Introduction
C-reactive protein (CRP), which was found to be a
representative acute-phase reactant in 1930, is the most widely
used marker of systemic inflammation (1). Inflammation plays an important role
during almost all stages of tumor development (2). For example, the proliferation of renal
cell carcinoma (RCC) is associated with increased levels of
interleukin-6 (IL-6), nuclear factor-κB, and other inflammatory
factors (3,4), and the serum CRP levels were found to
be correlated with the levels of proinflammatory cytokines, such as
IL-6 (5). Numerous studies have
demonstrated that CRP is a significant prognostic factor among
patients with RCC at various stages, whether they are being treated
by surgery, systemic therapy, or both (6).
Sunitinib malate (Sutent, Pfizer Inc., New York, NY,
USA) is an orally administered, small-molecule, multitargeted
inhibitor of tyrosine kinases (e.g., vascular endothelial growth
factor receptor, platelet-derived growth factor receptor,
phosphorylation of stem cell factor receptor, Fms-like tyrosine
kinase-3, colony-stimulating factor-1 receptor, and RET receptor
tyrosine kinases). In a phase III clinical trial including patients
with advanced RCC, sunitinib achieved significantly better results
compared with interferon (IFN)-α and is approved worldwide for the
treatment of advanced RCC (7,8). The
number of patients treated with sunitinib is increasing and,
therefore, there is an urgent need to identify biomarkers that may
be used to predict its efficacy.
Recent studies have revealed a prognostic role for
CRP in the outcome of sunitinib treatment (9–12).
Normal pretreatment CRP levels predict a higher response rate and
better survival among patients undergoing sunitinib treatment
(9,11). However, the prognostic role of CRP
kinetics after sunitinib initiation has not been investigated to
date. Previous studies have demonstrated that postoperative
normalization of the CRP level is associated with better prognosis
in patients with localized or advanced RCC who receive nephrectomy,
metastasectomy, or both (13–16). In
addition, CRP kinetics has been found to be a prognostic factor for
the effect of cytokine therapies (13,15,17). We
hypothesized that CRP kinetics may be an important predictor of the
efficacy of sunitinib treatment in patients with advanced RCC.
Patients and methods
Patients and treatment
The present study was performed with the approval of
the Kitasato University Medical Ethics Organization (approval no.
KMEO B15-125). The requirement for informed consent was waived due
to the retrospective nature of the analyses. Between December, 2008
and December, 2012, 56 consecutive patients with advanced RCC who
were treated with sunitinib at the Department of Urology, Kitasato
University Hospital (Sagamihara, Japan) were enrolled. Eligible
patients had measurable tumors, metastatic or primary. All the
patients underwent surgical treatment or biopsy of the primary
lesion and had histologically proven RCC. The sample group
comprised 40 men and 16 women, with a median age of 65 years
(range, 36–80 years) at the time of sunitinib initiation. Of the 56
patients, 53 (94.6%) presented with clear-cell RCC and 3 (5.4%)
with papillary RCC. In general, sunitinib at a dose of 50 mg was
administered orally once daily in a 6-week cycle consisting of 4
weeks of treatment followed by 2 weeks without treatment. Dose
reductions were permitted depending on individual tolerance.
Response and progression were assessed by the
treating physician according to the Response Evaluation Criteria in
Solid Tumors (RECIST), version 1.1 (https://www.eortc.be/Recist/documents/RECISTGuidelines.pdf),
determined by means of computed tomography or magnetic resonance
imaging performed every 4–8 weeks. Adverse events were evaluated by
means of physical examination and laboratory assessments, including
hematological and serum chemistry, every 2–4 weeks during sunitinib
treatment, and were graded according to the National Cancer
Institute Common Terminology Criteria for Adverse Events, version
4.0 (https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf).
Assessment of serum CRP
Serum CRP was measured by latex agglutination
immunoassay using the Nanopia CRP kit (Daiichi Pure Chemicals,
Tokyo, Japan). Serum CRP was assessed prior to sunitinib treatment
and every 2–4 weeks during treatment. The normal cut-off value
specified by the manufacturer is 0.30 mg/dl and, therefore,
patients with a serum CRP level of ≤0.30 mg/dl were assigned to the
normal CRP cohort, as previously described (9).
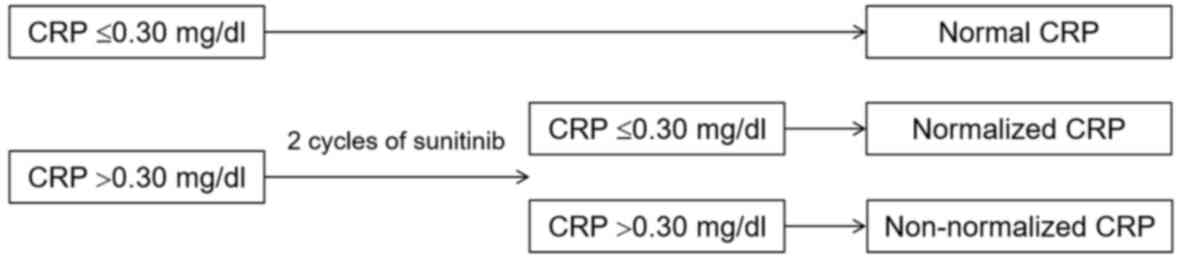
The patient charts were retrospectively reviewed and
the patients were divided into 3 cohorts according to the
pretreatment CRP level and CRP kinetics as follows: i) Normal CRP
cohort (pretreatment CRP ≤0.30 mg/dl); ii) normalized CRP cohort
(pretreatment CRP >0.30 mg/dl that normalized within 2 cycles of
treatment); and iii) non-normalized CRP cohort (pretreatment CRP
>0.30 mg/dl that did not normalize at any point after the
initiation of sunitinib treatment) (Fig.
1). Disease control rates, namely complete response, partial
response (PR) and stable disease (SD), were evaluated.
Non-parametric estimates of progression-free and overall survival
were compared.
Statistical analysis
Analysis of variance and post hoc Fisher's protected
least-significant difference test were used to evaluate differences
of means between cohorts. The Chi-squared test was used to evaluate
differences for categorical variables. Non-parametric estimates of
survival were made by means of Kaplan-Meier curves. Survival curves
were generated on the basis of progression-free and overall
survival from the initiation of sunitinib treatment to the date of
disease progression or death from any cause. Log-rank tests were
used for statistical comparisons. The effects on survival were
assessed by means of univariate and multivariate regression
analysis using the Cox proportional hazards model. All the analyses
were performed with StatView software, version 5.0 (SAS Institute,
Cary, NC, USA) and differences were considered statistically
significant if P<0.05.
Results
Patient characteristics
The pretreatment characteristics of the patients are
listed in Table I by CRP cohort. The
median follow-up period was 15.5 months (range, 1–56 months). The
normal, normalized and non-normalized CRP groups comprised 30.4,
14.3 and 55.4% of the patients, respectively. Compared with the
other two cohorts, the non-normalized CRP cohort exhibited
significantly higher pretreatment CRP levels (P=0.0002) and
included a significantly higher proportion of patients with an
Eastern Cooperative Oncology Group (ECOG) performance status (PS)
of ≥1, Memorial Sloan-Kettering Cancer Center (MSKCC) poor risk
classification, no prior nephrectomy, and first-line treatment
(P=0.0051, 0.0129, 0.0289 and 0.0116, respectively). There were no
statistically significant differences in any of the other
pretreatment characteristics, tumor characteristics, or relative
dose intensity.
 | Table I.Patient characteristics of the three
CRP cohorts. |
Table I.
Patient characteristics of the three
CRP cohorts.
| Characteristics | Normal CRP | Normalized CRP | Non-normalized
CRP | P-value |
|---|
| Total patients, n
(%) | 17 (30.4) | 8 (14.3) | 31 (55.4) |
|
| Pretreatment serum
CRP level (mean ± SD; mg/dl) | 0.09±0.08 | 0.80±0.72 | 5.89±6.19 | 0.0002 |
| Gender, n (%) |
|
|
| 0.7682 |
| Male | 13 (76.5) | 5 (62.5) | 22 (71.0) |
|
|
Female | 4 (23.5) | 3 (37.5) | 9 (29.0) |
|
| Age, years |
|
|
| 0.7603 |
|
Median | 67 | 67 | 64 |
|
|
Range | 46–77 | 51-§80 | 36–78 |
|
| Mean ±
SD | 65±9.4 | 65.8±8.7 | 63.5±8.8 |
|
| ECOG PS, n (%) |
|
|
| 0.0051 |
| 0 | 16 (94.1) | 6 (75.0) | 15 (48.4) |
|
| ≥1 | 1 (5.9) | 2 (25.0) | 16 (51.6) |
|
| MSKCC risk
classification, n (%) |
|
|
| 0.0129 |
|
Favorable | 4 (23.5) | 2 (25.0) | 5 (16.1) |
|
|
Intermediate | 13 (76.5) | 4 (50.0) | 11 (35.5) |
|
| Poor | 0 (0) | 2 (25.0) | 15 (48.4) |
|
| Prior nephrectomy, n
(%) |
|
|
| 0.0289 |
| Yes | 16 (94.1) | 7 (87.5) | 19 (61.3) |
|
| No | 1 (5.9) | 1 (12.5) | 12 (38.7) |
|
| T stage, n (%) |
|
|
| 0.8936 |
| T1,2 | 8 (47.1) | 4 (50.0) | 13 (41.9) |
|
| ≥T3 | 9 (52.9) | 4 (50.0) | 18 (58.1) |
|
| Grade, n (%) |
|
|
| 0.3490 |
|
1,2 | 11 (64.7) | 7 (87.5) | 15 (48.4) |
|
| 3 | 5 (29.4) | 1 (12.5) | 10 (32.2) |
|
| Prior
immunotherapy, n (%) |
|
|
| 0.0662 |
|
IFN-α | 8 (52.9) | 3 (37.5) | 6 (19.4) |
|
| IL-2
and IFN-α | 4 (23.5) | 1 (12.5) | 5 (16.1) |
|
| Prior targeted
therapy, n (%) |
|
|
| 0.9191 |
|
Sorafenib | 5 (29.4) | 2 (25.0) | 10 (32.2) |
|
| Metastatic site,
n |
|
|
|
|
|
Lung | 14 | 4 | 21 |
|
|
Bone | 2 | 2 | 15 |
|
| Lymph
nodes | 3 | 3 | 6 |
|
|
Pancreas | – | 2 | 4 |
|
|
Liver | 1 | 1 | 4 |
|
|
Adrenal | 2 | – | 3 |
|
|
Brain | 1 | 1 | 2 |
|
|
Local | 1 | 1 | 1 |
|
|
Kidney | – | 1 | 2 |
|
|
Skin | – | – | 3 |
|
|
Prostate | 1 | – | – |
|
| No. of metastatic
sites, n (%) |
|
|
| 0.4976 |
| 1 | 8 (47.1) | 2 (25.0) | 12 (38.7) |
|
| ≥2 | 8 (47.1) | 6 (75.0) | 18 (58.1) |
|
| Treatment, n
(%) |
|
|
| 0.0116 |
|
First-line | 4 (23.5) | 3 (37.5) | 19 (61.3) |
|
|
Second-line | 9 (52.9) | 4 (50.0) | 3 (9.7) |
|
|
Third-line | 4 (23.5) | 1 (12.5) | 9 (29.0) |
|
| RDI (%) |
|
|
| 0.7128 |
|
Median | 61.1 | 63.0 | 65.4 |
|
|
Range | 33.3–100 | 27.1–75 | 16.1–100 |
|
| Mean ±
SD | 62.5±20.0 | 56.1±16.7 | 62.7±22.1 |
|
Disease control rate
In the normal CRP cohort, 8 patients (47.06%)
exhibited PR to treatment and 8 patients (47.06%) had SD, according
to RECIST. In the normalized CRP cohort, 4 patients (50.0%)
exhibited PR to treatment and 2 patients (25.0%) had SD, whereas in
the non-normalized CRP cohort, 4 patients (12.9%) exhibited PR to
treatment and 7 patients (22.6%) had SD. The normal and normalized
CRP cohorts exhibited significantly better disease control rates
compared with the non-normalized CRP cohort (P<0.0001 and
P=0.0445, respectively; Table
II).
 | Table II.Disease control rates by cohort. |
Table II.
Disease control rates by cohort.
| Cohorts | PR + SD (%) | P-value |
|---|
| Normal CRP | 94.1 | <0.0001 |
| Normalized CRP | 75.0 | 0.0445 |
| Non-normalized
CRP | 35.5 | – |
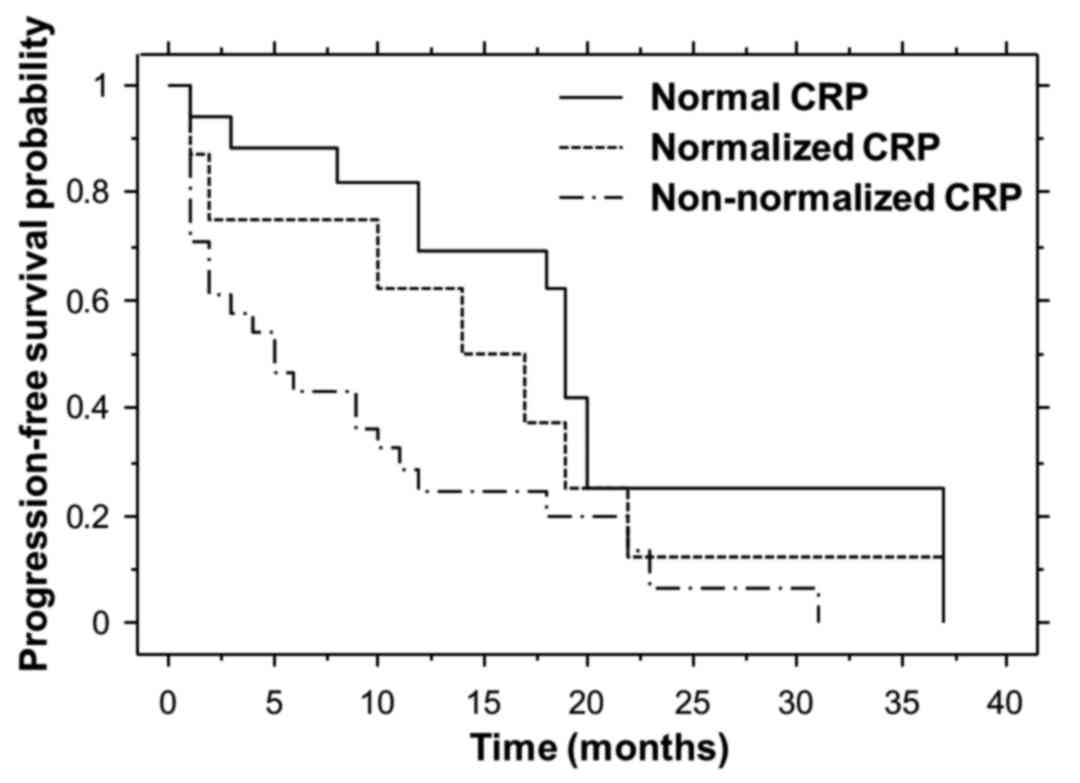
Progression-free survival
Non-parametric estimates of progression-free
survival were analyzed by means of Kaplan-Meier curves for each
cohort (Fig. 2). The median
progression-free survival times for the normal, normalized and
non-normalized CRP cohorts were 19.0, 14.0 and 5.0 months,
respectively. The median progression-free survival for the normal
CRP cohort was significantly longer compared with that for the
non-normalized CRP cohort (P=0.0050).
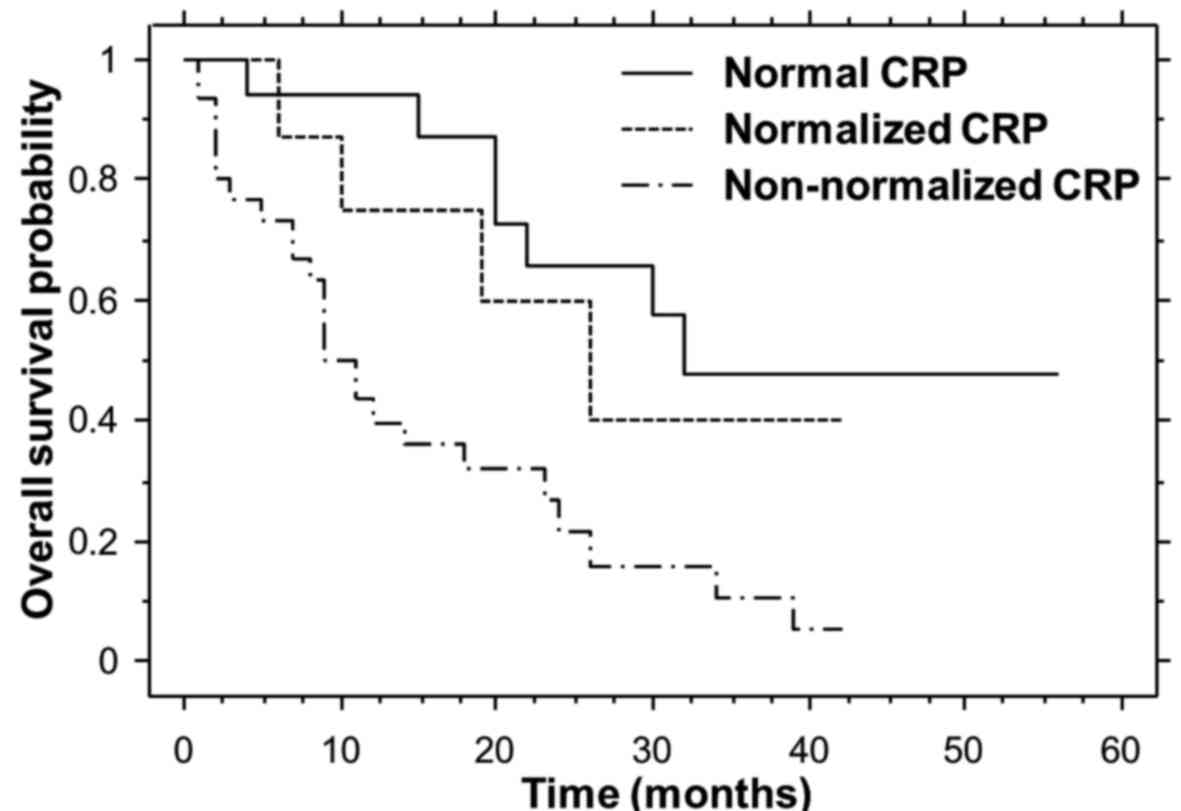
Overall survival
Non-parametric estimates of overall survival were
analyzed by means of Kaplan-Meier curves for each cohort (Fig. 3). The median overall survival times
for the normal, normalized and non-normalized CRP cohorts were
32.0, 26.0 and 11.0 months, respectively. The median overall
survival times for the normal and normalized CRP cohorts were
significantly longer compared with that of the non-normalized CRP
cohort (P=0.0005 and 0.0466, respectively).
Cox proportional hazards model
To assess the overall prognostic significance of
selected variables, univariate and multivariate Cox proportional
hazards regression analyses were performed (Table III). On univariate analysis, a
significantly longer overall survival was predicted by male gender
[hazard ratio (HR)=0.480; 95% confidence interval (CI):
0.242–0.953; P=0.0358], ECOG PS 0 (HR=0.207; 95% CI: 0.096–0.447;
P<0.0001), MSKCC non-poor (favorable and intermediate) risk
classification (HR=0.208; 95% CI: 0.096–0.449; P<0.0001) and
normal plus normalized CRP (HR=0.275; 95% CI: 0.133–0.567;
P=0.0005).
 | Table III.Univariate and multivariate analyses
with Cox proportional hazards model for predicting overall
survival. |
Table III.
Univariate and multivariate analyses
with Cox proportional hazards model for predicting overall
survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Gender (male) | 0.480
(0.242–0.242) | 0.0358 | 0.597
(0.264–1.264) | 0.2167 |
| ECOG PS 0 | 0.207
(0.096–0.096) | <0.0001 | 0.441
(0.139–1.139) | 0.1642 |
| MSKCC non-poor | 0.208
(0.096–0.096) | <0.0001 | 0.795
(0.230–2.230) | 0.7169 |
| Normal, normalized
CRP | 0.275
(0.133–0.133) | 0.0005 | 0.334
(0.148–0.148) | 0.0084 |
| Prior nephrectomy
(yes) | 0.552
(0.255–1.255) | 0.1318 |
|
|
| T1,2 | 1.127
(0.585–2.585) | 0.7210 |
|
|
| Grade 1,2 | 0.683
(0.332–1.332) | 0.2993 |
|
|
| Prior immunotherapy
(yes) | 0.619
(0.320–1.320) | 0.1551 |
|
|
| Prior sorafenib
(yes) | 0.916
(0.449–1.449) | 0.8091 |
|
|
| Single metastatic
site | 0.596
(0.285–1.285) | 0.1678 |
|
|
| First-line
treatment | 1.776
(0.920–3.920) | 0.0868 |
|
|
On multivariate analysis, variables associated with
significantly better overall survival included male gender, ECOG PS
0, MSKCC non-poor risk classification and normal plus normalized
CRP (Table III). Following
adjustment for differences in these variables, normal plus
normalized CRP was a predictor of better overall survival
(HR=0.334; 95% CI: 0.148–0.755; P=0.0084).
Discussion
CRP has been shown to be a non-specific biomarker in
patients with various stages of RCC who receive surgery (13–16,18–21),
immunotherapy (13,15,17,22,23) and
molecular-targeted therapy (9–12). CRP
has a promising role in predicting survival among patients with
localized and metastatic RCC. The kinetics of CRP levels in RCC was
first described by Fujikawa et al in 1999 (13), in a retrospective study of 58
patients with metastatic RCC, among whom 34 had elevated
pretreatment CRP (≥1.0 ng/ml). A total of 21 patients with elevated
CRP levels received cytoreductive surgery combined with
postoperative immunotherapy. Patients whose postoperative nadir CRP
decreased to within normal limits (<1.0 ng/ml) exhibited
significantly better disease-specific survival compared with
patients whose CRP remained elevated (P=0.0025) (13). Subsequently, Tatokoro et al
(14) reported that the prognosis of
patients whose CRP normalized (to <0.5 mg/dl) following
cytoreductive nephrectomy and the prognosis of patients without
preoperative elevated CRP were better compared with the prognosis
of patients whose CRP did not normalize after surgery. These
investigators concluded that CRP kinetics may predict the clinical
course of patients with metastatic RCC who undergo cytoreductive
nephrectomy (14). Saito et
al (15) reported that CRP
kinetics affect survival in patients with metastatic RCC treated
with immunotherapy, metastasectomy, or both. A decrease in CRP
level (to <0.5 mg/dl) during treatment predicts better prognosis
in patients with metastatic RCC, and a prolonged period of normal
CRP level is associated with prolonged survival (15). Ito et al (16) reported that non-normalization of
postoperative CRP (≥0.3 mg/dl) strongly predicted recurrence and
prognosis in 263 patients with N0M0 RCC who underwent nephrectomy.
Shinohara et al (17)
reported that response rate and 1-year progression-free survival
were significantly higher in patients with normalized CRP compared
with those in non-normalized patients treated with IFN-α
combination therapy. The combination of natural IFN-α and meloxicam
reduced post-treatment CRP level in nearly half of the patients in
the high CRP group (≥0.4 mg/dl) and exhibited therapeutic efficacy
in those patients (17).
We previously demonstrated that normal pretreatment
CRP level is an independent prognostic factor for patients with
advanced RCC treated with sunitinib; specifically, among 41
patients, the normal CRP cohort (≤0.30 mg/dl, 31.7%) exhibited a
significantly higher disease control rate (P=0.0022) and longer
progression-free survival (P=0.0361) compared with the elevated CRP
cohort (>0.30 mg/dl, 68.3%). However, 35.7% of the patients in
the elevated CRP cohort still experienced clinical benefit (PR +
SD) from sunitinib (9). On the basis
of these previous results, the present study of CRP kinetics was
designed. The normalized CRP cohort comprised patients with a
pretreatment CRP level of >0.30 mg/dl that normalized (to ≤0.30
mg/dl) within 2 cycles of treatment; the cohort was defined this
way as most therapeutic evaluations were performed 2 cycles after
the initiation of sunitinib treatment. The normal CRP cohort (CRP
≤0.30 mg/dl, 30.4% of the patients) exhibited a significantly
better disease control rate, longer progression-free survival and
longer overall survival (P<0.0001, P=0.0050 and P=0.0005,
respectively) compared with the non-normalized CRP cohort. The
normalized CRP cohort (14.3%) also exhibited a significantly better
disease control rate and longer overall survival (P=0.0445 and
0.0466, respectively) compared with the non-normalized CRP cohort.
The multivariate analysis revealed that normal plus normalized CRP
predicted better overall survival (HR=0.334; 95% CI: 0.148–0.755;
P=0.0084). There were no significant differences between the
cohorts with regard to relative dose intensity of sunitinib. To the
best of our knowledge, the present study is the first to describe
the prognostic effect of CRP kinetics in patients with advanced RCC
treated with sunitinib.
The mechanisms of CRP normalization after initiation
of sunitinib have not been explained in detail to date. CRP
production in the liver is strongly induced by proinflammatory
cytokines, such as IL-1, tumor necrosis factor and particularly
IL-6 (24). Experimental studies
have demonstrated that RCC cells may produce IL-6, which is
recognized as a growth promotor in RCC cells (3,25). By
reducing tumor volume, sunitinib treatment may reduce the total
amount of IL-6 secreted by the tumor, which in turn may contribute
to CRP normalization following initiation of sunitinib
treatment.
The reported proportions of patients who experience
CRP normalization vary between studies. For example, Tatokoro et
al (14) reported that CRP
decreased to normal after cytoreductive nephrectomy in 74% of
patients who had elevated CRP prior to surgery, and Ito et
al (16) reported that 65.8% of
patients achieved CRP normalization following nephrectomy. By
contrast, Saito et al (15)
reported that, among patients who underwent immunotherapy,
metastasectomy, or both, only 49% achieved CRP normalization.
Shinohara et al (17)
reported that, among patients with a high baseline CRP level, 50%
had CRP levels within the normal range following IFN-α combination
therapy. In the present study, CRP normalization after initiation
of sunitinib treatment was observed in only 20.5% of the patients
with elevated pretreatment CRP levels. The differences between
these studies may have been due to the different types of treatment
that were applied.
There were potential limitations to the present
study. First, this was a retrospective, single-institutional study.
Second, the sample size of the present study was small, as the
normalized CRP cohort only included 8 patients. However,
significant results of disease control rate and overall survival
were obtained and we do not consider that these limitations
affected the validity of our results.
Several clinical and molecular markers predicting
the outcome of sunitinib treatment have been identified to date.
CRP kinetics may play an important role in predicting the outcome
of sunitinib treatment and may be an informative marker to guide
early changes to the chemotherapeutic agent if CRP does not
decrease after the initiation of sunitinib. Another important issue
is treatment selection in patients with non-normalized CRP. Further
studies are required to determine the optimal treatment for the
non-normalized CRP cohort.
In conclusion, pretreatment normal CRP predicted a
better disease control rate, longer progression-free survival and
longer overall survival in patients with advanced RCC treated with
sunitinib. Post-treatment CRP normalization also predicted a better
disease control rate and longer overall survival. CRP kinetics as
well as pretreatment CRP level were found to be prognostic
indicators in patients with advanced RCC treated with
sunitinib.
Acknowledgements
The authors would like to thank Neil M. Singer for
providing expert editorial assistance.
References
|
1
|
Tillett WS and Francis T: Serological
reactions in pneumonia with a non-protein somatic fraction of
pneumococcus. J Exp Med. 52:561–571. 1930. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koo AS, Armstrong C, Bochner B,
Shimabukuro T, Tso CL, deKernion JB and Belldegrum A: Interleukin-6
and renal cell cancer: Production, regulation, and growth effects.
Cancer Immunol Immunother. 35:97–105. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oya M, Takayanagi A, Horiguchi A, Mizuno
R, Ohtsubo M, Marumo K, Shimizu N and Murai M: Increased nuclear
factor-kappa B activation is related to the tumor development of
renal cell carcinoma. Carcinogenesis. 24:377–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blay JY, Negrier S, Combaret V, Attali S,
Goillot E, Merrouche Y, Mercatello A, Ravault A, Tourani JM,
Moskovtchenko JF, et al: Serum level of interleukin 6 as a
prognosis factor in metastatic renal cell carcinoma. Cancer Res.
52:3317–3322. 1992.PubMed/NCBI
|
|
6
|
Saito K and Kihara K: Role of C-reactive
protein in urological cancers: A useful biomarker for predicting
outcomes. Int J Urol. 20:161–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik
C, Kim ST, et al: Sunitinib versus interferon alfa in metastatic
renal-cell carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili
R, Bjarnason GA, et al: Overall survival and updated results for
sunitinib compared with interferon alfa in patients with metastatic
renal cell carcinoma. J Clin Oncol. 27:3584–3590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujita T, Iwamura M, Ishii D, Tabata K,
Matsumoto K, Yoshida K and Baba S: C-reactive protein as a
prognostic marker for advanced renal cell carcinoma treated with
sunitinib. Int J Urol. 19:908–913. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yasuda Y, Saito K, Yuasa T, Kitsukawa S,
Urakami S, Yamamoto S, Yonese J, Takahashi S and Fukui I:
Prognostic impact of pretreatment C-reactive protein for patients
with metastatic renal cell carcinoma treated with tyrosine kinase
inhibitors. Int J Clin Oncol. 18:884–889. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beuselinck B, Vano YA, Oudard S, Wolter P,
De Smet R, Depoorter L, Teghom C, Karadimou A, Zucman-Rossi J,
Debruyne PR, et al: Prognostic impact of baseline serum C-reactive
protein in patients with metastatic renal cell carcinoma (RCC)
treated with sunitinib. BJU Int. 114:81–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Teishima J, Kobatake K, Hayashi T, Seno Y,
Ikeda K, Nagamatsu H, Hieda K, Shoji K, Miyamoto K, Inoue S, et al:
Prognostic significance of C-reactive protein in patients with
intermediate-risk metastatic renal cell carcinoma treated with
molecular targeted therapy. Oncol Lett. 8:881–885. 2014.PubMed/NCBI
|
|
13
|
Fujikawa K, Matsui Y, Oka H, Fukuzawa S
and Takeuchi H: Serum C-reactive protein level and the impact of
cytoreductive surgery in patients with metastatic renal cell
carcinoma. J Urol. 162:1934–1937. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tatokoro M, Saito K, Iimura Y, Fujii Y,
Kawakami S and Kihara K: Prognostic impact of postoperative
C-reactive protein level in patients with metastatic renal cell
carcinoma undergoing cytoreductive nephrectomy. J Urol.
180:515–519. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saito K, Tatokoro M, Fujii Y, Iimura Y,
Koga F, Kawakami S and Kihara K: Impact of C-reactive protein
kinetics on survival of patients with metastatic renal cell
carcinoma. Eur Urol. 55:1145–1154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ito K, Yoshii H, Sato A, Kuroda K, Asakuma
J, Horiguchi A, Sumitomo M and Asano T: Impact of postoperative
C-reactive protein level on recurrence and prognosis in patients
with N0M0 clear cell renal cell carcinoma. J Urol. 186:430–435.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shinohara N, Kumagai A, Kanagawa K,
Maruyama S, Abe T, Sazawa A and Nonomura K: Multicenter phase II
trial of combination therapy with meloxicam, a cox-2 inhibitor, and
natural interferon-alpha for metastatic renal cell carcinoma. Jpn J
Clin Oncol. 39:720–726. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lamb GW, McMillan DC, Ramsey S and
Aitchison M: The relationship between the preoperative systemic
inflammatory response and cancer-specific survival in patients
undergoing potentially curative resection for renal clear cell
cancer. Br J Cancer. 94:781–784. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ito K, Asano T, Yoshii H, Satoh A,
Sumitomo M and Hayakawa M: Impact of thrombocytosis and C-reactive
protein elevation on the prognosis for patients with renal cell
carcinoma. Int J Urol. 13:1365–1370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Komai Y, Saito K, Sakai K and Morimoto S:
Increased preoperative serum C-reactive protein level predicts a
poor prognosis in patients with localized renal cell carcinoma. BJU
Int. 99:77–80. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karakiewicz PI, Hutterer GC, Trinh QD,
Jeldres C, Perrotte P, Gallina A, Tostain J and Patard JJ:
C-reactive protein is an informative predictor of renal cell
carcinoma-specific mortality: A European study of 313 patients.
Cancer. 110:1241–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Atzpodien J, Royston P, Wandert T and
Reitz M: DGCIN-German Cooperative Renal Carcinoma
Chemo-Immunotherapy Trials Group: Metastatic renal carcinoma
comprehensive prognostic system. Br J Cancer. 88:348–353. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Casamassima A, Picciariello M, Quaranta M,
Berardino R, Ranieri C, Paradiso A, Lorusso V and Guida M:
C-reactive protein: A biomarker of survival in patients with
metastatic renal cell carcinoma treated with subcutaneous
interleukin-2 based immunotherapy. J Urol. 173:52–55. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gauldie J, Richards C, Harnish D, Lansdorp
P and Baumann H: Interferon beta 2/B-cell stimulatory factor type 2
shares identity with monocyte-derived hepatocyte-stimulating factor
and regulates the major acute phase protein response in liver
cells. Proc Natl Acad Sci USA. 84:7251–7255. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miki S, Iwano M, Miki Y, Yamamoto M, Tang
B, Yokokawa K, Sonoda T, Hirano T and Kishimoto T: Interleukin-6
(IL-6) functions as an in vitro autocrine growth factor in renal
cell carcinomas. FEBS Lett. 250:607–610. 1989. View Article : Google Scholar : PubMed/NCBI
|