lymphadenectasis or metastasis to other
organs, including the liver and lung. The intrahepatic and
extrahepatic bile ducts and the main pancreatic duct were clearly
dilated, with obvious atrophy of the pancreatic parenchyma
(Fig. 1A). Consequently, classical
pancreatoduodenectomy was performed, accompanied with
cholecystectomy. Macroscopically, a solid tumor sized 42×40×20 mm
was identified in the head of the pancreas, with unclear borders.
On cross-section the tumor was solid, gray and brown, and
accompanied by cavitation, hemorrhage and necrosis. Mucin
production was not observed in the tumor specimens.
The tumor specimens were fixed in a 4% solution of
neutral formaldehyde. Following dehydration, the fixed specimens
were embedded in paraffin and cut into 3–4-µm sections. The
technique of EnVision Immunity immunohistochemical staining
(ZSGB-BIO Co., Beijing, China) was applied with automated
instrumentation. The antibodies in the EnVision staining procedure
included antibodies targeted against cytokeratin (CK) (ZM-0069;
mouse; dilution, 1:100), mucin (MUC) 1 and MUC2 (ZM-0391 and
ZM-0392, respectively; mouse; dilution, 1:100), carcinoembryonic
antigen (ZM-0062; mouse; dilution, 1:100), CD56 (ZM-0057; mouse;
dilution, 1:100), chromogranin A (CgA) (ZM-0076; mouse; dilution,
1:100) and Ki-67 (ZM-0166; mouse; dilution, 1:100). All the
antibodies were purchased from ZSGB-BIO. Detailed operating
procedures were conducted according to the manufacturer's
instructions. The resultant black and brown granules in the cells
were considered as a positive reaction.
Microsatellite instability (MSI) analysis and
fluorogenic quantitative polymerase chain reaction methods were
applied to detect the expression of epidermal growth factor
receptor (EGFR), Kirsten rat sarcoma viral oncogene homolog (KRAS),
neuroblastoma RAS viral oncogene homolog (NRAS), B-Raf
proto-oncogene (BRAF) and P53 gene mutations. Detailed operative
procedures were conducted according to the manufacturer's
instructions and standard operating procedures.
Discussion
An electronic search was performed through PubMed
(National Library of Medicine, NIH, Bethesda, MD, USA) and the
Chinese Periodical Database (search date up to and including June
6, 2016) for ITPN cases. In total, 44 English and 3 Chinese
articles were identified using the key words ‘intraductal
tubulopapillary neoplasms’. After screening, which involved the
deletion of significantly irrelevant information, 36 ITPN cases and
50 random cases of IPMN were selected.
Microscopically, the morphological characteristics
of the tumor was basophilic cuboidal cells of uniform size in the
pancreatic ducts, mostly arranged in a tubular and papillary
pattern. The tumor cells were cuboidal, with intense nuclear
staining and obvious presence of a nucleolus, exhibiting moderate
dysplasia. The majority of the tumor cells were arranged in a
cribriform or back-to-back pattern; additionally, normal pancreatic
intraductal epithelium was clearly seen. The tubular glands were
also closely packed, without secretion of mucin (Fig. 1B and D). The neoplastic cells had
partially invaded the smooth muscle wall of the duodenum and were
found proliferating around the small blood vessels and extending
into the connective tissue (Fig.
1C).
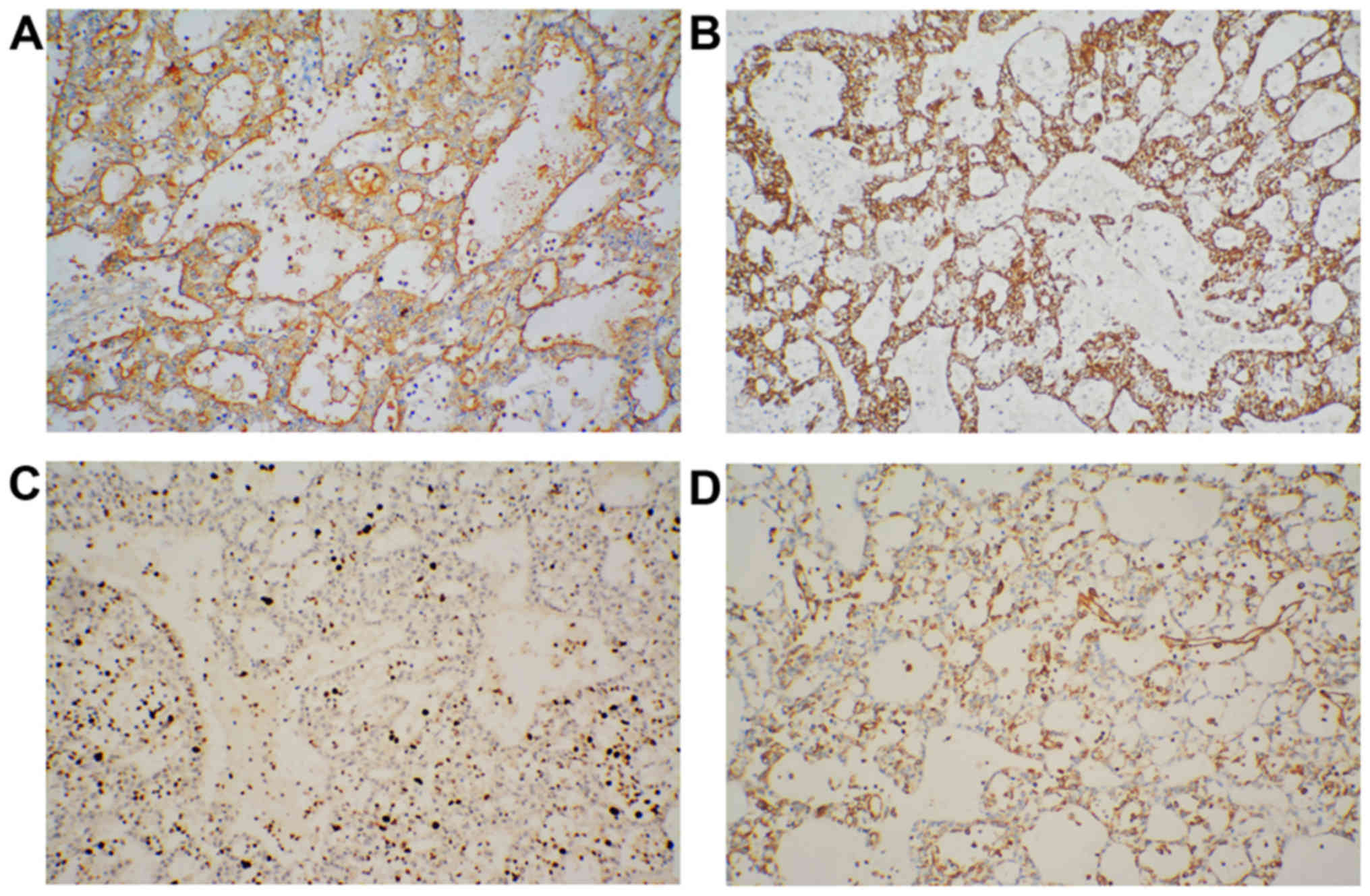
Furthermore, immunohistochemical staining of the
tumors showed positive reactions for CK and MUC1 and a partially
positive reaction for vimentin, but not MUC2. The Ki-67 index was
20%, indicating proliferative activity (Fig. 2). However, staining for CD56 and CgA
was negative in the tumor specimens (Fig. 3).
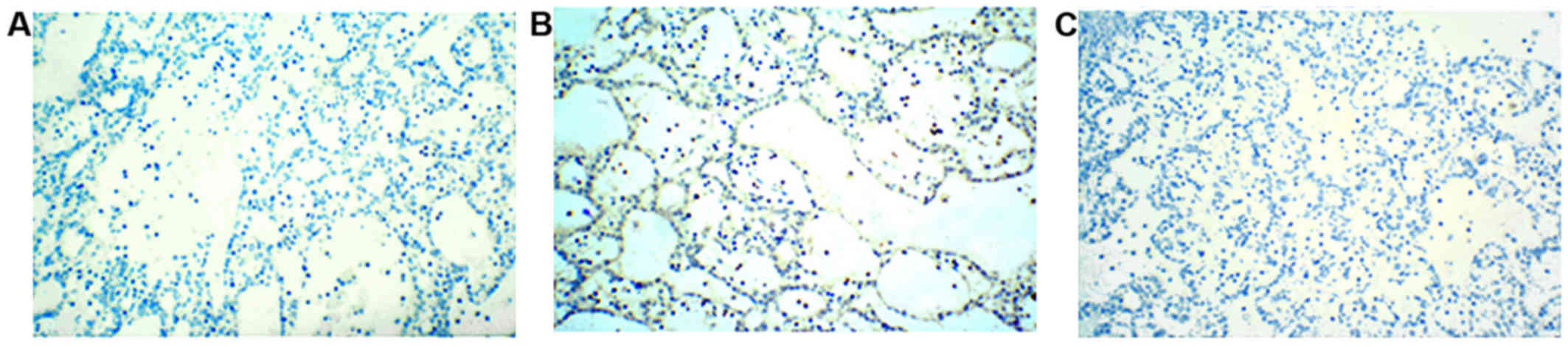
MSI analysis revealed microsatellite stability
(Fig. 4A); however, no gene
mutations were detected for EGFR, KRAS, NRAS, BRAF and
phosphoinositide-3-kinase, catalytic subunit α (Fig. 4B). In an alternate mechanistic
pathway of tumor generation and associated mechanisms,
immunohistochemical staining showed positive protein expression
staining patterns for P53 of ~50% (data not shown) and P53 gene
mutation analysis revealed a base mutation, namely a substitution
of A with T in the 2,030 position of the exon 9 DNA sequence of the
P53 gene in this tumor (Fig.
4C).
A comprehensive analysis that included the present
case and other ITPN cases that were previously reported in the
literature, suggested that there was immunoreactivity for CK and
MUC1 in tumor specimens. A number of cases clearly expressed P53;
by contrast, MUC2 and MUC5AC were not expressed (Table I). In addition, some cases exhibited
mutations in both the KRAS and P53 genes (Table I).
 | Table I.Immunohistochemical and mutation
analysis. |
Table I.
Immunohistochemical and mutation
analysis.
|
| Immunohistochemical
staining | Mutational
analysis |
|---|
|
|
|
|
|---|
| Case | MUC1 | MUC2 | MUC5AC | CK7 | CK19 | CDX2 | CgA | Ki-67(%) | P53 | TP53 | KRAS | NRAS | BRAF | PI3KCA |
|---|
| 1 | + | − | ND | − | ND | − | − | 20 | + | + | − | − | − | − |
| 2 | + | − | − | ND | − | ND | ND | 30 | + | ND | + | ND | ND | ND |
| 3 | + | − | − | ND | − | ND | ND | 35 | − | ND | + | ND | ND | ND |
| 4 | + | − | − | ND | − | ND | ND | 40 | + | ND | − | ND | ND | ND |
| 5 | + | − | − | ND | ND | ND | ND | 30.5 | − | ND | − | ND | ND | ND |
| 6 | + | − | − | ND | ND | ND | ND | 6.1 | − | ND | − | ND | ND | ND |
| 7 | + | − | − | ND | ND | ND | ND | 9.2 | − | ND | − | ND | ND | ND |
| 8 | + | − | − | ND | ND | ND | ND | 21.4 | + | ND | − | ND | ND | ND |
| 9 | + | − | − | ND | ND | ND | ND | 24.6 | − | ND | − | ND | ND | ND |
| 10 | + | − | − | ND | ND | ND | ND | 19.1 | − | ND | − | ND | ND | ND |
| 11 | + | − | − | ND | ND | ND | ND | 33.4 | − | ND | − | ND | ND | ND |
| 12 | + | − | − | ND | ND | ND | ND | 43 | − | ND | − | ND | ND | ND |
| 13 | + | − | − | ND | ND | ND | ND | 28.7 | − | ND | − | ND | ND | ND |
| 14 | + | − | − | ND | ND | ND | ND | 10.8 | − |
| − | ND | ND | ND |
| 15 | + | − | − | + | + | ND | ND | 30.5 | ND | − | − | ND | − | − |
| 16 | + | − | − | + | + | ND | ND | 6.1 | ND | − | − | ND | − | − |
| 17 | + | − | − | + | + | ND | ND | 9.2 | ND | − | − | ND | − | − |
| 18 | + | − | − | + | + | ND | ND | 21.4 | ND | + | − | ND | − | − |
| 19 | + | − | − | + | + | ND | ND | 24.6 | ND | − | − | ND | − | + |
| 20 | + | − | − | + | − | ND | ND | 19.1 | ND | − | − | ND | − | − |
| 21 | + | − | − | + | − | ND | ND | 33.4 | ND | − | − | ND | − | + |
| 22 | + | − | − | + | − | ND | ND | 43 | ND | − | − | ND | − | − |
| 23 | + | − | − | + | − | ND | ND | 28.7 | ND | ND | ND | ND | − | − |
| 24 | + | − | − | + | − | ND | ND | 10.8 | ND | − | ND | ND | ND | ND |
| 25 | + | − | − | + | − | ND | ND | 5–20 | ND | ND | ND | ND | ND | ND |
| 26 | + | − | − | + | ND | ND | ND | 10–15 | ND | − | − | ND | − | − |
| 27 | + | ND | − | + | − | ND | ND | 20–30 | ND | ND | − | ND | ND | ND |
| 28 | + | − | − | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 29 | + | − | − | + | + | ND | ND | 9 | ND | ND | − | ND | + | ND |
| 30 | + | + | + | + | + | ND | ND | 32 | ND | + | − | ND | + | − |
| 31 | + | − | − | + | + | ND | ND | 24.6 | ND | ND | ND | ND | ND | ND |
| 32 | + | − | − | + | + | ND | ND | 5 | ND | − | − | ND | ND | ND |
| 33 | + | − | − | + | + | ND | ND | ND | ND | − | ND | ND | ND | ND |
| 34 | ND | − | − | + | + | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 35 | + | − | − | + | + | ND | ND | ND | ND | ND | ND | ND | − | ND |
| 36 | ND | ND | ND | + | + | ND | ND | 10–60 | ND | + | − | ND | − | ND |
A large body of the literature has previously
demonstrated that tumor tissues in IPMN were mainly located in the
duct, exhibiting a papillary arrangement. The branches of the
papillary formation are intricate, and serve a function akin to
sieves. Most cells in IPMN are mucous epithelial cells, including
goblet cells, whereas only few cells are eosinophilic epithelial or
cuboidal epithelial (pancreatic duct type epithelium). Among 50
randomly selected patients with IPMN (defined according to the WHO
classification of digestive system tumors in 2010) 21 patients had
low-grade intraepithelial neoplasia, 9 had high-grade
intraepithelial neoplasia and 20 had invasive cancer. The
clinicopathological characteristics differentiating IPMN and ITPN
are summarized in Table II.
 | Table II.Clinicopathological characteristics
of ITPN and IPMN. |
Table II.
Clinicopathological characteristics
of ITPN and IPMN.
| Clinicopathological
characteristics | ITPN | IPMN |
|---|
| Site | Head of
pancreas | Head of
pancreas |
| Clinical
manifestations | Abdominal pain,
nausea and vomiting, weight loss, obstructive jaundice | Abdominal pain,
nausea and vomiting, weight loss, obstructive jaundice |
| Gross
pathology | Multiple nodular
lesions in the pancreatic ducts, no mucus secretion | Central type:
Papillary or cauliflower pattern, mucus secretion Peripheral type:
Polycystic change, mucus secretion |
| Histopathological
characteristics | Papillary and
sieve-like architecture, no mucus | Papillary and
sieve-like architecture, presence of mucus |
| Immune
phenotype | MUC2−,
MUC5AC− |
MUC2+,
MUC5AC+ |
| KRAS gene
mutation | Few KRAS gene
mutations | Several KRAS gene
mutations |
| Prognosis | Better expect
invasive carcinoma | Better expect
invasive carcinoma |
ITPN accounts for ~0.4% of pancreatic tumors, and it
is a rare pancreatic neoplasm with few reported cases investigating
its clinical and molecular pathology (3). Due to the low incidence rate of ITPN,
its diagnosis is often delayed and differential diagnosis may be
challenging. The present study demonstrated a wide age range at
onset for ITPN (35–82 years), with a median age of 55 years
(2). There was no particular gender
predilection (2). The majority of
the patients exhibited mild clinical symptoms, such as abdominal
pain, vomiting, weight loss and jaundice among others. In the
laboratory examinations, the blood biochemical parameters and tumor
markers had no clinical specificity. CT and MRI examinations
revealed the pancreatic duct lesions. The most common site was the
pancreatic head, accounting for 50%, followed by the pancreatic
body (34%); the least common site was the pancreatic tail,
accounting for only 15%.
ITPN is an intraductal neoplasm that is accompanied
by high-grade dysplasia. The gross pathological characteristics
include uniform solid nodular polyps in the dilated pancreatic
duct, with absence of mucin production (4). The size of the tumor is ~0.5–15 cm, and
the pancreatic tissue surrounding the tumor is usually solid and
hardened (4). Parts of the tumor
exhibit tubular or back-to-back gland-like formations. In terms of
morphology, the tumors display a sieve-like appearance rather than
papillary formations in the pancreatic ducts. The tumor cells are
cuboidal with a deeply stained nucleus, without mucus secretion in
the cytoplasm. The proportion of patients with accompanying
invasive carcinoma is ~40%.
Immunohistochemical analysis is helpful in
diagnosing ITPNs. CK is an important marker differentiating the
origin of the cell, as it is positive in tissues of epithelial
origin. In the case reported herein, the immunohistochemical
staining for CK was found to be positive, which confirmed that the
neoplasm originated from the pancreatic duct epithelium (5). On this basis, other immunohistochemical
targets have been used to distinguish the tumor origins, and
included expression of MUC1 and MUC2, which are members of a family
of high molecular weight proteins (5). Moreover, MUC1 encoded transmembrane
mucin proteins and MUC2 encoded secretory mucin proteins. Previous
case reports suggested that the tumors originated from pancreatic
ductal epithelium when they stained positive for MUC1; however,
MUC2-positive staining usually suggests that the tumor most likely
arises from ampullary or colorectal tissues (5,6).
Therefore, in the case report presented herein, the CK-positive,
MUC1-positive and MUC2-negative expression confirmed the ductal
epithelial origin of the tumor.
The monoclonal antibody Ki-67 is an important marker
in cell cycle analysis, reflecting the proliferative activity of
the carcinoma (7). The expression of
Ki-67 provides an important reference in distinguishing benign from
malignant tumors. The Ki-67 index is associated with the
histological grade of the tumor and patient prognosis, i.e., the
higher the Ki-67 index, the higher the histological grade of the
tumor and, thus, the poorer is the prognosis. In benign tumors of
the pancreas, the Ki-67 index is usually <2%. In our patient,
the Ki-67 index was 20%, which suggests a neoplastic tumor with low
histological grade.
Subsequently, additional analyses were performed to
exclude pancreatic endocrine tumors through the expression of CD56,
CgA and vimentin. CD56 is a neural cell adhesion factor, which
mainly characterizes tumors of neuroectodermal origin and is
expressed by a number of endocrine tumors (8). Cg is divided into three subtypes,
namely A, B and C, which comprise a group of soluble acidic
proteins. CgA is most widely distributed in endocrine cells
containing secretory granules and carcinomas of endocrine origin
(9). Thus, CgA is currently used as
an endocrine tumor marker. Vimentin is a marker of normal
mesenchymal cells, which is also expressed in some epithelial cells
or epithelial tumors (5). A number
of pancreatic endocrine carcinomas are positive for the expression
of CgA and CD56, but not necessarily vimentin. Therefore, the
expression of vimentin, CD56 and CgA, suggests that the neoplastic
origin in this case was not endocrine. CDX2 is a protein that is
composed of 311 amino acids, containing a short sequence-specific
binding region (10). Approximately
95% of colon or rectal carcinomas are CDX2-positive. However, MUC2
and CDX2 were not expressed in the intestinal epithelial specimens
in the case reported herein.
According to previous studies, the EGFR pathway,
including downstream proteins such as KRAS, NRAS and BRAF, is a
common signal transduction pathway in digestive system tumors
(11). EGFR is a type of
glycoprotein, which belongs to the tyrosine kinase receptor family.
Abnormal expression of EGFR is closely associated with
proliferation, angiogenesis, invasion, metastasis and apoptosis of
tumor cells (12). Under normal
physiological conditions, the functional expression of the KRAS
protein is rapidly inactivated following stimulation by EGFR.
However, mutation of the KRAS gene leads to its sustained
activation, as well as of the EGFR gene, which promotes tumor cell
proliferation (13). Detection of
KRAS gene mutations is clinically important as it plays an
important role in identifying the specific characteristics of the
tumor, and may help elucidate the process underlying the
development of various cancers and administer the appropriate
chemotherapy in clinical practice. In addition, RAF is activated by
its upstream activator protein RAS, which binds to the
Raf-1N-terminal. The RAS/RAF/MEK/ERK pathway is one of the most
important signal transduction pathways, as it is involved in
several cellular physiological functions and also plays an
important role in the pathogenesis and pathophysiology of a number
of diseases (14). In this study,
mutational analyses did not reveal any DNA sequence mutation in the
common exons of EGFR, KRAS, NRAS or BRAF genes, indicating that the
pathogenesis of ITPN is likely not related to the EGFR pathway.
P53 is a tumor suppressor gene, and P53 mutations
occur in ~50% of all malignant tumors (15). The P53 protein is one of the
transcription factors that regulates the cell cycle. Similar to all
other tumor suppressor genes, the P53 gene plays a role in
monitoring cell division under steady-state conditions (16). The P53 gene inhibits malignant
transformation, determines the extent of variation of cellular DNA
sequences and induces cellular repair by itself if the extent of
cell damage is mild; by contrast, the P53 gene may induce apoptosis
if the extent of cell damage is considerable (17).
Under conditions of mutation, the proper function of
P53 is lost, as has been observed across a wide variety of
mutations in a number of different tumors. The P53 gene is
associated with 50% of human cancers, such as cancers of the liver,
breast, bladder, gastric tissues, colon and prostate gland
(18). The site of the P53 mutation
in human cancer is usually found in the highly conserved regions
that include positions 175, 248, 249, 282 and 273. However, the
different types of P53 mutations found in tumors at different
mutation sites, as is commonly found in colon and breast cancer,
have a similar epidemiology. However, the P53 mutation spectrum is
not consistent across tumor types. In the case reported herein,
positive expression of P53 and DNA sequence analysis of the P53
gene introduced at a novel idea for the therapy of ITPN, which
dominantly features P53 tumor gene therapy as a promising starting
point that may offer new hope for combating ITPN.
Of note, IPMN may be confused with ITPN due to its
similar morphology and clinical symptoms, such as jaundice and
epigastric pain. However, the main characteristics of IPMN are
mucin production that is accompanied by obvious ductal dilation,
and the presence of mucous columnar epithelium, which are not
present in ITPN. Moreover, serous cystadenoma (SCA), also referred
to as microcystic adenoma, exhibited similarities to ITPN in terms
of histological patterns. However, SCA often occurs in the
pancreatic body or tail, the diameter of the tumor on cross-section
is 1–2 mm and it is filled with a colorless fluid that
differentiates it from ITPN.
Acknowledgements
The present study was supported by the Natural
Foundation of Hubei Province (grant no. 2013CFB267) and the Wuhan
Science and Technology Key Project (grant no.
2013060602010248).
References
|
1
|
Kasugai H, Tajiri T, Takehara Y, Mukai S,
Tanaka J and Kudo SE: Intraductal tubulopapillary neoplasms of the
pancreas: Case report and review of the literature. J Nippon Med
Sch. 80:224–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamaguchi H, Kuboki Y, Hatori T, Yamamoto
M, Shimizu K, Shiratori K, Shibata N, Shimizu M and Furukawa T: The
discrete nature and distinguishing molecular features of pancreatic
intraductal tubulopapillary neoplasms and intraductal papillary
mucinous neoplasms of the gastric type, pyloric gland variant. J
Pathol. 231:335–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kölby D, Thilén J, Andersson R, Sasor A
and Ansari D: Multifocal intraductal tubulopapillary neoplasm of
the pancreas with total pancreatectomy: Report of a case and review
of literature. Int J Clin Exp Pathol. 8:9672–9680. 2015.PubMed/NCBI
|
|
4
|
Yamaguchi H, Shimizu M, Ban S, Koyama I,
Hatori T, Fujita I, Yamamoto M, Kawamura S, Kobayashi M, Ishida K,
et al: Intraductal tubulopapillary neoplasms of the pancreas
distinct from pancreatic intraepithelial neoplasia and intraductal
papillary mucinous neoplasms. Am J Surg Pathol. 33:1164–1172. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Modi Y, Shaaban H, Gauchan D, Maroules M,
Parikh N and Guron G: Primary clear cell ductal adenocarcinoma of
the pancreas: A case report and clinicopathologic literature
review. J Cancer Res Ther. 10:773–776. 2014.PubMed/NCBI
|
|
6
|
Yokoyama S, Kitamoto S, Higashi M, Goto Y,
Hara T, Ikebe D, Yamaguchi T, Arisaka Y, Niihara T, Nishimata H, et
al: Diagnosis of pancreatic neoplasms using a novel method of DNA
methylation analysis of mucin expression in pancreatic juice. PLoS
One. 9:e937602014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garcia-Carracedo D, Yu CC, Akhavan N, Fine
SA, Schönleben F, Maehara N, Karg DC, Xie C, Qiu W, Fine RL, et al:
Smad4 loss synergizes with TGFα overexpression in promoting
pancreatic metaplasia, PanIN development and fibrosis. PLoS One.
10:e01208512015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stelow EB, Shaco-Levy R, Bao F, Garcia J
and Klimstra DS: Pancreatic acinar cell carcinomas with prominent
ductal differentiation: Mixed acinar ductal carcinoma and mixed
acinar endocrine ductal carcinoma. Am J Surg Pathol. 34:510–518.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gurung B, Hua X, Runske M, Bennett B,
LiVolsi V, Roses R, Fraker DA and Metz DC: PTCH 1 staining of
pancreatic neuroendocrine tumor (PNET) samples from patients with
and without multiple endocrine neoplasia (MEN-1) syndrome reveals a
potential therapeutic target. Cancer Biol Ther. 16:219–224. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumari N, Prabha K, Singh RK, Baitha DK
and Krishnani N: Intestinal and pancreatobiliary differentiation in
periampullary carcinoma: The role of immunohistochemistry. Hum
Pathol. 44:2213–2219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nagathihalli NS, Beesetty Y, Lee W,
Washington MK, Chen X, Lockhart AC and Merchant NB: Novel
mechanistic insights into ectodomain shedding of EGFR ligands
amphiregulin and TGF-α: Impact on gastrointestinal cancers driven
by secondary bile acids. Cancer Res. 74:2062–2072. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sreekumar BK, Belinsky GS, Einwachter H,
Rhim AD, Schmid R and Chung C: Polarization of the vacuolar
adenosine triphosphatase delineates a transition to high-grade
pancreatic intraepithelial neoplasm lesions. Pancreas.
43:1256–1263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mackinnon AC Jr, Luevano A, de Araujo LC,
Rao N, Le M and Suster S: Cribriform adenocarcinoma of the lung:
Clinicopathologic, immunohistochemical, and molecular analysis of
15 cases of a distinctive morphologic subtype of lung
adenocarcinoma. Mod Pathol. 27:1063–1072. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schultz NA, Roslind A, Christensen IJ,
Horn T, Høgdall E, Pedersen LN, Kruhøffer M, Burcharth F, Wøjdemann
M and Johansen JS: Frequencies and prognostic role of KRAS and BRAF
mutations in patients with localized pancreatic and ampullary
adenocarcinomas. Pancreas. 41:759–766. 2012.PubMed/NCBI
|
|
15
|
Guan H, Gurda G, Lennon AM, Hruban RH and
Erozan YS: Intraductal tubulopapillary neoplasm of the pancreas on
fine needle aspiration: Case report with differential diagnosis.
Diagn Cytopathol. 42:156–160. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Navas C, Hernández-Porras I, Schuhmacher
AJ, Sibilia M, Guerra C and Barbacid M: EGF receptor signaling is
essential for k-ras oncogene-driven pancreatic ductal
adenocarcinoma. Cancer Cell. 22:318–330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shin SH, Kim SC, Hong SM, Kim YH, Song KB,
Park KM and Lee YJ: Genetic alterations of K-ras, p53, c-erbB-2,
and DPC4 in pancreatic ductal adenocarcinoma and their correlation
with patient survival. Pancreas. 42:216–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Søreide K and Sund M:
Epidemiological-molecular evidence of metabolic reprogramming on
proliferation, autophagy and cell signaling in pancreas cancer.
Cancer Lett. 356:281–288. 2015. View Article : Google Scholar : PubMed/NCBI
|