Introduction
Molecularly targeted agents for cancer have followed
the same clinical development process as cytotoxic agents,
targeting tumor location and histology (1–3). The
majority of molecular alterations in tumor genetics exist across
different tumor types and histologies, although the incidence
varies (4). This observation
challenges existing drug development strategies for molecularly
targeted agents and raises the possibility of a shift towards
histology-agnostic molecularly-based treatment (5). Of these targets, the
phosphatidylinositide-3-kinase (PIK3CA) pathway is essential in the
metabolism, proliferation and apoptosis of cells (6). Relatively common mutations of the
PIK3CA associated pathway are 4 hotspots: H1047R, E542 K, E545K and
phosphatase and tensin homolog (PTEN) loss (7). The phosphoinositide 3-kinase (PI3K)-v
Akt murine thymoma viral oncogene homolog (AKT)-mechanistic target
of rapamycin (mTOR) signaling cascade is one of the most important
intracellular pathways to be frequently activated in diverse cancer
types (8,9). The association between the activation
of the PI3K-AKT-mTOR pathway and tumorigenesis and cancer
progression in numerous types of tumors is well established, and
may contribute to acquire resistance to various anti-neoplastic
agents (8,10). Activation of the PIK3CA pathway
increases the signaling of the AKT/mTOR pathway and stimulates
proliferation of the cell (11–13).
mTOR inhibitors interrupts translation and
expression of major proteins that regulate gene expression, cell
cycle and angiogenesis (14). In
vitro, these agents inhibit growth and proliferation of tumor
cells including cancer of the ovary, breast, lung, prostate and
kidney (15). Of the first
generation mTOR inhibitors, sirolimus has been approved by the Food
and Drug Administration as an agent that prevent rejection of
kidney transplantation (16,17). Certain previous studies compared the
effects of sirolimus vs. placebo in a mouse model of colon cancer
with PIK3CA mutation (17,18). A marked difference in the ratio of
change from baseline volume in PET-CT was identified between
sirolimus and placebo (−83.1 vs. +96%, respectively) (18,19).
Although several chemotherapeutic agents have been
developed, their effects on refractory cancer are limited and
clinical benefits are only obtained in specific
patient-subpopulations (20–22). Therefore, specific biomarkers for
various patient subpopulations are required to identify the
patients who would receive the most benefit from sirolimus
treatment. Several previous studies have suggested that PIK3CA
genomic aberrations may be strong predictors of efficacy of mTOR
inhibitors (11,23). Therefore, the advancement of targeted
agents against specific pathways associated with cancer-progression
are required for patients with refractory cancer. Thus, the present
study assessed the efficacy and safety of sirolimus in patients
with refractory cancer with PIK3CA mutation/amplification.
Materials and methods
Eligibility
The present study was an open-labeled, single arm,
prospective single-center clinical trial to evaluate efficacy and
safety of sirolimus in patients with refractory cancer with PIK3CA
mutation/amplification. Patients were enrolled between October 2014
and April 2015 at Samsung Medical Center (Seoul, Korea). Patients
were eligible if they had a histologically confirmed refractory
solid cancer with PIK3CA mutation/amplification. The inclusion
criteria were: An age between 18 and 75 years, ≥1 measurable
lesion, Eastern Cooperative Oncology Group performance status of
0–2 and a life expectancy ≥3 months. Adequate hematologic function
[absolute neutrophil count (ANC) ≥1,500 mm3 (normal
range, 1,500–7,500 mm3), platelet count ≥75,000
mm3 (normal range, 150,000–450,000 mm3)],
hepatic function [aspartate aminotransferase/alanine
aminotransferase ≤3.0 times the upper normal limit (UNL), bilirubin
≤1.5 times the UNL], and renal function (serum creatinine ≤1.5
times the UNL) were also required. Patients receiving sirolimus as
a prior treatment were excluded. All patients provided written
informed consent according to the guidelines provided by the
institutional review board and all procedures were carried out
according to guidelines from the Declaration of Helsinki. The
Institutional Review Board at Samsung Medical Center approved the
protocol.
Study design and objectives
PIK3CA amplification/mutation was detected by
targeted deep sequencing by CancerSCAN™ (24). Briefly, extracted genomic DNA was
sheared to 150–200 bp fragments using Covaris S220 (Covaris,
Woburn, MA, USA) and targeted genes were captured using a custom
panel capture library (Agilent Technologies, Inc., Santa Clara, CA,
USA) for 2.5 Mb of exonic regions with an Illumina Paired-End
Sequencing Library kit. DNA sequencing of 100 or 101-bp paired-end
reads was performed using the Illumina HiSeq 2,500 sequencer
(Illumina, Inc., San Diego, CA, USA). There is no established dose
of sirolimus, although several studies reported effective oral
daily doses of 0.5–10 mg (25,26). The
dose and schedule of sirolimus based was determined based on
previous phase I studies (27,28).
Sirolimus was administered orally at a daily dose of 1 mg
continuously (28 day cycles). Treatment was continued from day 1,
and was terminated due to progression of the disease, unacceptable
toxicity or the patient's request. Thereafter, the patients were
followed up. Toxicity was assessed each cycle using the National
Cancer Institute Common Terminology Criteria for Adverse Events,
version 4.0 (29). Patients
underwent radiological evaluation every 4 weeks and clinical tumor
response was assessed using the Response Evaluation Criteria in
Solid Tumors (RECIST version 1.1) (30).
The primary objective of this study was the
progression free survival (PFS). The second objectives were
evaluated overall survival (OS), overall response rate, disease
control rate (DCR) and safety. Following an analysis of the
feasibility of sirolimus for refractory cancer patients with PIK3CA
mutation/amplification in this pilot study, a further phase II
trial will be considered.
Statistical analysis
Patient characteristics were presented using
descriptive statistics. PFS was calculated from the first day of
treatment to the date on which progressive disease was first
observed or on the day of the last follow-up. OS was calculated
from the first day of treatment to the date of mortality or last
follow-up. PFS and OS were estimated using the Kaplan-Meier method.
Response rate was calculated as the ratio of the number of patients
who achieved a complete response or partial response (PR) to the
number of assessable patients. DCR was defined as ratio of patients
achieved more than stable disease at 8 weeks. Statistical data were
analyzed using SPSS version 18 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
This study enrolled 4 patients who met the inclusion
criteria. The baseline characteristics of patients are shown in
Table I. The median age of the
patients was 53.3 years (range, 49–61), and all the patients were
male. Three patients were diagnosed with advanced gastric cancer
and one patient had hilar cholangiocarcinoma. The median time from
initial diagnosis was 16.0 months (range, 14.5–19.5). All patients
received more than second- line chemotherapy. According to the
results of next generation sequencing, one patient had an E542K
mutation and the remaining patients had an E545K mutation.
 | Table I.Baseline patient characteristics. |
Table I.
Baseline patient characteristics.
| Variables | Patient no. 1 | Patient no. 2 | Patient no. 3 | Patient no. 4 |
|---|
| Age | 51 | 49 | 61 | 56 |
| Sex | Male | Male | Male | Male |
| ECOG PS | 1 | 1 | 1 | 1 |
| Primary cancer | AGC | AGC | Hilar CCC | AGC |
| Metastatic site | Lymph node | Adrenal gland | Liver Lung | Liver |
|
| Bone | Peritoneal
seeding | Peritoneal
seeding |
|
|
|
|
| Pleural
seeding |
|
| Prior
chemotherapy |
|
|
|
|
| 1st
line | FOLFOX +
Onartuzumab | FOLFIRI | CCRT with 5FU | XELOX |
| 2nd
line | Paclitaxel | Docetaxel | GP | Paclitaxel |
| 3rd
line | – | – | XP | FOLFIRI |
| PIK3CA
mutation | E545K mutation | E542K mutation | E545K mutation | E545K mutation |
|
| PTEN loss >90%
of tumor cells | PTEN loss in 100%
of tumor cells | No PTEN loss | PTEN loss in 100%
of tumor cells |
Efficacy and treatment response
A median of 2.5 cycles of sirolimus was
administered. There was no patient with more than a PR. Three
patients had stable disease after one cycle of sirolimus. Patient
no. 1 had progressive disease following one cycle of sirolimus.
Patients nos. 2 and 3 experienced disease progression following the
second cycle of sirolimus and patient no. 4 had disease progression
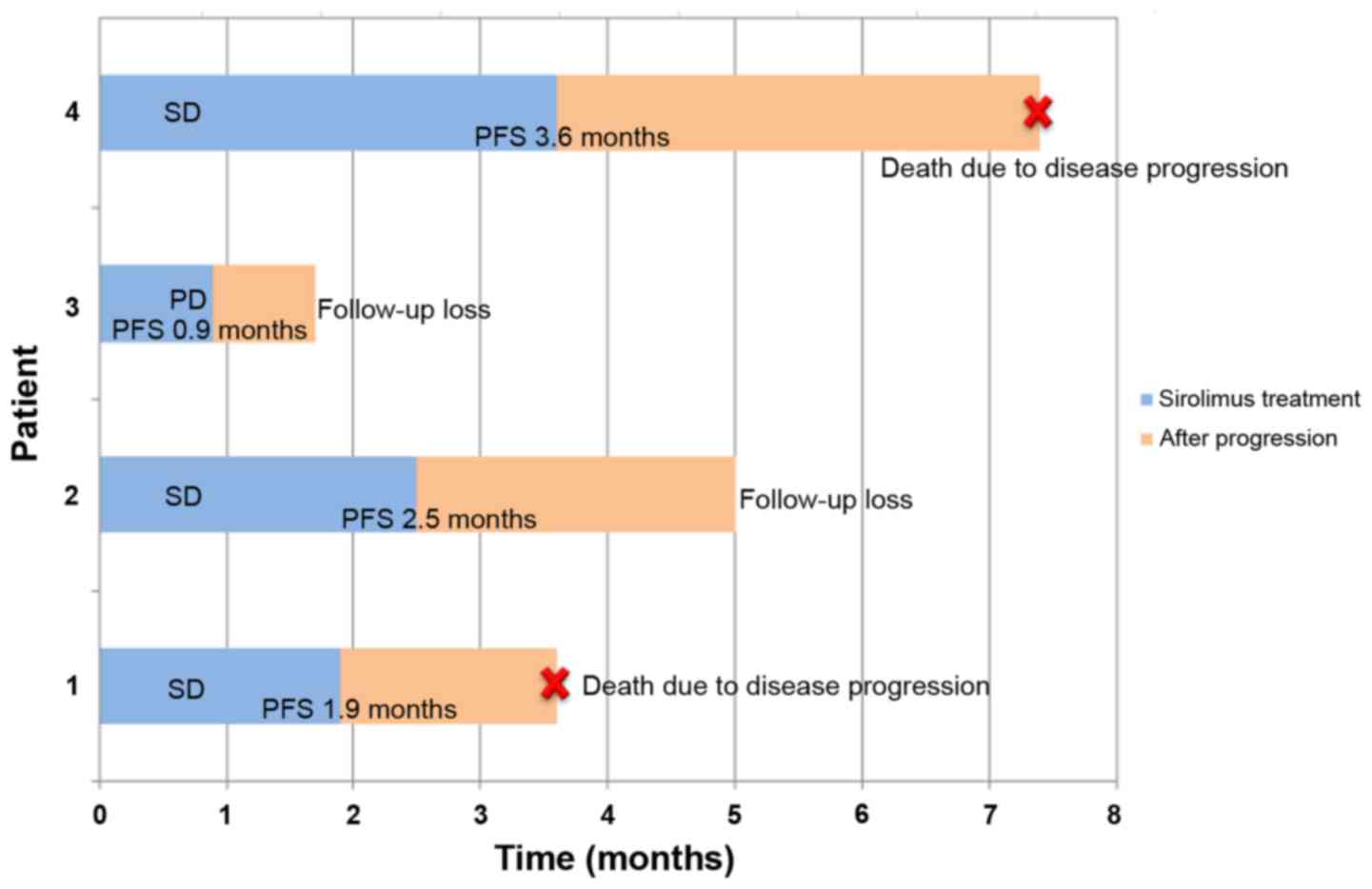
following the 4th cycle. The DCR at 8 weeks was 25%. The clinical
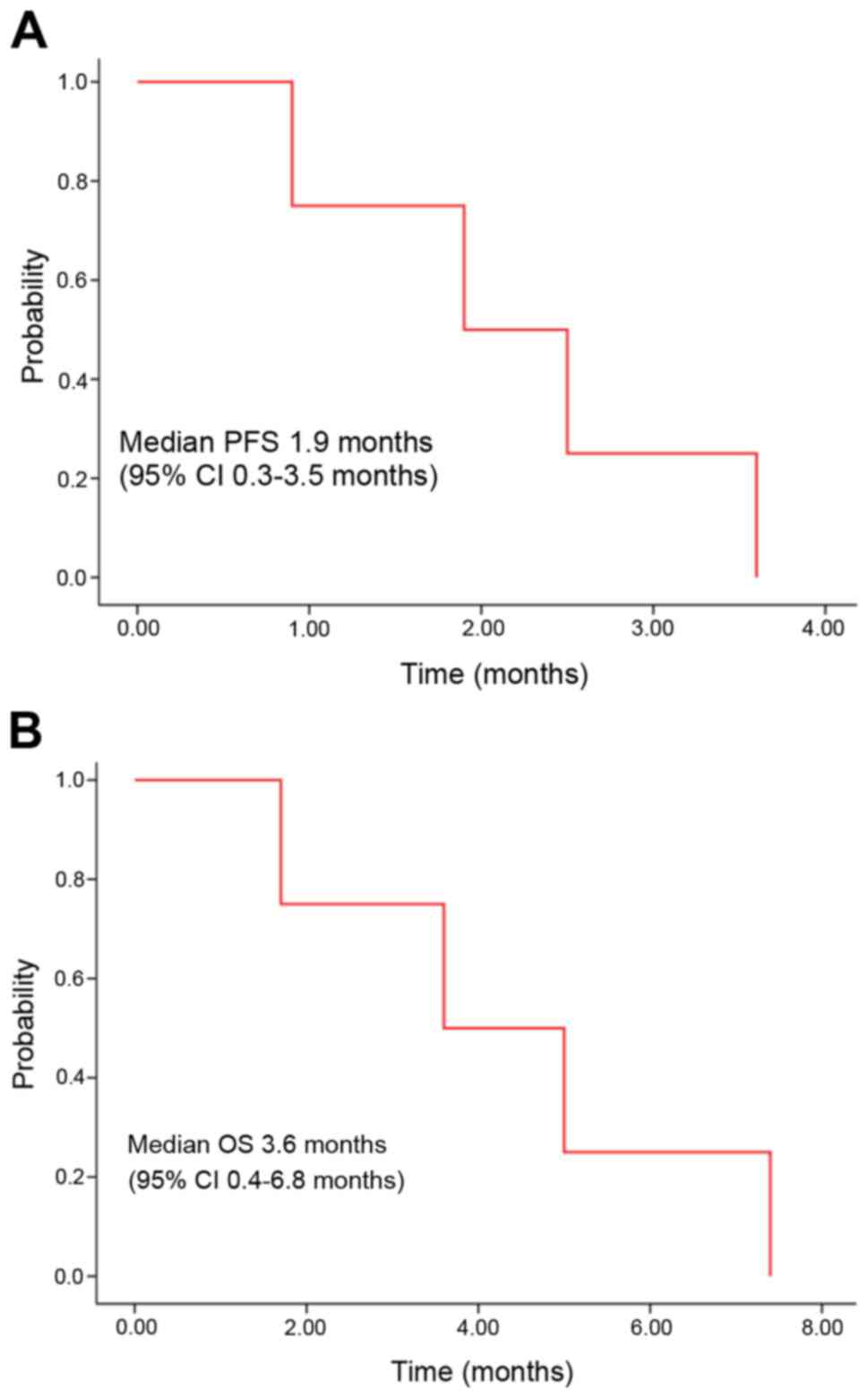
course of the 4 patients is presented in Fig. 1. The median PFS was 1.9 months [95%
confidence interval (CI); 0.3–3.5 months; Fig. 2A], and the median OS was 3.6 months
(95% CI, 0.4–6.8 months; Fig.
2B).
Safety
Dose reduction or treatment delay was not required
in the enrolled patients. Grade 3 or greater
hematologic/non-hematologic toxicity was not observed. Grade 1
nausea was reported in one patient. There were no
treatment-associated mortalities.
Discussion
PIK3CA is a key down-stream protein kinase of the
PI3K-AKT signaling pathway, and sirolimus is a novel macrolide
derivative of rapamycin that inhibits mTOR, thereby preventing
phosphorylation of its downstream molecules. In the present study,
the anti-tumor activity of sirolimus was investigated in solid
tumors with a specific genotypes concerning PIK3CA
amplification/mutation. In the current study, sirolimus had modest
clinical benefits and a tolerable toxicity-profile in patients with
refractory cancer with PIK3CA mutation/amplification.
In the present study, 3 of 4 patients had metastatic
gastric cancer. In the second-line chemotherapy setting, numerous
phase III clinical trials of targeted agents for advanced gastric
cancer are ongoing presently (31,32). Of
several studies, one phase III trial with an mTOR inhibitor was the
GRANITE-1 study (33). In this
previous study, the efficacy of everolimus, an oral mTOR
serine/threonine kinase inhibitor, was compared with the efficacy
of best supportive care only. The median PFS was 1.7 months with
everolimus vs. 1.4 months with placebo (hazard ratio, 0.66; 95% CI,
0.56–0.78; P<0.0001) (25). PFS
in 3 gastric cancer patients of the current study was 1.9, 2.5 and
3.6 months. These patients received sirolimus as a third or fourth
line therapy. Sirolimus may be a potential option to consider for
heavily pretreated patients with gastric cancer.
There have been several studies concerning mTOR
inhibitors in patients with a PIK3CA mutation (12). PIK3CA mutation has been reported in a
number of cancer types, including malignancies of the colon,
breast, liver and ovary (11), with
the most common mutations being E545 K, E542K in exon 9 and H1047R
in exon 20 (7). According to certain
studies, patients with a PIK3CA mutation and/or PTEN aberrations
have a greater response to mTOR inhibitors compared with patients
with wild type tumors, and in particular, H1047R mutation was
associated with a high response to mTOR inhibitors (11,12). On
the other hand, certain studies reported that PTEN loss was
associated with reduced sensitivity to mTOR inhibitors due to Akt
activation, limiting the effect of mTOR inhibition (34,35). In
the present study, the genotype of PIK3CA mutations was E545K in 3
patients and E542K in 1 patient, and there was no H1047R mutation.
In addition, three gastric cancer patients revealed PTEN loss in
the immunohistochemistry (IHC).
Although tumors had a PIK3CA mutation or PTEN loss,
there are a number of factors that may underlie differing responses
to mTOR inhibitors. Varying tumor types may have different
subgroups of PIK3CA mutation and co-existing mutations (36). In colorectal cancer, PIK3CA exon 9
mutations are associated with K-ras mutation but exon 20 mutations
are not associated with K-ras mutation (37,38). Due
to concomitant MARK mutations, these subgroups do not respond well
to mTOR inhibitor therapy (37,38). In
another case series, patients with PIK3CA mutation combined with
pS6 over expression were associated with a good response to mTOR
inhibitors and long duration of disease control (39). Therefore, clinical features and
co-existing mutations alongside PIK3CA mutation, dependent on the
cancer type, require further investigation to elucidate the
applications of mTOR inhibitors. Although the present study used
biomarker-driven patient selection, PIK3CA mutation/amplification
and/or PTEN loss were not sufficient for predicting the anti-tumor
activity of sirolimus. Thus, a more comprehensive molecular
analysis is required to fully realize the potential of personalized
medicine using mTOR inhibitors including sirolimus.
In conclusion, as the present research was a pilot
study, the sample size was small and the patient population was
heterogeneous. Nevertheless, sirolimus had modest efficacy and a
tolerable toxicity profile in patients with refractory cancer with
PIK3CA mutation/amplification. These findings support the premise
of further investigations. Therefore, a phase II clinical trial of
sirolimus in patients with refractory cancer with PIK3CA
mutation/amplification is currently being conducted.
References
|
1
|
Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó
L, Ahn MJ, De Pas T, Besse B, Solomon BJ, Blackhall F, et al:
Crizotinib versus chemotherapy in advanced ALK-positive lung
cancer. N Engl J Med. 368:2385–2394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sosman JA, Kim KB, Schuchter L, Gonzalez
R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ,
Flaherty KT, et al: Survival in BRAF V600-mutant advanced melanoma
treated with vemurafenib. N Engl J Med. 366:707–714. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sekulic A, Migden MR, Oro AE, Dirix L,
Lewis KD, Hainsworth JD, Solomon JA, Yoo S, Arron ST, Friedlander
PA, et al: Efficacy and safety of vismodegib in advanced basal-cell
carcinoma. N Engl J Med. 366:2171–2179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ciriello G, Miller ML, Aksoy BA,
Senbabaoglu Y, Schultz N and Sander C: Emerging landscape of
oncogenic signatures across human cancers. Nat Genet. 45:1127–1133.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karakas B, Bachman KE and Park BH:
Mutation of the PIK3CA oncogene in human cancers. Br J Cancer.
94:455–459. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burris HA III: Overcoming acquired
resistance to anticancer therapy: Focus on the PI3K/AKT/mTOR
pathway. Cancer Chemother Pharmacol. 71:829–842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Janku F, Hong DS, Fu S, Piha-Paul SA,
Naing A, Falchook GS, Tsimberidou AM, Stepanek VM, Moulder SL, Lee
JJ, et al: Assessing PIK3CA and PTEN in early-phase trials with
PI3K/AKT/mTOR inhibitors. Cell Rep. 6:377–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Janku F, Wheler JJ, Naing A, Stepanek VM,
Falchook GS, Fu S, Garrido-Laguna I, Tsimberidou AM, Piha-Paul SA,
Moulder SL, et al: PIK3CA mutations in advanced cancers:
Characteristics and outcomes. Oncotarget. 3:1566–1575. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zaytseva YY, Valentino JD, Gulhati P and
Evers BM: mTOR inhibitors in cancer therapy. Cancer Lett. 319:1–7.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aoki M, Blazek E and Vogt PK: A role of
the kinase mTOR in cellular transformation induced by the
oncoproteins P3k and Akt. Proc Natl Acad Sci USA. 98:pp. 136–141.
2001; View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guba M, von Breitenbuch P, Steinbauer M,
Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S,
Anthuber M, et al: Rapamycin inhibits primary and metastatic tumor
growth by antiangiogenesis: Involvement of vascular endothelial
growth factor. Nat Med. 8:128–135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dupont P and Warrens AN: The evolving role
of sirolimus in renal transplantation. QJM. 96:401–409. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leystra AA, Deming DA, Zahm CD, Farhoud M,
Olson TJ, Hadac JN, Nettekoven LA, Albrecht DM, Clipson L, Sullivan
R, et al: Mice expressing activated PI3K rapidly develop advanced
colon cancer. Cancer Res. 72:2931–2936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deming DA, Leystra AA, Farhoud M,
Nettekoven L, Clipson L, Albrecht D, Washington MK, Sullivan R,
Weichert JP and Halberg RB: mTOR inhibition elicits a dramatic
response in PI3K-dependent colon cancers. PLoS One. 8:e607092013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park HS, Lim SM, Kim S, Kim S, Kim HR,
Kwack K, Lee MG, Kim JH and Moon YW: Pilot study of a
next-generation sequencing-based targeted anticancer therapy in
refractory solid tumors at a Korean Institution. PLoS One.
11:e01541332016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
von Hoff DD, Stephenson JJ Jr, Rosen P,
Loesch DM, Borad MJ, Anthony S, Jameson G, Brown S, Cantafio N,
Richards DA, et al: Pilot study using molecular profiling of
patients' tumors to find potential targets and select treatments
for their refractory cancers. J Clin Oncol. 28:4877–4883. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsimberidou AM, Iskander NG, Hong DS,
Wheler JJ, Falchook GS, Fu S, Piha-Paul S, Naing A, Janku F, Luthra
R, et al: Personalized Medicine in a phase i clinical trials
program: The MD Anderson cancer center initiative. Clin Cancer Res.
18:6373–6383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
PIK3CA mutations predict response to
PI3K/AKT/mTOR inhibitors. Cancer Discovery. 2:205. 2012.
|
|
24
|
Kim ST, Lee J, Park SH, Park JO, Park YS,
Kang WK and Lim HY: Prospective phase II trial of everolimus in
PIK3CA amplification/mutation and/or PTEN loss patients with
advanced solid tumors refractory to standard therapy. BMC Cancer.
17:2112017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rizell M, Andersson M, Cahlin C, Hafström
L, Olausson M and Lindnér P: Effects of the mTOR inhibitor
sirolimus in patients with hepatocellular and cholangiocellular
cancer. Int J Clin Oncol. 13:66–70. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reardon DA, Quinn JA, Vredenburgh JJ,
Gururangan S, Friedman AH, Desjardins A, Sathornsumetee S, Herndon
JE II, Dowell JM, McLendon RE, et al: Phase 1 trial of gefitinib
plus sirolimus in adults with recurrent malignant glioma. Clin
Cancer Res. 12:860–868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martin-Liberal J, Gil-Martín M,
Sáinz-Jaspeado M, Gonzalo N, Rigo R, Colom H, Muñoz C, Tirado OM
and García del Muro X: Phase I study and preclinical efficacy
evaluation of the mTOR inhibitor sirolimus plus gemcitabine in
patients with advanced solid tumours. Br J Cancer. 111:858–865.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Desar IM, Timmer-Bonte JN, Burger DM, van
der Graaf WT and van Herpen CM: A phase I dose-escalation study to
evaluate safety and tolerability of sorafenib combined with
sirolimus in patients with advanced solid cancer. Br J Cancer.
103:1637–1643. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dueck AC, Mendoza TR, Mitchell SA, Reeve
BB, Castro KM, Rogak LJ, Atkinson TM, Bennett AV, Denicoff AM,
O'Mara AM, et al: Validity and reliability of the US national
cancer institute's patient-reported outcomes version of the common
terminology criteria for adverse Events (PRO-CTCAE). JAMA Oncol.
1:1051–1059. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matsuoka T and Yashiro M: The role of
PI3K/Akt/mTOR signaling in gastric carcinoma. Cancers. 6:1441–1463.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu L, Wu N and Li J: Novel targeted
agents for gastric cancer. J Hematol Oncol. 5:312012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung
HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K, et al: Everolimus
for previously treated advanced gastric cancer: Results of the
randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol.
31:3935–3943. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Seront E, Pinto A, Bouzin C, Bertrand L,
Machiels JP and Feron O: PTEN deficiency is associated with reduced
sensitivity to mTOR inhibitor in human bladder cancer through the
unhampered feedback loop driving PI3K/Akt activation. Br J Cancer.
109:1586–1592. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Weigelt B, Warne PH and Downward J: PIK3CA
mutation, but not PTEN loss of function, determines the sensitivity
of breast cancer cells to mTOR inhibitory drugs. Oncogene.
30:3222–3233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chaft JE, Arcila ME, Paik PK, Lau C, Riely
GJ, Pietanza MC, Zakowski MF, Rusch V, Sima CS, Ladanyi M and Kris
MG: Coexistence of PIK3CA and other oncogene mutations in lung
adenocarcinoma-rationale for comprehensive mutation profiling. Mol
Cancer Ther. 11:485–491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Janku F, Wheler JJ, Westin SN, Moulder SL,
Naing A, Tsimberidou AM, Fu S, Falchook GS, Hong DS, Garrido-Laguna
I, et al: PI3K/AKT/mTOR inhibitors in patients with breast and
gynecologic malignancies harboring PIK3CA mutations. J Clin Oncol.
30:777–782. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
de Roock W, Claes B, Bernasconi D, De
Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V,
Papamichael D, Laurent-Puig P, et al: Effects of KRAS, BRAF, NRAS,
and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy
in chemotherapy-refractory metastatic colorectal cancer: A
retrospective consortium analysis. Lancet Oncol. 11:753–762. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park JH, Ryu MH, Park YS, Park SR, Na YS,
Rhoo BY and Kang YK: Successful control of heavily pretreated
metastatic gastric cancer with the mTOR inhibitor everolimus
(RAD001) in a patient with PIK3CA mutation and pS6 overexpression.
BMC Cancer. 15:1192015. View Article : Google Scholar : PubMed/NCBI
|