Introduction
The term primitive neuroectodermal tumor (PNET) was
first introduced by Hart and Earle to describe tumors composed of
small round cells with different degrees of neural, glial and
ependymal differentiation (1). PNETs
have been classified according to the World Health Organization as
central- and peripheral-type. Central PNETs usually involve the
brain and spinal cord, whereas peripheral PNET involve the
sympathetic nervous system, skeleton and soft tissues (2).
PNETs of the cervix are extremely rare. To the best
of our knowledge, only 14 cases have been described between 1987
and 2015 in the English literature (3–14) and
there are currently no universally accepted standard treatment
guidelines. Data on long-term follow-up are not available and the
clinical outcome of PNET patients remains elusive (4–6). The aim
of this study was to present two cases of primary PNET of the
cervix and describe the diagnostic and treatment procedures.
Case reports
Case 1. A 48-year-old woman, gravida 3, para 2,
presented with irregular uterine bleeding over the course of 1
year. On gynecological examination, a cervical mass rich in blood
vessels was identified, measuring 6.0 cm in its greatest dimension
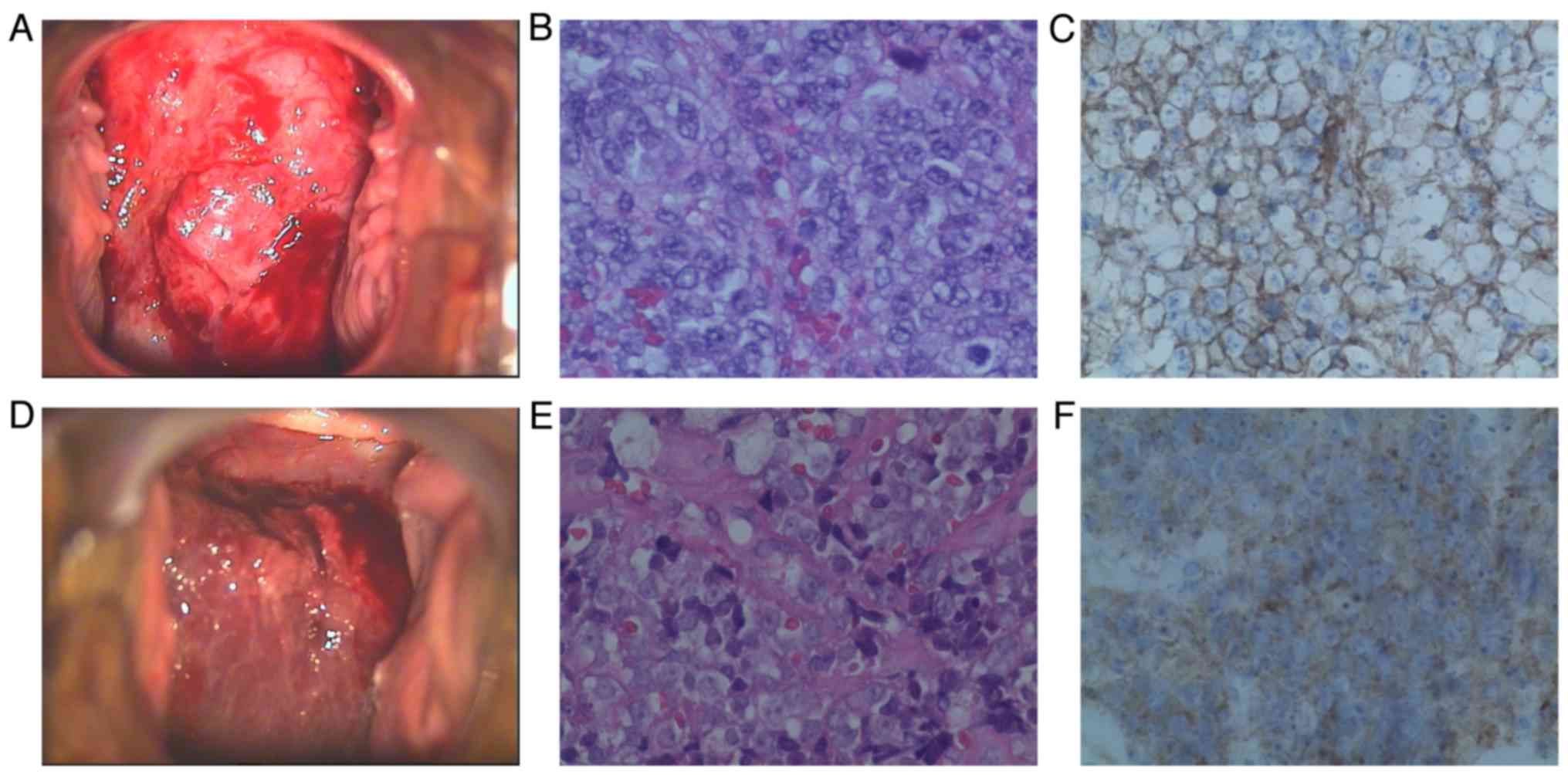
(Fig. 1). The left parametrium was
also involved, and the tumor was staged as IIb. A cervical biopsy
was performed and the initial diagnosis was small-cell
carcinoma.
Histologically, the tumor comprised small
blue-stained tumor cells with scant cytoplasm, arranged in dense
sheets, without rosette or gland formation. Areas of necrosis were
present. The neoplastic cells, some of which exhibited prominent
nucleoli, infiltrated several capillaries. The cells uniformly
expressed CD99 and vimentin, whereas synaptophysin (Syn), CD56,
S-100 and epithelial membrane antigen were focally positive.
Neuron-specific enolase (NSE), chromogranin A (CgA), cytokeratin
(CK), CK5, CK8/18, P16, leukocyte common antigen (LCA), desmin,
actin, myogenic differentiation 1 and Wilms tumor 1 were not
expressed. The Ki-67 labeling index was ~80%.
The laboratory tests evaluating hematological
parameters, electrolyte levels, and hepatic and renal functions,
were normal. The levels of tumor markers, including squamous cell
carcinoma antigen (SCC), human epididymis protein 4 (HE4),
carbohydrate antigen (CA)125, CA199, α-fetoprotein (AFP), and
β-human chorionic gonadotrophin (β-HCG) were within the normal
range. A positron emission tomography-computed tomography scan
showed a cervical tumor with abnormally increased metabolism and
hypermetabolic lymph nodes in the area of the left iliac
vessels.
After one course of induction chemotherapy
(pirarubicin + cisplatin + ifosfamide) and internal radiation
(iridium-192,17 Gy), the patient underwent radical hysterectomy
with bilateral salpingo-oophorectomy and bilateral pelvic
lymphadenectomy. The postoperative histopathological examination
revealed a tumor embolus in a vessel and involvement of 5 of the 28
lymph nodes. Five courses of consolidation chemotherapy with the
previous regimen were completed. The patient also received
postoperative radiotherapy with a dose of 200 cGy 5 days per week
for 4 weeks.
The patient is currently followed up in our
outpatient clinic every 3 months; at the 27-month follow-up, she
remained asymptomatic and clinically disease-free.
Case 2. A 43-year-old woman, gravida 2, para 2,
presented with urinary frequency for 1 month. On gynecological
examination, a mass was identified in the cervix, measuring 10.0 cm
in its greatest dimension, with left parametrial involvement
(Fig. 1). The tumor was staged as
IIb. A cervical biopsy was performed and the initial diagnosis was
small-cell carcinoma. The patient underwent radical hysterectomy
with bilateral salpingo-oophorectomy and lymph node dissection.
Histologically, the tumor consisted of small blue
tumor cells with scant cytoplasm arranged in dense sheets, without
rosette or gland formation. Areas of necrosis were present. The
neoplastic cells, some of which exhibited prominent nucleoli,
infiltrated several capillaries (Fig.
1). Some cells had indistinct cytoplasmic borders with
vesicular nuclei. Immunohistochemistry confirmed the diagnosis, as
the cells expressed the CD99 antigen and were negative for CgA,
NSE, Syn, LCA, CK, human melanoma black 45, melan-A, S-100 and P16.
The Ki-67 labeling index was ~90%.
The fasting blood glucose level of the patient was
11.37 mmol/l. The tumor markers were as follows: SCC 2.4 ng/ml
(normal, <1.5 ng/ml) and HE4 218.7 pmol/l (normal, <140
pmol/l). CA125, CA199, AFP and β-HCG were within the normal
range.
After one course of induction chemotherapy
(pirarubicin + cisplatin + ifosfamide) and internal radiation
(iridium-192, 24 Gy), the patient underwent radical hysterectomy
with bilateral salpingo-oophorectomy and bilateral pelvic
lymphadenectomy. An accessory ureter was found in the left pelvic
cavity. The postoperative histopathological examination revealed no
residual tumor. Due to financial difficulties, the patient received
only 1 course of consolidation chemotherapy with the previous
regimen, and postoperative radiotherapy was not completed.
The patient is currently followed up in our
outpatient clinic discontinuously; at the 12-month follow-up, she
remained asymptomatic and clinically disease-free.
Discussion
Peripheral PNET is a rare and aggressive malignancy
of the female genital tract. The ovaries are the most common and
the uterine corpus the second most common location of PNET
(15,16), followed by the cervix (8), vulva and vagina (17). Overall, 14 cases of PNET of the
uterine cervix have been reported in the English literature to
date, in patients aged 21–51 years (Table I). As previously reported, the main
presenting symptoms of cervical PNET are irregular vaginal
bleeding, lower abdominal distension and pain, uterine enlargement
and a mass increasing in size (16).
The incidence of PNET is difficult to ascertain, due to its
rarity.
 | Table I.Management of patients with PNET of
the cervix. |
Table I.
Management of patients with PNET of
the cervix.
|
| Age, | FIGO |
| Follow-up, | Refs. |
|---|
| Authors | years | stage | Treatment | months |
|
|---|
| Sato et
al | 44 | Ib2 | TAH + BSO + PL+
CT | AWD, 6 | (3) |
| Horn et
al | 26 | Ib1 | TAH + BSO + PL+ RT +
CT | DOD, 50 | (4) |
| Cenacchiet al | 36 | Ib2 | TAH | AWD, 18 | (5) |
| Pauwels et
al | 45 | Ib1 | TAH + RT | AWD, 42 | (6) |
| Tsao et
al | 24 | NA | CT + RH + CT +
RT | NA | (7) |
| Malpica et
al |
|
|
|
| (8) |
| Case 1 | 35 | Ib1 | TAH + BSO
+ PL+ CT | AWD, 5 |
|
| Case 2 | 50 | Ib2 | TAH + BSO + PL+
CT | AWD, 18 |
|
| Snijders-Keilholz
et al | 21 | Ib2 | CT + TAH + CT | AWD, 27 | (9) |
| Farzaneh et
al | 45 | Ib2 | CT + RH + PL +
CT | AWD, 48 | (10) |
| Masoura et
al | 23 | IVb | TAH + BSO + CT | DODa | (11) |
| Arora et
al | 23 | NA | CT + RH + PL+ RT | AWD, 36 | (12) |
| Li et al | 27 | IIIb | CT + RT | AWD, 6 | (13) |
| Xiao et
al |
|
|
|
| (14) |
| Case 1 | 52 | IIa | TAH + BSO + PL + CT +
CRS | DOD, 9 |
| Case 2 | 59 | IVb | TAH + BSO + PL +
partial small | DODb |
|
|
|
| intestinal
excision |
| Present |
| Case 1 | 48 | IIb | CT + brachytherapy +
RH + PL + CT + RT | AWD, 27 |
| Case 2 | 43 | IIb | CT + brachytherapy +
RH + PL + CT | AWD, 12 |
The diagnosis of PNET is difficult by routine
hematoxylin and eosin staining alone, due to the overlapping
clinical, imaging, histological and immunophenotypic
characteristics with other small round-cell tumors, such as primary
small-cell tumor, osteosarcoma, non-Hodgkin lymphoma, malignant
melanoma and metastases (18). The
diagnosis is based on a combination of morphological and
immunohistochemical characteristics and electron microscopy.
Rosettes are formed from the cytoplasmic extensions in several
cases. CD99, a specific immunohistochemical marker for the
diagnosis of PNET, is present in >97% of the cases (19). In the two cases presented in this
study, PNET was strongly expressed on the tumor cell membranes. It
has been reported that some tumor cells express vimentin and
focally NSE, CgA, Syn, and S-100 (20). Molecular genetic analysis may
identify chromosome translocations that help distinguish peripheral
PNETs from other round-cell tumors. Approximately 85% of peripheral
PNET cases have a balanced t(11;22)(q24;q12) translocation that
results in the formation of a chimerical fusion of the EWS-FLI1
gene (21).
A standard management protocol for this tumor is
currently unavailable (22). The
treatment strategies for PNET include surgical resection, adjuvant
chemotherapy and radiation therapy. Adjuvant chemotherapy was
considered to play an important part in PNET management, as the
tumors were associated with a 80–90% relapse rate with surgery
alone (23). Radiation therapy has
been typically used for patients with inoperable tumors and/or
positive surgical margins, and in those with a poor histological
response (24). The majority of the
chemotherapy regimens reported are based on trials with bone PNETs
(25). The commonly used
chemotherapeutic agents include cisplatin, vincristine,
doxorubicin, ifosfamide, cyclophosphamide, dactinomycin and VP-16,
but there is currently no consensus on the optimal chemotherapy
treatment. Among the previously reported cases, only 1 patient was
treated with chemotherapy and radiotherapy alone; 13 patients were
treated with hysterectomy, whereas bilateral salpingo-oophorectomy,
with or without pelvic lymphadenectomy, was also performed in a
proportion of those patients. Of the 13 patients, 7 were previously
reported to receive chemotherapy alone and 1 received radiotherapy
alone. In 3 patients, a combination of chemotherapy and
radiotherapy was administered postoperatively. In our cases, both
patients received neoadjuvant chemotherapy and radiation to reduce
tumor burden.
The most unfavorable prognostic factor is the
presence of distant metastasis at the time of diagnosis (26). Of the 14 cases reported, 2 stage IVb
patients succumbed to the disease soon after diagnosis. Other
unfavorable prognostic factors include late-stage disease,
insufficient surgical resection, large tumor size, central location
of the lesions (pelvis or spine) and poor response to chemotherapy
(26).
Overall, peripheral PNET is a rare finding,
particularly in the cervix, and requires early detection, correct
diagnosis and multimodal therapies, including total excision,
adjuvant chemotherapy and/or radiotherapy. The study of more cases
of primary peripheral PNET with longer follow-up periods is
required to facilitate the formulation of treatment protocols.
Acknowledgements
The authors wish to thank Editage (http://www.aje.com/) for the English language editing,
and the staff of the Tianjin Central Hospital of Gynecology and
Obstetrics, particularly the staff at the Department of
Gynecological Oncology.
References
|
1
|
Hart MN and Earle KM: Primitive
neuroectodermal tumors of the brain in children. Cancer.
32:890–897. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sato S, Yajima A, Kimura N, Namiki T,
Furuhashi N and Sakuma H: Peripheral neuroepithelioma (peripheral
primitive neuroectodermal tumor) of the uterine cervix. Tohoku J
Exp Med. 180:187–195. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Horn LC, Fischer U and Bilek K: Primitive
neuroectodermal tumor of the cervix uteri. A case report. Gen Diagn
Pathol. 142:227–230. 1997.PubMed/NCBI
|
|
5
|
Cenacchi G, Pasquinelli G, Montanaro L,
Cerasoli S, Vici M, Bisceglia M, Giangaspero F, Martinelli GN and
Derenzini M: Primary endocervical extraosseous Ewing's
sarcoma/PNET. Int J Gynecol Pathol. 17:83–88. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pauwels P, Ambros P, Hattinger C, Lammens
M, Dal Cin P, Ribot J, Struyk A and van den Berghe H: Peripheral
primitive neuroectodermal tumour of the cervix. Virchows Arch.
436:68–73. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsao AS, Roth LM, Sandler A and Hurteau
JA: Cervical primitive neuroectodermal tumor. Gynecol Oncol.
83:138–142. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Malpica A and Moran CA: Primitive
neuroectodermal tumor of the cervix: A clinicopathologic and
immunohistochemical study of two cases. Ann Diagn Pathol.
6:281–287. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Snijders-Keilholz A, Ewing P, Seynaeve C
and Burger CW: Primitive neuroectodermal tumor of the cervix uteri:
A case report-changing concepts in therapy. Gynecol Oncol.
98:516–519. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Farzaneh F, Rezvani H, Boroujeni PT and
Rahimi F: Primitive neuroectodermal tumor of the cervix: A case
report. J Med Case Reports. 5:4892011. View Article : Google Scholar
|
|
11
|
Masoura S, Kourtis A, Kalogiannidis I,
Kotoula V, Anagnostou E, Angelidou S and Agorastos T: Primary
primitive neuroectodermal tumor of the cervix confirmed with
molecular analysis in a 23-year-old woman: A case report. Pathol
Res Pract. 208:245–249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arora N, Kalra A, Kausar H, Ghosh TK and
Majumdar A: Primitive neuroectodermal tumour of uterine cervix - a
diagnostic and therapeutic dilemma. J Obstet Gynaecol. 32:711–713.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li B, Ouyang L, Han X, Zhou Y, Tong X,
Zhang S and Zhang Q: Primary primitive neuroectodermal tumor of the
cervix. Onco Targets Ther. 6:707–711. 2013.PubMed/NCBI
|
|
14
|
Xiao C, Zhao J, Guo P, Wang D, Zhao D, Ren
T, Yang J, Shen K, Lang J, Xiang Y, et al: Clinical analysis of
primary primitive neuroectodermal tumors in the female genital
tract. Int J Gynecol Cancer. 24:404–409. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kleinman GM, Young RH and Scully RE:
Primary neuroectodermal tumors of the ovary. A report of 25 cases.
Am J Surg Pathol. 17:764–778. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Euscher ED, Deavers MT, Lopez-Terrada D,
Lazar AJ, Silva EG and Malpica A: Uterine tumors with
neuroectodermal differentiation: A series of 17 cases and review of
the literature. Am J Surg Pathol. 32:219–228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McCluggage WG, Sumathi VP, Nucci MR,
Hirsch M, Dal Cin P, Wells M, Flanagan AM and Fisher C: Ewing
family of tumours involving the vulva and vagina: Report of a
series of four cases. J Clin Pathol. 60:674–680. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang J, Guo Q, Yang Y, Zhang J, Lang J and
Shi H: Primary vulvar Ewing sarcoma/primitive neuroectodermal
tumor: A report of one case and review of the literature. J Pediatr
Adolesc Gynecol. 25:e93–e97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vural C, Uluoğlu O, Akyürek N, Oğuz A and
Karadeniz C: The evaluation of CD99 immunoreactivity and EWS/FLI1
translocation by fluorescence in situ hybridization in central
PNETs and Ewing's sarcoma family of tumors. Pathol Oncol Res.
17:619–625. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dadhwal V, Bahadur A, Gupta R, Bansal S
and Mittal S: Peripheral neuroectodermal tumor of the vulva: A case
report. J Low Genit Tract Dis. 14:59–62. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cetiner H, Kir G, Gelmann EP and Ozdemirli
M: Primary vulvar Ewing sarcoma/primitive neuroectodermal tumor: A
report of 2 cases and review of the literature. Int J Gynecol
Cancer. 19:1131–1136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kimber C, Michalski A, Spitz L and Pierro
A: Primitive neuroectodermal tumours: Anatomic location, extent of
surgery, and outcome. J Pediatr Surg. 33:39–41. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bose P, Murugan P, Gillies E and Holter
JL: Extraosseous Ewing's sarcoma of the pancreas. Int J Clin Oncol.
17:399–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carvajal R and Meyers P: Ewing's sarcoma
and primitive neuroectodermal family of tumors. Hematol Oncol Clin
North Am. 19:501–525, vi-vii. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shah JP, Jelsema J, Bryant CS, Ali-Fehmi R
and Malone JM Jr: Carboplatin and paclitaxel adjuvant chemotherapy
in primitive neuroectodermal tumor of the uterine corpus. Am J
Obstet Gynecol. 200:e6–e9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iwamoto Y: Diagnosis and treatment of
Ewing's sarcoma. Jpn J Clin Oncol. 37:79–89. 2007. View Article : Google Scholar : PubMed/NCBI
|