Introduction
Despite great improvements in disease management
that have allowed more than half of the patients to reach
adulthood, cystic fibrosis (CF) remains a life-threatening
condition if major complications affect lung and gut function
(1,2). Diagnosis of cancer, in addition to CF,
is rare, and challenges multidisciplinary treatment algorithms in
terms of newly diagnosed patients and those with the pre-existing
condition. In children, malignancies are rare, and thus far, no
case of treatment of osteosarcoma in a pediatric CF patient has
been described. Osteoblastic osteosarcoma is the most frequently
occurring osseous malignancy in children and adolescents.
Typically, this tumor type is localized in the lower long bones
(75%), and less frequently in the upper long bones (11%) (3), and generally there is an improved
prognosis in childhood. However, the 6-year overall survival rate
has not improved during the last 40 years, remaining at a plateau
of ~70–80% (4). Aggressive and
long-lasting neoadjuvant and adjuvant antineoplastic chemotherapies
are essential, although these treatments are associated with
prolonged neutropenia and harbor the risk of bacterial infection
(5). The total duration of
chemotherapy usually exceeds 6 months on the condition that the
tumor response to neoadjuvant treatment was satisfactory. Grade 3–4
toxicity according to the World Health Organization (WHO) common
toxicity criteria (6) is quite
common: 83, 58, and 27% of patients experience neutropenia,
infectious complications, and mucositis, respectively. The
essential local treatment approach in osteosarcoma is total
compartment resection, ideally with limb salvage by endoprothesis
or autologous bone implantation instead of amputation. Although the
survival rate is similar with either approach, an improved function
is achievable with limb salvage (7).
However, local complications are more common in patients undergoing
limb salvage. In the present study, the case of a boy with CF who
was successfully treated for osteoblastic osteosarcoma is
described.
Case report
CF was suspected in the Guthrie blood spot screening
test from a male newborn, which revealed elevated levels of
immunoreactive trypsinogen and pancreatitis-associated protein,
followed by a positive sweat test. Diagnosis was confirmed by
molecular analysis, unraveling the homozygous carrier status of
mutation ΔF508. The boy's condition was maintained at an excellent
level under a treatment comprising secretolysis using inhalation
with hypertonic saline and acetylcysteine, salbutamol and
ipratropium bromide, paralelled with physiotherapy, pancreatic
enzyme replacement (PERT) and vitamin replacement therapies,
including pancreatic amylases, lipases, proteases, vitamins A, B1,
B2, B6, B12, C, D, E, folic acid, biotin, niacin and pantothenic
acid. Although the lungs had been permanently colonized with
Staphylococcus aureus since the boy was one year old,
antibiotic prophylaxis was performed as maintenance therapy for
only three years. Regular lung function tests did not reveal any
evidence of obstructive or restrictive ventilation disorder
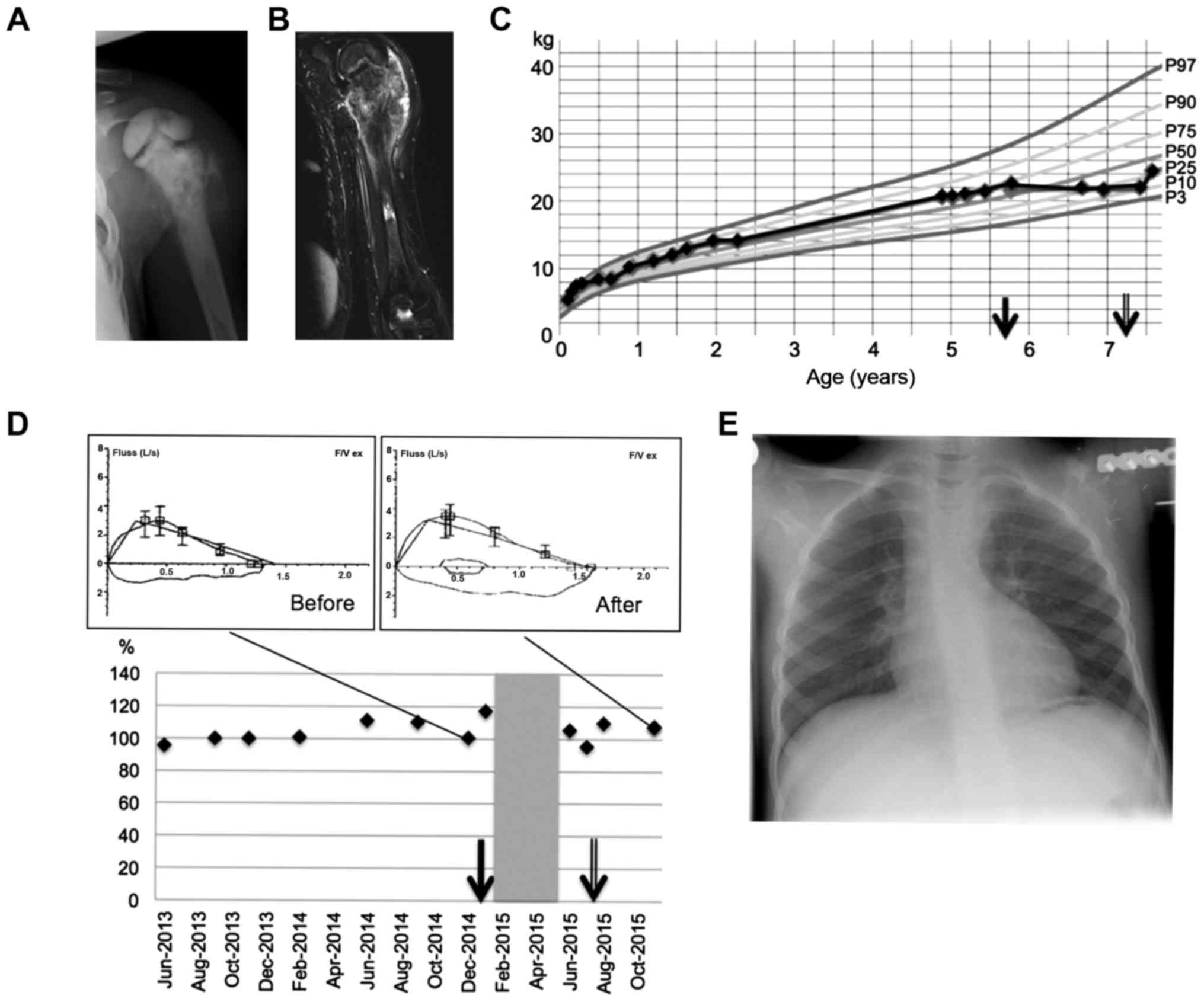
(Fig. 1). A chest X-ray revealed no
pathological findings. Therefore, hospitalization was never
required while the patient presented at the specialized ambulance
for CF in the children's hospital (University Hospital ‘Carl Gustav
Carus’) as required, or every 3 months for a regular follow-up. At
the age of 5 years, following a minimal trauma, an indolent
swelling of the left shoulder occurred without impaired motility.
No further complaints were reported. Conventional X-radiography
revealed an irregularly shaped calcified mass at the proximal
humerus, suggestive of osteosarcoma. Magnetic resonance imaging
(MRI) findings supported this suspicion (Fig. 1). No metastatic disease was detected.
Tumor biopsy confirmed a diagnosis of highly malignant osteoblastic
osteosarcoma. Prior to treatment, antibiotic prophylaxis with
cefuroxime (20 mg/kg body weight per day, twice daily) was
initiated to avoid pulmonary infections. Neoadjuvant chemotherapy
(the EURAMOS-1 trial) (8) was
initiated, featuring the MAP regimen [adriamycin, 75
mg/m2 (A), cisplatin, 120 mg/m2 (P) and
methotrexate, 12 g/m2 (M)]. Surgery was scheduled
following two cycles of MAP, i.e., 10 weeks following the start of
the chemotherapy (9). The first
febrile episode of unknown origin (FUO) during neutropenia occurred
at the ninth week. The patient was treated initially with
piperacillin (200 mg/kg body weight per day, thrice daily) and
tazobactam (25 mg/kg body weight per day, thrice daily), and
subsequently after 48 h with meropenem (60 mg/kg body weight per
day, thrice daily) and teicoplanin (on the first day, 20 mg/kg body
weight per day qd; thereafter 10 mg/kg body weight per day qd),
resulting in defervescence. Neoadjuvant chemotherapy was delayed
for one week, and completed thereafter. MRI imaging revealed
satisfactory tumor regress, therefore making a limb salvage
surgical approach possible. Compartment resection of the proximal
humerus was performed, and replacement of the humerus was performed
by the left clavicula method (termed the ‘clavicula pro humero’
method, after Winkelmann) (10).
Tumor histology following resection revealed a good response (grade
3, according to Salzer-Kuntschik) (11) to neoadjuvant treatment. The
postoperative course was complicated by compartmental syndrome 2
days after surgery. Fasciotomy was performed, followed by a
stepwise wound closure. This complication caused a transient
deficiency of sensory and motoric function of the left forearm and
hand, which were markedly improved following intensive
physiotherapy. At 2 weeks postoperatively, the patient experienced
2 days of FUO, which was resolved promptly by antibiotic treatment.
As adjuvant treatment, the patient received four cycles of MAP
regimen. Up to week 27, the patient tolerated this chemotherapy
without delay. In week 28, the patient experienced fever again, and
antibiotics (piperacillin and tazobactam; dosages as detailed
above) were administered for 5 days. Treatment was concluded at
week 29 without further complications. The follow-up thus far is 19
months, in ongoing complete remission. The mobility of the elbow
and wrist remain satisfactory.
Discussion
While the occurrence of osteosarcoma at a young age,
as for our patient, is very uncommon (it typically occurs at
puberty), the coincidence of CF and malignancy is an extremely rare
event in childhood. The worldwide incidence in Caucasians of
osteosarcoma is 4.4:1,000,000 at an age of 0–22 years (3) while the incidence of CF is 1:2,500
(12). Therefore, the coincidence of
CF and osteosarcoma can be calculated to be as low as
1.76×10−9. The case presented here is, to the best of
our knowledge, the first case of a pediatric patient with CF
challenged by osteoblastic osteosarcoma. As cancers occur
predominantly at an older age, experience of how chemotherapy is
tolerated by CF patients is rather limited. However, colorectal or
pancreatic carcinoma represent exceptions that are observed as
comorbidity of CF in early adulthood. This coincidence may be
attributed to chronic inflammation in CF (13–16), and
the frequency increases, particularly following lung
transplantation due to immunosuppression. Previous reports
(17,18) have emphasized that a high caloric
fatty-based food intake, including by means of percutaneous
endoscopic gastrostomy, becomes very important in cancer therapy
once mucositis hinders oral food intake. In CF patients with
malignancies, this represents a major challenge, as the recommended
additional energy intake in CF itself compared with healthy
controls should be increased by 100 kcal/kg daily. However, no
detailed guidelines exist to date. Under continuous PERT, the
patient in the present case report thrived well, until
antineoplastic chemotherapy was started (Fig. 1). However, the
chemotherapy-associated loss of weight issue was resolved quickly
following the end of treatment. Longitudinal growth remained
unimpaired. Pulmonary infection during neutropenia also represents
a major challenge in oncology. Younger CF patients with only
little, or no, pre-damaged lung tissue have an improved initial
constitution. Our patient had no permanent airway colonization with
Pseudomonas. As the patient's lungs had been colonized
intermittently with typical bacteria, such as S. aureus,
Haemophilus influenza, Serratia marcescens, and
Escherichia coli, since he was 1 year old, cefuroxime was
administered during the neutropenic phases. No pulmonary
complications were observed, and the patient's lung function
remained stable and unchanged during the chemotherapy. The present
authors therefore conclude that this prophylaxis worked
successfully, although no general recommendations may be deduced
from this single case. Immobility following surgery hindered the
physiotherapy required daily for respiratory hygiene. In the
opinion of the present authors, taking this risk was considered to
have been justified, when balancing temporary immobility caused by
surgical procedures with avoiding limb amputation. Overall, our
patient tolerated treatment for osteosarcoma well, experiencing
only two FUO episodes during neutropenia. The present case is
intriguing, not only due to the rare coincidence of CF with
osteosarcoma in young childhood, but also since antineoplastic
treatment was manageable without exacerbation of CF. In conclusion,
it may be speculated that chemotherapy against osteosarcoma may be
applied in pediatric patients with CF, with the caveat that defined
precautions are followed.
References
|
1
|
Welsh MJ and Smith AE: Molecular
mechanisms of CFTR chloride channel dysfunction in cystic fibrosis.
Cell. 73:1251–1254. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dodge JA: A millennial view of cystic
fibrosis. Dev Period Med. 19:9–13. 2015.PubMed/NCBI
|
|
3
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mueller EL, Walkovich KJ, Mody R,
Gebremariam A and Davis MM: Hospital discharges for fever and
neutropenia in pediatric cancer patients: United States, 2009. BMC
Cancer. 15:3882015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Trotti A, Byhardt R, Stetz J, Gwede C,
Corn B, Fu K, Gunderson L, McCormick B, Morrisintegral M, Rich T,
et al: Common toxicity criteria: Version 2.0. an improved reference
for grading the acute effects of cancer treatment: Impact on
radiotherapy. Int J Radiat Oncol Biol Phys. 47:13–47. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mavrogenis AF, Abati CN, Romagnoli C and
Ruggieri P: Similar survival but better function for patients after
limb salvage versus amputation for distal tibia osteosarcoma. Clin
Orthop. 470:1735–1748. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Whelan JS, Bielack SS, Marina N, Smeland
S, Jovic G, Hook JM, Krailo M, Anninga J, Butterfass-Bahloul T,
Böhling T, et al: EURAMOS-1, an international randomised study for
osteosarcoma: Results from pre-randomisation treatment. Ann Oncol.
26:407–414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bielack SS, Smeland S, Whelan JS, Marina
N, Jovic G, Hook JM, Krailo MD, Gebhardt M, Pápai Z, Meyer J, et
al: Methotrexate, doxorubicin and cisplatin (MAP) plus maintenance
pegylated interferon alfa-2b versus MAP alone in patients with
resectable high-grade osteosarcoma and good histologic response to
preoperative MAP: First results of the EURAMOS-1 good response
randomized controlled trial. J Clin Oncol. 33:2279–2287. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Winkelmann WW: Clavicula pro humero-a new
surgical method for malignant tumors of the proximal humerus. Z
Orthop Ihre Grenzgeb. 130:197–201. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salzer-Kuntschik M, Delling G, Beron G and
Sigmund R: Morphological grades of regression in osteosarcoma after
polychemotherapy - study COSS 80. J Cancer Res Clin Oncol. 106
Suppl:S21–S24. 1983. View Article : Google Scholar
|
|
12
|
Scotet V, Duguépéroux I, Saliou P, Rault
G, Roussey M, Audrézet MP and Férec C: Evidence for decline in the
incidence of cystic fibrosis: A 35-year observational study in
Brittany, France. Orphanet J Rare Dis. 7:142012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Demeyer S, De Boeck K, Witters P and
Cosaert K: Beyond pancreatic insufficiency and liver disease in
cystic fibrosis. Eur J Pediatr. 175:881–894. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fink AK, Yanik EL, Marshall BC,
Wilschanski M, Lynch CF, Austin AA, Copeland G, Safaeian M and
Engels EA: Cancer risk among lung transplant recipients with cystic
fibrosis. J Cyst Fibros. 16:91–97. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maisonneuve P, Marshall BC and Lowenfels
AB: Risk of pancreatic cancer in patients with cystic fibrosis.
Gut. 56:1327–1328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Neglia JP, Wielinski CL and Warwick WJ:
Cancer risk among patients with cystic fibrosis. J Pediatr.
119:764–766. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yen EH, Quinton H and Borowitz D: Better
nutritional status in early childhood is associated with improved
clinical outcomes and survival in patients with cystic fibrosis. J
Pediatr. 162:530–535.e1. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Steinkamp G and von der Hardt H:
Improvement of nutritional status and lung function after long-term
nocturnal gastrostomy feedings in cystic fibrosis. J Pediatr.
124:244–249. 1994. View Article : Google Scholar : PubMed/NCBI
|