Introduction
Breast cancer is a major health concern worldwide
and the most common type of cancer among women (1), with a reported 458,000 deaths annually,
making it the most common cause of cancer-related mortality among
women in developed as well as developing countries (2). Therefore, there is an urgent need for
novel biomarkers for the prognosis and effective treatment of
breast cancer. The T-cell immunoglobulin and mucin domain
(TIM) gene family was positionally cloned in 2001 from
within the T-cell and airway phenotype regulator locus as novel
allergy and asthma susceptibility genes (3). The TIM gene family consists of
eight members (TIM-1-8) on mouse chromosome 11B1.1, and
three members (TIM-1, TIM-3 and TIM-4) on
human chromosome 5q33.2, a chromosomal region that has been
repeatedly associated with asthma, allergy and autoimmunity
(4).
Tim-3 was found to be particularly expressed in T
helper type 1 (Th1) cells, CD8+ T cells and Th17 cells.
At present, Tim-3 expression may be found in innate immune cells,
including natural killer (NK) cells, dendritic cells (DCs),
monocytes, mast cells and other lymphocyte subpopulations (5–8).
In recent years, Tim-3 has been considered to be a
negative regulatory molecule, which plays a crucial role in
antitumor immunity. However, the mechanism underlying its antitumor
properties remains unknown. The aim of this study was to
investigate the expression of Tim-3 in 150 invasive ductal breast
cancer (IDC) and 100 normal breast tissue samples by
immunohistochemistry and determine the expression of Tim-3 in
breast cancer tissue and its association with clinicopathological
parameters and cytotoxic lymphocyte (CTL) infiltration.
Patients and methods
Patient selection
A total of 150 breast cancer tissue specimens were
collected from female patients at the Department of Breast Surgery
of the Southwest Medical University Affiliated Hospital (Sichuan,
China) between April, 2013 and May, 2014; all the cases were
pathologically diagnosed postoperatively. Following selection of
the paraffin blocks, 4-µm sections were prepared, stained with
hematoxylin and eosin (HE), and the diagnosis was confirmed by two
pathologists. Sections with inflammation, hemorrhage and incisional
biopsies with insufficient tissue were excluded from the study.
Clinical information, including age, primary tumor size, axillary
lymph node metastasis and TNM stage, World Health Organization
(WHO) grade, Ki-67, molecular classification and location of the
lesion, was extracted from patient files and recorded in tables.
The median age of the patients was 49.6 years (range, 33–68 years).
Patients without any complications did not receive chemotherapy or
other therapies prior to surgery. Furthermore, 100 pathologically
confirmed normal breast tissue or benign lesion samples were also
obtained, located at a distance 3.0–5.0 cm from the tumor. All the
participants provided written informed consent and the study
protocol was approved by the Ethics Committee of the Affiliated
Hospital of Xinan Medical University. The study was conducted over
a period of 6 months.
Reagents and instruments
Rabbit anti-human Tim-3 polyclonal antibody
(dilution, 1:500; catalog no., 185703; Abcam, Cambridge, UK);
murine monoclonal anti-human CD8 antibody (dilution 1:500,
GM710301, Gene Biology Company, Shanghai, China), 10% neutral
buffered formalin, xylene, serial concentrations of ethanol,
phosphate-buffered solution (PBS), 3% H2O2
and diaminobenzidine (DAB) (all from Jinshan Chemical Reagent
Company, Chengdu, China).
Immunohistochemistry
To quantify Tim-3 cells in large numbers of
patients, paraffin-embedded IDC samples were processed for
immunohistochemistry. The specimens were fixed in 10% neutral
buffered formalin, embedded in paraffin and cut into 4-µm serial
sections. Paraffin-embedded tissues were dewaxed in xylene,
rehydrated by serial concentrations of ethanol and rinsed in PBS,
followed by treatment with 3% H2O2 to block
endogenous peroxidase. Following heating in a microwave at 750 W
for 15 min to retrieve the tissue antigen, the sections were
incubated with 10% normal goat serum at room temperature for 10 min
to block non-specific reactions. This was followed by washing with
PBS and incubation with polyclonal rabbit anti-human Tim-3 antibody
(dilution, 1:500, clone 185703, IgG2a; Abcam), murine monoclonal
anti-human CD8 antibody, for 12 h at 4°C, and with horseradish
peroxidase-conjugated goat anti-rat IgG (dilution, 1:500; GM710301;
Gene Biology Company, Shanghai, China). Following washing with PBS,
the sections were developed in DAB substrate. The sections were
then counterstained with hematoxylin for 2 min and dehydrated in
ethanol and xylene prior to mounting on slides. The sections were
subjected to EnVision immunohistochemical staining. PBS instead of
primary antibodies was used as negative control. Visualization was
achieved with ABC-Elite Reagent (Sigma, St. Louis, MO, USA). The
sections were counterstained with Mayer's hematoxylin (Sigma). The
nuclei were stained with 1% ammonium hydroxide. The number of Tim-3
cells was counted in five fields at a magnification of ×400.
Statistical analysis
All the data were analyzed with the SPSS software
package, version 17.0 (SPSS Inc., Chicago, IL, USA). Due to the
non-normal distribution, the Mann-Whitney U-test was used for
comparison between groups. Independent samples t-test was used for
the comparison of two means; the Chi-squared test was used for rate
comparison. P-values <0.05 were considered to indicate
statistically significant differences.
Results
Expression of Tim-3 in the tissue of
invasive breast carcinoma and normal breast tissue
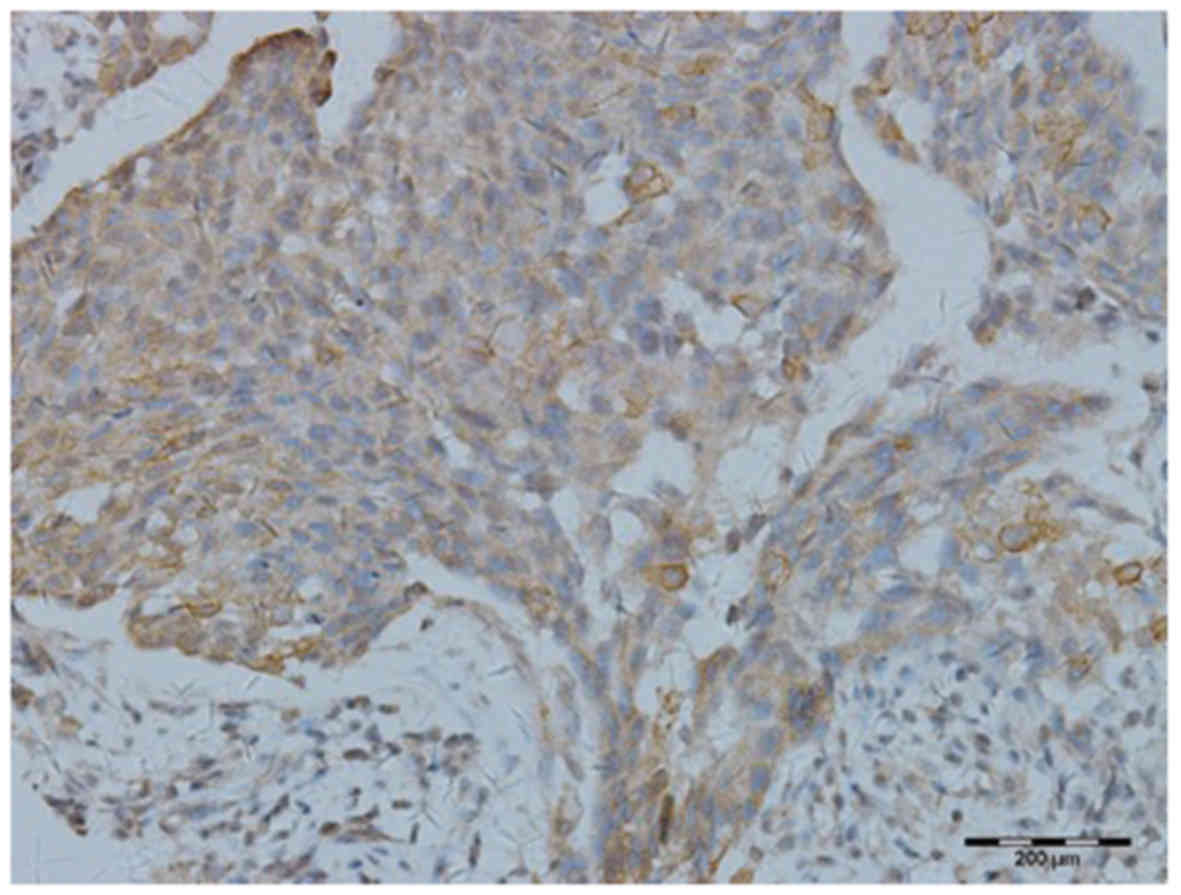
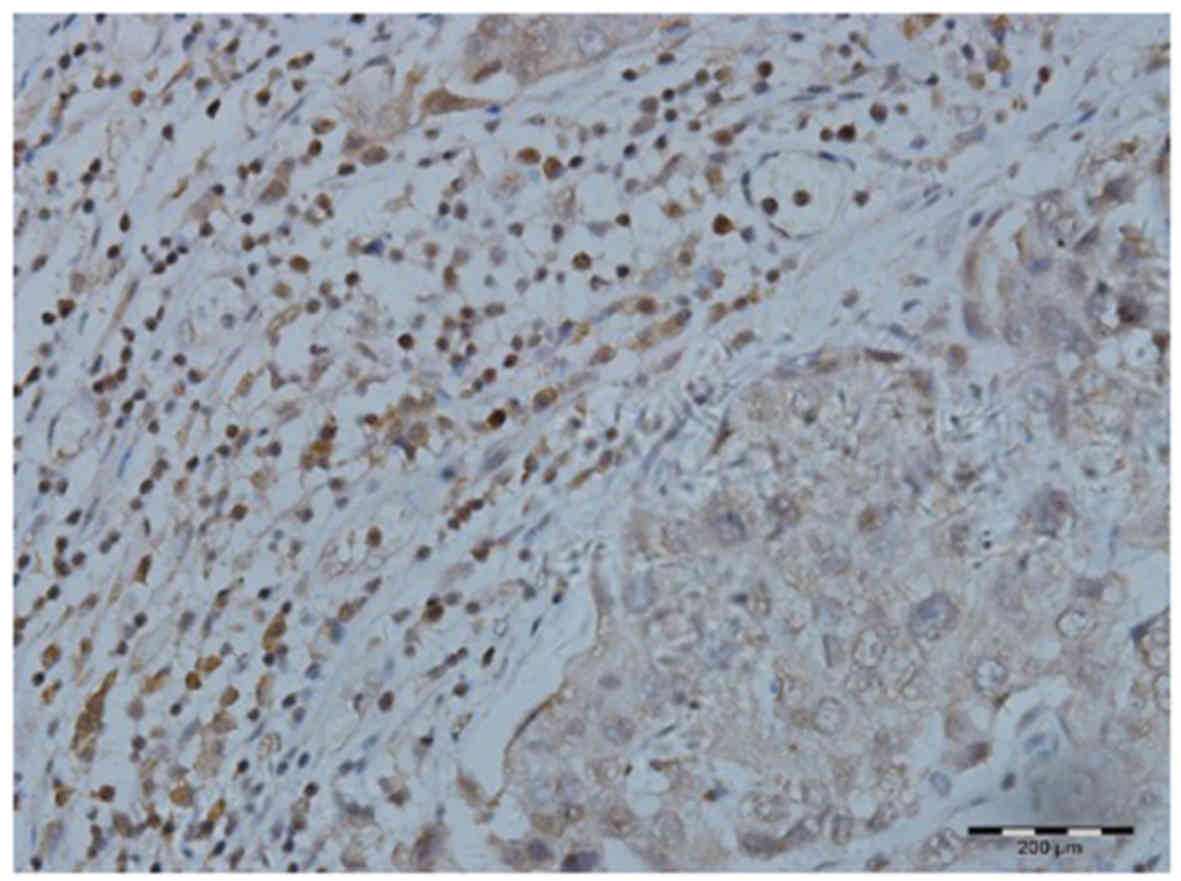
Tim-3 was found to be expressed on the surface of
the tumor cells (Fig. 1) and
CD8+ T cells (Fig. 2).
Tim-3 expression on IDC cells (98%) was significantly higher
compared with that in normal breast tissue (13%;
χ2=0.195, P=0.000). Similarly, the expression of Tim-3
on CD8+ T cells in IDC tissue (90%) was also
significantly increased compared with that in normal breast tissue
(23%; χ2=11.11, P=0.000; Table I).
 | Table I.Expression of Tim-3 on breast tissue
cells and on CTLs in IDC and normal breast or benign lesions. |
Table I.
Expression of Tim-3 on breast tissue
cells and on CTLs in IDC and normal breast or benign lesions.
|
|
| Expression of Tim-3
on breast tissue cells | Expression of Tim-3
on CTLs |
|---|
|
|
|
|
|
|---|
| Variables | Patient no. | High (n) | Rate (%) | High (n) | Rate (%) |
|---|
| IDC | 150 | 147 | 98 | 135 | 90 |
| Normal breast/benign
lesions | 100 | 13 | 13 | 23 | 23 |
| χ2 |
|
| 0.195 |
| 11.11 |
| P-value |
|
| 0.000 |
| 0.000 |
Association between Tim-3 expression
and clinicopathological characteristics in IDC patients
The expression of Tim-3 on tumor cells was
significantly associated with clinicopathological characteristics,
such as gender, age, lymph node metastasis and TNM stage (P=0.015,
0.001 and 0.027, respectively). Our study indicated that the median
expression level of Tim-3 on CD8+ T cells was
significantly associated with clinicopathological parameters such
as lymph node metastasis, TNM stage, WHO grade and molecular
classification (P=0.000, 0.004, 0.009 and 0.000, respectively),
whereas it was not correlated with other clinicopathological
parameters (all P-values >0.05; Table II).
 | Table II.Association between the expression of
Tim-3 and the clinicopathological characteristics of patients with
breastinfiltrating ductal carcinoma. |
Table II.
Association between the expression of
Tim-3 and the clinicopathological characteristics of patients with
breastinfiltrating ductal carcinoma.
|
|
| Tim-3 expression |
|---|
|
|
|
|
|---|
|
|
| On tumor cells | On CTLs |
|---|
|
|
|
|
|
|---|
| Variables | Patient no.
(n=150) | Low (%) | High (%) | P-value | Low (%) | High (%) | P-value |
|---|
| Age, years |
|
|
| 0.015 |
|
| 0.991 |
|
<45 | 48 | 27 (56.25) | 21 (43.75) |
| 24 (50.00) | 24 (50.00) |
|
|
≥45 | 102 | 36 (35.29) | 66 (64.71) |
| 52 (50.98) | 50 (49.02) |
|
| Tumor size |
|
|
| 0.422 |
|
| 0.114 |
| T1 | 51 | 24 (47.06) | 27 (52.94) |
| 31 (60.78) | 20 (39.21) |
|
| T2 | 36 | 15 (41.67) | 21 (58.33) |
| 18 (50.00) | 18 (50.00) |
|
| T3 | 60 | 24 (40.00) | 36 (60.00) |
| 27 (45.00) | 33 (55.00) |
|
| T4 | 3 | 0
(0.00) | 3
(100.00) |
| 0
(0.00) | 3
(100.00) |
|
| Lymph node
status |
|
|
| 0.001 |
|
| 0.000 |
| N0 | 42 | 21 (50.00) | 21 (50.00) |
| 36 (85.71) | 6
(14.29) |
|
| N1 | 69 | 36 (52.17) | 33 (47.82) |
| 28 (40.58) | 41 (59.42) |
|
| N2 | 24 | 3
(12.50) | 21 (87.50) |
| 6
(25.00) | 18 (75.00) |
|
| N3 | 15 | 3
(20.00) | 12 (80.00) |
| 6
(40.00) | 9
(60.00) |
|
| TNM stage |
|
|
| 0.027 |
|
| 0.004 |
| I | 18 | 12 (66.67) | 6
(33.33) |
| 10 (55.56) | 8
(44.44) |
|
| II | 42 | 21 (50.00) | 21 (50.00) |
| 30 (71.43) | 12 (28.57) |
|
|
III | 60 | 18 (30.00) | 42 (70.00) |
| 27 (45.00) | 33 (55.00) |
|
| IV | 30 | 12 (40.00) | 18 (60.00) |
| 9
(30.00) | 21 (70.00) |
|
| WHO grade |
|
|
| 0.536 |
|
| 0.009 |
| I | 15 | 6
(40.00) | 9
(50.00) |
| 3
(20.00) | 12 (80.00) |
|
| II | 99 | 39 (39.39) | 60 (60.61) |
| 49 (49.49) | 50 (50.51) |
|
|
III | 36 | 18 (50.00) | 18 (50.00) |
| 24 (66.67) | 12 (33.33) |
|
| Ki-67, % |
|
|
| 0.630 |
|
| 0.512 |
|
<14 | 39 | 15 (38.46) | 24 (61.24) |
| 18 (46.15) | 21 (53.85) |
|
|
≥14 | 111 | 48 (43.24) | 63 (56.76) |
| 58 (52.25) | 53 (47.75) |
|
| Molecular
classification |
|
|
| 0.314 |
|
| 0.000 |
| Luminal
A | 24 | 9
(37.50) | 15 (62.50) |
| 15 (62.50) | 9
(37.50) |
|
| Luminal
B | 60 | 30 (50.00) | 30 (50.00) |
| 18 (30.00) | 42 (60.00) |
|
|
HER2-overexpressing | 36 | 15 (41.67) | 21 (58.33) |
| 19 (52.78) | 17 (47.22) |
|
|
Basal-like | 30 | 9
(30.00) | 21 (70.00) |
| 24 (80.00) | 6
(20.00) |
|
Association between Tim-3 expression
and the degree of CD+8 T-cell infiltration
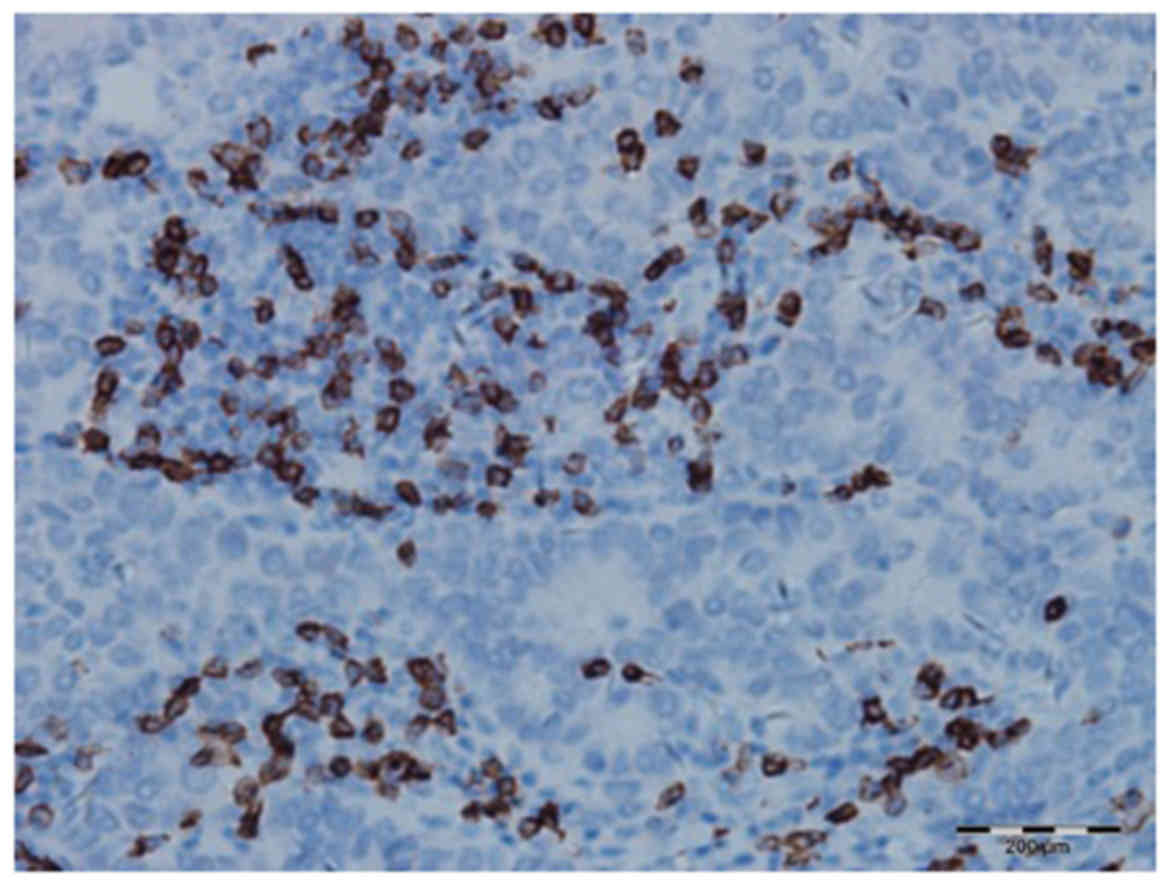
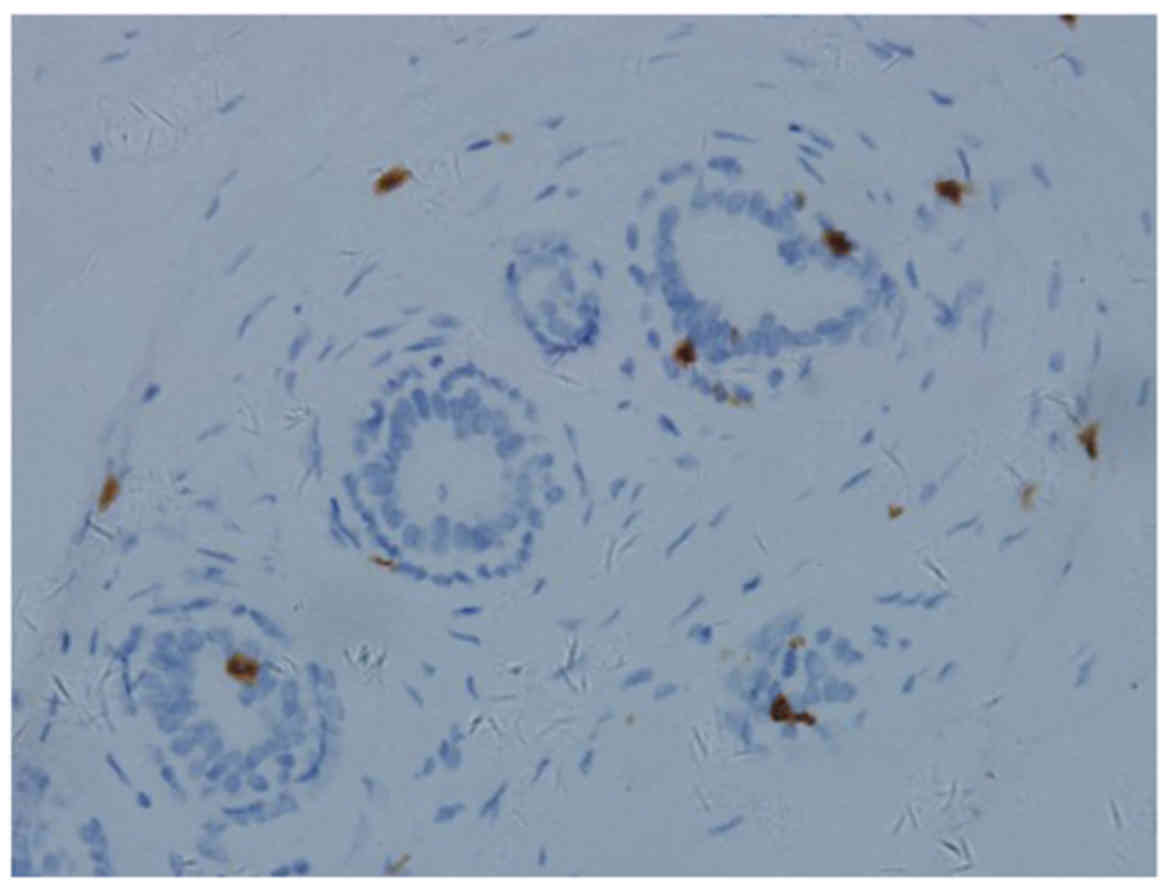
The degree of CD8+ T-cell infiltration in
IDC (Fig. 3) was higher compared
with that in normal breast tissue (Fig.
4). Further analysis revealed that the degree of
CD8+ T-cell infiltration was significantly correlated
with primary tumor size, lymph node metastasis, WHO grade, Ki-67
and molecular classification (P=0.017, 0.002, 0.007, 0.003 and
0.000, respectively), whereas it was not correlated with other
clinicopathological parameters (all P-values >0.05; Table III).
 | Table III.Association between expression of
Tim-3 on CTLs and the clinicopathological parameters of breast
infiltrating ductal carcinoma. |
Table III.
Association between expression of
Tim-3 on CTLs and the clinicopathological parameters of breast
infiltrating ductal carcinoma.
|
|
| Expression of Tim-3
on CTLs |
|
|
|---|
|
|
|
|
|
|
|---|
| Variables | Patient no.
(n=150) | Low (%) | High (%) | χ2 | P-value |
|---|
| Age, years |
|
|
| 3.160 | 0.075 |
|
<45 | 48 | 18 (37.50) | 30 (62.50) |
|
|
|
≥45 | 102 | 24 (23.53) | 78 (76.47) |
|
|
| Tumor size |
|
|
| 10.177 | 0.017 |
| T1 | 51 | 15 (29.41) | 36 (70.59) |
|
|
| T2 | 36 | 6
(16.67) | 30 (83.33) |
|
|
| T3 | 60 | 18 (30.00) | 42 (70.00) |
|
|
| T4 | 3 | 3
(100.00) | 0
(0.00) |
|
|
| Lymph node
status |
|
|
| 14.482 | 0.002 |
| N0 | 42 | 3
(7.14) | 39 (92.87) |
|
|
| N1 | 69 | 27 (39.13) | 42 (60.87) |
|
|
| N2 | 24 | 6
(25.00) | 18 (75.00) |
|
|
| N3 | 15 | 6
(40.00) | 9
(60.00) |
|
|
| TNM stage |
|
|
| 3.564 | 0.313 |
| I | 18 | 3
(16.67) | 15 (83.33) |
|
|
| II | 42 | 12 (28.57) | 30 (71.43) |
|
|
|
III | 60 | 15 (25.00) | 45 (75.00) |
|
|
| IV | 30 | 12 (40.00) | 18 (60.00) |
|
|
| WHO grade |
|
|
| 9.939 | 0.007 |
| I | 15 | 9
(60.00) | 6
(40.00) |
|
|
| II | 99 | 27 (27.27) | 72 (72.72) |
|
|
|
III | 36 | 6
(16.67) | 30 (83.33) |
|
|
| Ki-67 |
|
|
| 8.615 | 0.003 |
|
<14% | 39 | 18 (46.15) | 21 (53.85) |
|
|
|
≥14% | 111 | 24 (21.62) | 87 (78.38) |
|
|
| Molecular
classification |
|
|
| 23.636 | 0.000 |
| Luminal
A | 24 | 9
(37.50) | 15 (62.50) |
|
|
| Luminal
B | 60 | 27 (45.00) | 33 (55.00) |
|
|
|
HER2-overexpressing | 36 | 6
(16.67) | 30 (83.33) |
|
|
|
Basal-like | 30 | 0
(0.00) | 30 100.00) |
|
|
| Tim-3 expression on
tumor |
|
|
| 0.946 | 0.331 |
|
Low | 63 | 15 (23.80) | 48 (76.20) |
|
|
|
High | 87 | 27 (31.03) | 60 (68.97) |
|
|
Furthermore, by evaluating the abovementioned
parameters that were statistically significant by logistic
multifactor regression analysis, axillary lymph node metastasis,
WHO grade, Ki-67 and molecular classification were found to
significantly affect the extent of the tumor infiltration by CTLs
(all P-values <0.05; Table
IV).
 | Table IV.Analysis of multiple factors
affecting the infiltration degree by CTLs. |
Table IV.
Analysis of multiple factors
affecting the infiltration degree by CTLs.
|
| Exp(B) CI 95% |
|---|
|
|
|
|---|
| Variables | B | SE | Wald | df | Sig | Exp(B) | Upper limit | Lower limit |
|---|
| Tumor size | −13.962 |
0.685 | 425.901 | 1 |
0.229 |
1.767 |
0.488 |
8.566 |
| Lymph node
status | −4.649 |
1.387 | 11.236 | 1 |
0.001 |
0.010 |
0.046 |
1.345 |
| WHO grade |
0.576 |
1.137 | .257 | 1 |
0.002 |
1.779 |
0.023 |
0.665 |
| Ki-67 |
1.240 |
0.860 | 39.34 | 1 |
0.038 |
3.456 |
1.020 | 29.720 |
| Molecular
classification | 16.910 | 985.39 |
0.000 | 1 |
0.000 |
2.200E9 |
7.140E8 |
4.791E10 |
| Tim-3 expression on
tumor | −0.983 |
0.640 |
2.359 | 1 |
0.125 |
0.374 |
1.07 |
1.312 |
Discussion
Co-opted immune checkpoint pathways are likely
involved in the mechanism underlying tumor immune suppression.
Tim-3 is a newly confirmed molecule with an important immunological
function in several physiological and pathological processes.
Tim-3 was initially found to be selectively
expressed on terminally differentiated interferon γ-producing
CD4+ Th1 cells and CD8+ cytotoxic T cells
(9,10), as well as on Th17 cells, DCs,
monocytes, regulatory T cells (Tregs), mast cells, NK cells and
tumor-infiltrating lymphocytes. It is also expressed on tumor
cells, such as melanoma, squamous cell carcinoma, gastric cancer
and non-small-cell lung cancer cells, but not on CD4+
Th2 cells (10–18).
Previous studies suggested that Tim-3 may modulate
the immune response of Th1 cells and regulate cell immune tolerance
(19,20). Tim-3 has also drawn significant
attention in autoimmune diseases, anaphylactic diseases, immune
tolerance and antitumor immunity (21,22). The
Tim-3/galectin-9 pathway plays an important role in the suppressive
tumor microenvironment (TME). In a variety of cancers, the
overexpression of Tim-3 is associated with poor prognosis (9).
Tim-3 marks the most suppressed or dysfunctional
population of CD8+ T cells in preclinical models of
solid as well as hematological malignancies; thus, Tim-3 may be a
key immune checkpoint in tumor-induced immune suppression (18,23).
The Tim-3/galectin-9 pathway contributes to the TME
in the human body and Tim-3 is also characterized as a key
regulator of the dysfunctional CD8+ T-cell phenotype
(18,24) early in the TME, where neoplastic
growth is promoted via induction of CD8+ T-cell
dysfunctionality (25).
Several recent studies demonstrated that Tim-3 is
highly expressed in a large number of tumor tissues types,
including cervical cancer (15),
gastric cancer (16), acute myeloid
leukemia (23) lung cancer (25), ovarian cancer (26) and glioma (27). In this study, the Tim-3 expression on
tumor cells and CD8+ T cells of IDC and normal breast
tissues was investigated by immunohistochemistry. The expression of
Tim-3 in IDC tissue was distinctly higher compared with that in
normal breast tissue (P=0.000), which suggested that Tim-3 was
involved in the pathogenesis of breast cancer via its regulatory
effect on various immune cells and tumor cells, indicating that
Tim-3 may play an important role in tumorigenesis. The expression
of Tim-3 on tumor cells may affect the malignant biological
behavior of the tumor.
In the present study, we observed that the
expression of Tim-3 in IDC was significantly associated with age
(P<0.05), reflecting the decrease in the overall immune ability
of the body with advancing age. The expression of the negative
regulatory immune molecule Tim-3 on breast cancer cells was clearly
increased.
Our test results also demonstrated that the strength
of the Tim-3 expression on tumor cells exhibited an increasing
trend with the increasing number of metastatic axillary lymph nodes
and advanced pathological stage. Furthermore, the expression of
Tim-3 on IDC cells was significantly correlated with local axillary
lymph node metastasis and pathological stage (P<0.05), but the
results of our study demonstrated that primary tumor size, WHO
grade, Ki-67 and molecular classification of breast IDC were not
significantly associated with the expression of Tim-3 on tumor
cells. The possible underlying mechanisms may be as follows: i) A
large amount of Tim-3 on the tumor cell surface forms a ‘shield’ to
protect Tim-3-positive tumor cells from the toxicity of
immunological effector cells; however, the expression of negative
immune regulatory factor Tim-3 does not affect the proliferation
and differentiation of tumor cells. ii) In some cases, the size of
the primary tumor is large at clinical diagnosis; thus, immune
adjustment factors are no longer able to interfere with the growth
of tumor cells, or there is a disproportional rate of tumor cell
proliferation and immune clearance. iii) The experimental data may
be insufficient; thus, larger samples are required to confirm the
findings.
In nearly all previous studies, immune cells were
identified by specific cluster of differentiation (CD) markers,
following immunohistochemical staining of the slides. It is
important to distinguish between different types of T lymphocytes,
as they all have different functions in the TME.
CD8+ T cells play an important role in
the TME. CTL is the main antitumor immune cell type, which may
identify tumor antigens and eliminate tumor cells with one of two
main mechanisms: i) The perforin pathway, or ii) the
Fas/FasL-mediated apoptosis pathway. Fourcade et al
(28) discovered that Tim-3 was
expressed on NY-ESO-1-specific CD8+ T cells in patients
with advanced melanoma. They found that the blockade of both the
Tim-3 and programmed cell death protein-1 (PD-1) pathways may
reverse tumor-induced T-cell exhaustion in patients with advanced
melanoma. Our results suggested that the Tim-3 expression on
CD8+ T cells was correlated with tumor invasion and TNM
stage, which may be due to Tim-3-induced T-cell exhaustion, leading
to tumor occurrence.
Our test data demonstrated that the expression of
Tim-3 on tumor-infiltrating CTLs increased as axillary lymph node
metastasis progressed. Lymph node metastasis and TNM stage, primary
tumor size, WHO grade and molecular classification are
significantly associated with the expression of Tim-3 on CTL cells
(P<0.05).
This indicates that the expression of Tim-3 on CTLs
may inhibit the tumor cell-killing function of CTLs. Even in the
TME, CD8+ CTLs may transform into CD8+ Tregs,
decreasing the tumor-infiltrating CTL immune surveillance,
decreasing the local immune function and promoting the growth of
tumor cells and metastasis.
The present study analyzed the association of
tumor-infiltrating CTLs and the pathological characteristics of
breast-infiltrating ductal carcinoma by single factor analysis of
variance and demonstrated that primary tumor size, axillary lymph
node metastasis, WHO grade, Ki-67 and molecular classification may
statistically significantly affect the degree of tumor CTL
infiltration (P<0.05).
Furthermore, the abovementioned statistically
significant parameters were assessed by multiple logistic
regression analysis and the results also demonstrated that axillary
lymph node metastasis, WHO grade, Ki-67 and molecular
classification significantly affect the extent of CTL infiltration
of the tumor. The possible underlying mechanism may be as follows:
i) High expression of the negative immunomodulatory molecule Tim-3
on tumor-infiltrating CTLs may result in immune malfunction; as the
tumor grows, CD8+ CTLs may transform into
CD8+ Tregs. ii) Low degree of CTL infiltration may lead
to poor local immunity, which may contribute to tumor growth. iii)
The lower the degree of tumor differentiation, the higher its
antigenicity, which may cause a stronger local immune response.
In conclusion, Tim-3 is expressed in the majority of
solid tumors and tumor-infiltrating CTLs. This finding suggests
that Tim-3 participates in the immune escape through the following
mechanisms: i) When it binds to its receptor, Tim-3 may induce
T-cell apoptosis or immune incompetence; ii) a large amount of
Tim-3 on the tumor cell surface may form a ‘shield’, which may
protect Tim-3-positive tumor cells from toxic injury or elimination
by CTLs; iii) Tim-3 positive cells may also affect T-cell secretion
of negative cytokines or Treg coordination.
Sakuishi et al (18) reported that, in lymphocytes
infiltrating solid tumors in mice, co-expression of Tim-3 and PD-1
may be detected. The multi-targeted therapy of Tim-3 and PD-1 has
been highly effective in controlling tumor growth. Our study
demonstrated that the expression of Tim-3 in breast cancer tissue
was negatively correlated with certain clinicopathological
parameters; however, the underlying mechanisms remain unclear.
Therefore, further research on the multi-targeted therapy of Tim-3
and PD-1 for the treatment of breast cancer.
In conclusion, Tim-3 is highly expressed in breast
IDC cells and tumo-infiltrating CTLs. The expression of Tim-3
exhibits a positive correlation with the malignant behavior of the
tumor. The expression of Tim-3 on CTLs is negatively correlated
with degree of CTL infiltration, and Tim-3 expression in breast
cancer exerts a negative effect on immune regulation. The largest
diameter of the primary tumor, the number of metastatic axillary
lymph nodes, degree of tumor cell differentiation and molecular
classification of breast cancer significantly afect the extent of
the CTL infiltration of the tumor tissue.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eccles SA, Aboagye EO, Ali S, Anderson AS,
Armes J, Berditchevski F, Blaydes JP, Brennan K, Brown NJ, Bryant
HE, et al: Critical research gaps and translational priorities for
the successful prevention and treatment of breast cancer. Breast
Cancer Res. 15:R922013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
McIntire JJ, Umetsu SE, Akbari O, Potter
M, Kuchroo VK, Barsh GS, Freeman GJ, Umetsu DT and DeKruyff RH:
Identification of Tapr (an airway hyperreactivity regulatory locus)
and the linked Tim gene family. Nat Immunol. 2:1109–1116. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McIntire JJ, Umetsu DT and DeKruyff RH:
TIM-1, a novel allergy and asthma susceptibility gene. Springer
Semin Immunopathol. 25:335–348. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hastings WD, Anderson DE, Kassam N,
Koguchi K, Greenfield EA, Kent SC, Zheng XX, Strom TB, Hafler DA
and Kuchroo VK: TIM-3 is expressed on activated human CD4+ T cells
and regulates Th1 and Th17 cytokines. Eur J Immunol. 39:2492–2501.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anderson AC, Anderson DE, Bregoli L,
Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW,
Hirashima M, et al: Promotion of tissue inflammation by the immune
receptor Tim-3 expressed on innate immune cells. Science.
318:1141–1143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khademi M, Illés Z, Gielen AW, Marta M,
Takazawa N, Baecher-Allan C, Brundin L, Hannerz J, Martin C, Harris
RA, et al: T Cell Ig- and mucin-domain-containing molecule-3
(TIM-3) and TIM-1 molecules are differentially expressed on human
Th1 and Th2 cells and in cerebrospinal fluid-derived mononuclear
cells in multiple sclerosis. J Immunol. 172:7169–7176. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakae S, Iikura M, Suto H, Akiba H, Umetsu
DT, Dekruyff RH, Saito H and Galli SJ: TIM-1 and TIM-3 enhancement
of Th2 cytokine production by mast cells. Blood. 110:2565–2568.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anderson AC: Tim-3, a negative regulator
of anti-tumor immunity. Curr Opin Immunol. 24:213–216. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anderson AC, Anderson DE, Bregoli L,
Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW,
Hirashima M, et al: Promotion of tissue inflammation by the immune
receptor Tim-3 expressed on innate immune cells. Science.
318:1141–1143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ngiow SF, Teng MW and Smyth MJ: Prospects
for TIM3-targeted antitumor immunotherapy. Cancer Res.
71:6567–6571. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wiener Z, Kohalmi B, Pocza P, Jeager J,
Tolgyesi G, Toth S, Gorbe E, Papp Z and Falus A: TIM-3 is expressed
in melanoma cells and is upregulated in TGF-beta stimulated mast
cells. J Invest Dermatol. 127:906–914. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhuang X, Zhang X, Xia X, Zhang C, Liang
X, Gao L, Zhang X and Ma C: Ectopic expression of TIM-3 in lung
cancers: A potential independent prognostic factor for patients
with NSCLC. Am J Clin Pathol. 137:978–985. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan J, Zhang Y, Zhang JP, Liang J, Li L
and Zheng L: Tim-3 expression defines regulatory T cells in human
tumors. PLoS One. 8:e580062013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao Y, Zhou X, Huang X, Li Q, Gao L, Jiang
L, Huang M and Zhou J: Tim-3 expression in cervical cancer promotes
tumor metastasis. PLoS One. 8:e538342013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang J, Jin MS, Kong F, Cao D, Ma HX, Jia
Z, Wang YP, Suo J and Cao X: Decreased galectin-9 and increased
Tim-3 expression are related to poor prognosis in gastric cancer.
PLoS One. 8:e817992013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang ZZ, Grote DM, Ziesmer SC, Niki T,
Hirashima M, Novak AJ, Witzig TE and Ansell SM: IL-12 upregulates
TIM-3 expression and induces T cell exhaustion in patients with
follicular B cell non-Hodgkin lymphoma. J Clin Invest.
122:1271–1282. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakuishi K, Apetoh L, Sullivan JM, Blazar
BR, Kuchroo VK and Anderson AC: Targeting Tim-3 and PD-1 pathways
to reverse T cell exhaustion and restore anti-tumor immunity. J Exp
Med. 207:2187–2194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sánchez-Fueyo A, Tian J, Picarella D,
Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T,
Kuchroo VK, et al: Tim-3 inhibits T helper type 1-mediated auto-
and alloimmune responses and promotes immunological tolerance. Nat
Immunol. 4:1093–1101. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sabatos CA, Chakravarti S, Cha E, Schubart
A, Sánchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ and
Kuchroo VK: Interaction of Tim-3 and Tim-3 ligand regulates T
helper type 1 responses and induction of peripheral tolerance. Nat
Immunol. 4:1102–1110. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Simmons WJ, Koneru M, Mohindru M, Thomas
R, Cutro S, Singh P, Dekruyff RH, Inghirami G, Coyle AJ, Kim BS and
Ponzio NM: Tim-3+ T-bet+ tumor-specific Th1 cells colocalize with
and inhibit development and growth of murine neoplasms. J Immunol.
174:1405–1415. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu WK, Lu XX, Yang S, Xu GP, Lan F, Chen
SX, Ni W, Xiong WN and Xiong SD: Expression of the Th1-specific
cell-surface protein Tim-3 increases in a murine model of atopic
asthma. J Asthma. 46:872–877. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou Q, Munger ME, Veenstra RG, Weigel BJ,
Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK
and Blazar BR: Coexpression of Tim-3 and PD-1 identifies a CD8+
T-cell exhaustion phenotype in mice with disseminated acute
myelogenous leukemia. Blood. 117:4501–4510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takamura S, Tsuji-Kawahara S, Yagita H,
Akiba H, Sakamoto M, Chikaishi T, Kato M and Miyazawa M: Premature
terminal exhaustion of Friend virus-specific effector CD8+ T cells
by rapid induction of multiple inhibitory receptors. J Immunol.
184:4696–4707. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang
F, Sun J, Yang Q, Zhang X and Lu B: TIM-3 expression characterizes
regulatory T cells in tumor tissues and is associated with lung
cancer progression. PLoS One. 7:e306762012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu J, Liu C, Qian S and Hou H: The
expression of Tim-3 in peripheral blood of ovarian cancer. DNA Cell
Biol. 32:648–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han S, Feng S, Xu L, Shi W, Wang X, Wang
H, Yu C, Dong T, Xu M and Liang G: Tim-3 on peripheral
CD4+ and CD8+ T cells is involved in the development of
glioma. DNA Cell Biol. 33:245–250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fourcade J, Sun Z, Benallaoua M, Guillaume
P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V and Zarour HM:
Upregulation of Tim-3 and PD-1 expression is associated with tumor
antigen-specific CD8+T cell dysfunction in melanoma patients. J Exp
Med. 207:2175–2186. 2010. View Article : Google Scholar : PubMed/NCBI
|