Introduction
Molecular-targeted therapy was recommended for the
systemic therapy of renal cell cancer (RCC) in the 2011 Japanese
Urological Association RCC guidelines (1,2);
however, these guidelines do not address the order of
administration of presently available multiple agents. The European
Association of Urology guidelines recommend either sunitinib or
everolimus as first-line therapy, and sorafenib or everolimus as
second-line therapy, although there are several aspects that remain
unknown regarding the optimal administration order and combination
of the multiple molecular-targeted drugs (3). At the 2015 ASCO annual meeting, the
results of a comparison test of sunitinib→everolimus vs.
everolimus→sunitinib were reported, indicating that the median
survival rates were 29.5 and 22.2 months, respectively, concluding
that sunitinib→everolimus was more effective (4,5).
Until clinical trials determine the optimal
treatment sequence, treatment individualization is required for
each patient based on patient and disease characteristics. In this
study, we investigated 12 cases of renal cancer in which axitinib
had been administered.
Patients and methods
Case series
A total of 12 patients who were diagnosed with RCC
between 2005 and 2011 were reviewed (Table I). Approval was obtained from the
Ethics Committee of our institution at the commencement of the
study. The patients included 9 men and 3 women, with a mean age of
66 years (range, 58–79 years). Axitinib was used as a first-line
drug in 4 cases, second-line in 5 cases, third-line in 1 case and
as a fourth-line drug in 2 cases. Partial response (PR) was
observed in 4 cases (30%) and stable disease in 4 cases (30%)
during axitinib treatment, with an overall response rate of 60%.
The duration of PR ranged from 6 to 19 months.
 | Table I.Summary of the investigated 12
cases. |
Table I.
Summary of the investigated 12
cases.
| Case no. | Age, years | Sex | Drugs (sequence) | Metastastic
sites | Effectiveness | PR duration,
months |
|---|
| 1. | 79 | Male | A | Lung, adrenal | PR | 6 |
| 2. | 59 | Male | Su→A→E | Lung, bone | PD |
|
| 3. | 73 | Male | Su→A | Lung, liver | PR | 9 |
| 4. | 69 | Male | Su→A | Kidney, lung,
pancreas | PR | 6 |
| 5. | 47 | Male | INF→Su→A | Lung, lymph
nodes | SD |
|
| 6. | 61 | Male | A→P | Bone, pleural
cavity | PR | 19 |
| 7. | 58 | Female | Su→A | Lung, lymph nodes,
brain, bone | PD |
|
| 8. | 76 | Female | Su→A | Liver | SD |
|
| 9. | 76 | Female | INF→So→UFT→A | Lung, lymph
nodes | SD |
|
| 10. | 64 | Male | A→E→INF→Su →So | Adrenal,
pancreas | PD |
|
| 11. | 67 | Male | GC→GDC→E→A | Lung, liver,
bone | SD→PD | 8 (SD, collecting
ductal carcinoma) |
| 12. | 63 | Male | A | Bone, lung | PD |
|
Of the 4 PR cases, the case 1 patient had received
axitinib as first-line therapy, and he had not received any other
molecular-targeted drugs; in 2 PR cases, axitinib was administered
as second-line treatment; in the remaining PR case, axitinib had
been followed by panitumab, with which a clinical response was
achieved.
Case reports
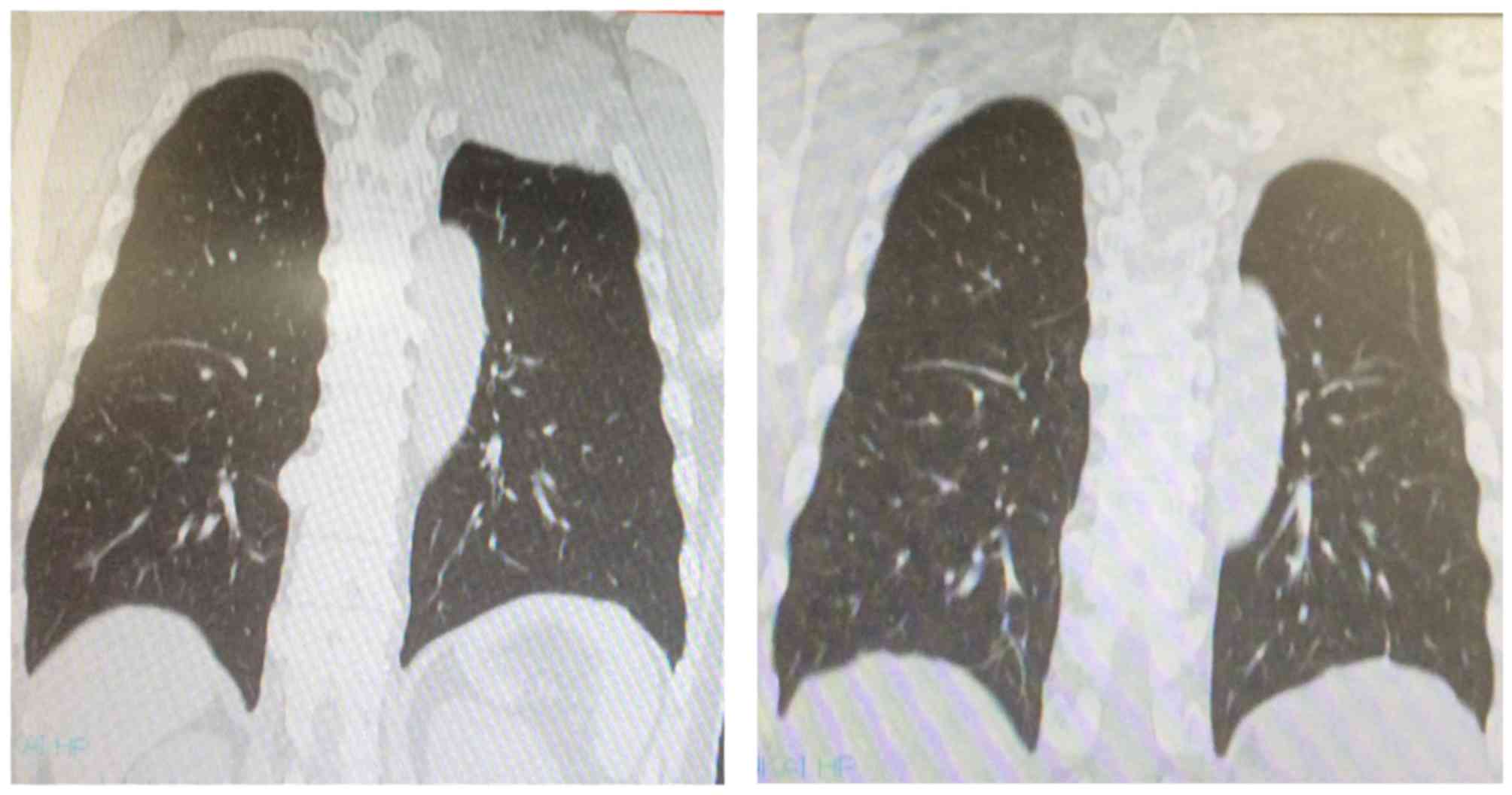
Case 6 was a 61-year-old male patient. At the
initial visit, an 8-cm mass was identified, extending from the
posterior aspect of the upper left lung to the chest wall,
infiltrating the ribs and the Th1 vertebral body, with partial
compression of the spinal cord. The computed tomography (CT) scan
revealed a 3-cm tumor in the inferior pole of the right kidney
(Fig. 1). CT-guided tumor biopsy of
the lung mass revealed RCC metastasis. Axitinib was administered at
10 mg/day; after 10 days, the dose was increased to 12 mg/day, and
to 14 mg/day 2 weeks later. At the time of admission to the
hospital the patient was bedridden. Following intensive
rehabilitation, the patient was able to use a wheelchair and even
to leave his house. The treatment was continued on an outpatient
basis. However, due to progression of metastatic bone disease
(Fig. 2), the patient was readmitted
to the hospital. He currently remains alive and is on pazopanib
treatment.
We encountered one case of collecting duct
carcinoma, which is a type of RCC refractory to cytotoxic and
molecular-targeted therapy. However, case 11, a 67-year-old male
patient, achieved PR. The patient visited our hospital complaining
of hematuria. Based on the results of the pathology, he was
diagnosed with renal collecting duct carcinoma with renal hilar
lymph node metastasis. After 4 months, the patient underwent left
nephrectomy and the subsequent pathological diagnosis was pT3, ly1,
v1, INFβ, pN2. Four weeks after the operation, the patient was
started on adjuvant GC chemotherapy (gemcitabine 1,200 mg and
cisplatin 300 mg) (6) and completed
four cycles. Six months postoperatively, multiple pulmonary
metastases were identified on CT, and metastasis to the right
pelvic bone was identified by bone scintigraphy. GDC chemotherapy
(gemcitabine 1,200 mg, docetaxel 80 mg and cisplatin 300 mg) was
initiated 4 weeks later (7). The
results of the CT conducted on June 20, 2012 confirmed peritoneal
dissemination, progression of multiple lung metastases and revealed
metastatic liver disease. Two months later the patient was
administered 10 mg everolimus. After administration of everolimus
for 3 months, the blood sugar level was found to be high, which was
considered to be an adverse event (AE) and the drug was
discontinued, followed by normalization of the patient's blood
sugar levels, after which time everolimus was resumed at a reduced
dose of 5 mg. At the 8-month follow-up the patient remained
progression-free, which is unusually long for renal collecting duct
cancer.
Discussion
Based on our cases, axitinib demonstrated reasonable
therapeutic efficacy as first- as well as second-line treatment.
However, to draw a firm conclusion, more cases must be
accumulated.
Procopio et al reviewed 13 cases of renal
collecting duct carcinoma. Renal collecting duct carcinoma patients
(median age, 57 years) comprise 3.4% of all metastatic RCC (mRCC)
patients, with a median survival time of 4 months, with only 3
cases having survived 6–33 months (8–11). The
disease-specific survival of our patient (case 11) was 13 months
from the time of the appearance of metastases. In a study on the
administration of sunitinib, axitinib, sorafenib, interferon and
temsirolimus as first-line drugs in 4,736 mRCC cases, a reduction
ratio of >7–8% of the tumor was associated with a relatively
good prognosis (12). Moreover, in a
study on the combined use of tyrosine kinase inhibitors (TKIs) and
mammalian target of rapamycin inhibitors in 153 cases of metastatic
clear cell RCC (ccRCC), comparing the combined use of lenvatinib
and everolimus with the use of lenvatinib alone and with the use of
everolimus alone, achieved a survival of 13.1, 7.5 and 8.5 months,
respectively, indicating that combination therapy was superior to
single-agent treatment (13).
Furthermore, in a study of 108 cases of non-ccRCC, survival with
sunitinib was 8.3 months and that with everolimus 5.6 months,
indicating that sunitinib was superior; however, for poor-risk RCC,
survival was 4.0 and 6.1 months for sunitinib and everolimus,
respectively, indicating that everolimus was better for poor-risk
cases (14).
A study of the programmed cell death protein-1
(PD-1) antibodies for RCC was also conducted (15–17). The
therapeutic effects of pazopanib and sunitinib in RCC cases
expressing PD-1 were significantly inferior to the PD-1
low-expressing cases, and the median survival time was also shorter
(18). Nivolumab (PD-1-inhibiting
antibodies) was investigated in 91 cases, and it was effective in
programmed death-ligand-1 (PD-L1)-positive as well as -negative
cases (based on immunostaining of cancer cells). While a 71% 1-year
survival was attained by both positive and negative cases, the
2-year survival rates were 64 and 48%, respectively (19). In addition, the mPFS of the cases in
whom anti-PD-1 treatment had not been effective and in whom TKIs
had been administered was 6.9 months, indicating that it may be
safely administered (20).
Immunostaining for PD-L1 was positive in 51% of spindle cell cancer
cases, whereas 100% of the cases that contained a ccRCC component
were positive (18). However, due to
the fact that only 17% of the ccRCCs that did not contain a spindle
cell carcinoma component were PD-L1 positive, there is a
possibility that PD-1 antibodies are effective in spindle cell
cancer, which has poor prognosis (21). A study to predict therapeutic effect
based on immunostaining for PD-L1 of tumor cells has been
conducted. However, cases were successfully treated irrespective of
positive or negative immunostaining; thus, no conclusion was
reached at that time (22).
Moreover, it is considered that the more gene mutations the cancer
cells harbour, the more successful the immunotherapy. Further
research on immune therapy that includes PD-L1 antibodies is
expected in the near future.
References
|
1
|
Naito S, Tatsugami K, Shinohara N, Tomita
Y, Mizokami A, Fujisawa M, Hashine K, Nishikido M, Nakagawa M,
Tsukamoto T and Akaza H: Final results of a phase II study of S-1
in patients with cytokine-refractory metastatic renal cell
carcinoma. Jpn J Clin Oncol. 44:122–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Japanese Urological Association: Guideline
on renal cell carcinoma 66–75. 2011 http://www.urol.or.jp/info/guideline/data/07_kidney_cancer_2011.pdfAccessed.
March 17–2015.
|
|
3
|
Ljungberg B, Albiges L, Bensalah K, Bex A,
Giles RH, Hora M, Kuczyk MA, Lam T, Marconi L, Merseburger AS,
Powles T, et al: Guideline on renal cell carcinoma. European
Association of Urology. http://uroweb.org/wp-content/uploads/10-Renal-Cell-Carcinoma_LR1.pdfAccessed.
March 17–2015.
|
|
4
|
Knox JJ, Barrios CH, Kim TM, Cosgriff T,
Srimuninnimit V, Pittman K, Sabbatini R, Rha SY, Flaig TW, Page RD,
et al: Final overall survival analysis for the phase II RECORD-3
study of first-line everolimus followed by sunitinib versus
first-line sunitinib followed by everolimus in metastatic RCC. Ann
Oncol. 28:1339–1345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Motzer RJ, Barrios CH, Kim TM, Falcon S,
Cosgriff T, Harker WG, Srimuninnimit V, Pittman K, Sabbatini R, Pha
SY, et al: Phase II randomized trial comparing sequential
first-line everolimus and second-line sunitinib versus first-line
sunitinib and second-line everolimus in patients with metastatic
renal cell carcinoma. J Clin Oncol. 32:2765–2771. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshinaga A and Kamata S: Long survival in
a patient with advanced ureteral carcinoma treated with TIN Regimen
(Paclitaxel, Ifosfamide, Nedaplatin) and Radiotherapy: A Case
Report. Hinyokika Kiyo. 60:435–437. 2014.(In Japanese). PubMed/NCBI
|
|
7
|
Hoshi S, Ohyama C, Ono K, Takeda A,
Yamashita S, Yamato T, Itoh A, Satoh M, Saito S, Okada Y, et al:
Gemcitabine plus carboplatin; and gemcitabine, docetaxel and
carboplatin combined chemotherapy regimens in patients with
metastatic urothelial carcinoma previously treated with a
platinum-based regimen: Preliminary report. Int J Clin Oncol.
9:125–129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Procopio G, Testa I, Iacovelli R, Grassi
P, Verzoni E, Garanzini E, Colecchia M, Torelli T and De Braud F:
Treatment of collecting duct carcinoma: Current status and future
perspectives. Anticancer Res. 34:1027–1030. 2014.PubMed/NCBI
|
|
9
|
Biondani P, Verzoni E, Torri V, Porcu L,
Grassi P, Testa I, DE Braud F and Procopio G: Sequential tyrosine
kinase inhibitors (TKIs) in metastatic renal cell carcinoma:
Results from a large cohort of patients. Anticancer Res.
34:2395–2398. 2015.
|
|
10
|
Bracarda S, Castellano D, Procopio G,
Sepúlveda JM, Sisani M, Verzoni E and Schmidinger M: Axitinib
safety in metastatic renal cell carcinoma: Suggestions for daily
clinical practice based on case studies. Expert Opin Drug Saf.
13:497–510. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Procopio G, Verzoni E, Biondani P, Grassi
P, Testa I, Garanzini E and de Braud F: Rationale and protocol of
RESORT, a randomized, open-label, multicenter phase II study to
evaluate the efficacy of sorafenib in patients with advanced renal
cell carcinoma after radical resection of the metastases. Tumori.
100:e28–e30. 2014.PubMed/NCBI
|
|
12
|
Grüenwald V, Lin X, Kalanovic D and
Simantov R: Early tumor shrinkage (eTS) as a predictive and
prognostic factor in metastatic renal cell carcinoma (mRCC). J Clin
Oncol. 33 (suppl; abstr 4551):2015.
|
|
13
|
Motzer R, Hutson T, Glen H, Michaelson D,
Molina AM, Eisen T, Jassem J, Zolnierek J, Maroto P, Mellado B, et
al: Randomized phase II, three-arm trial of lenvatinib (LEN),
everolimus (EVE) and LEN+EVE in patients (pts) with metastatic
renal cell carcinoma (mRCC). J Clin Oncol. 33 (suppl; abstr
4506):2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choueiri TK, Figueroa DJ, Fay AP,
Signoretti S, Liu Y, Gagnon R, Deen K, Carpenter C, Benson P, Ho
TH, et al: Correlation of PD-L1 tumor expression and treatment
outcomes in patients with renal cell carcinoma receiving sunitinib
or pazopanib: Results from COMPARZ, a randomized controlled trial.
Clin Cancer Res. 21:1071–1077. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Armstrong AJ, Broderick S, Eisen T,
Stadler WM, Jones RJ, Garcia JA, Vaishampayan UN, Picus J, Hawkins
RE, Hainsworth JD, et al: Final clinical results of a randomized
phase II international trial of everolimus vs. sunitinib in
patients with metastatic non-clear cell renal cell carcinoma
(ASPEN). J Clin Oncol. 33 (suppl; abstr 4507):2015.
|
|
16
|
Thoma C: Kidney cancer: CheckMate for
advanced-stage ccRCC? Nivolumab and cabozantinib aMETEORate poor
survival. Nat Rev Clin Oncol. 12:6212015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Michel Ortega RM and Drabkin HA: Nivolumab
in renal cell carcinoma. Expert Opin Biol Ther. 15:1049–1060. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Robert C, Long GV, Brady B, Dutriaux C,
Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C,
Kalinka-Warzocha E, et al: Nivolumab in previously untreated
melanoma without BRAF mutation. N Engl J Med. 372:320–330. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choueiri TK, Fishman M, Escudier B,
McDermott DF, Drake CG, Kluger HM, Stadler WM, Perez-Gracia JL,
McNeel DG, Curti BD, et al: Immunomodulatory activity of nivolumab
in metastatic renal cell carcinoma. Clin Cancer Res May.
11:2016.(Epub ahead of print).
|
|
20
|
Nadal R, Amin A, Geynisman DM, Voss Martin
HM, Weinstock M, Doyle J, Zhang Z, Viudez A, Plimack ER, McDermott
DF, et al: Efficacy and safety of endothelial growth factor
receptor (VEGFR)-tyrosine kinase inhibitors (TKI) after programmed
cell death 1 (PD-1) inhibitor treatment in patients with metastatic
clear cell renal cell carcinoma (mccRCC). J Clin Oncol.
33:45662015.
|
|
21
|
Ho TH, Millis SZ, Bryant D, Gatalica Z,
Reddy SK, Stanton ML, Castle EP, Joseph RW and Vogelzang NJ:
Molecular analysis of sarcomatoid renal cell carcinoma (sRCC). J
Clin Oncol. 33 (suppl; abstr 4556):2015. View Article : Google Scholar
|
|
22
|
Albiges L, Fay AP, Xie W, Krajewski K,
McDermott DF, Heng DY, Dariane C, DeVelasco G, Lester R, Escudier B
and Choueiri TK: Efficacy of targeted therapies after PD-1/PD-L1
blockade in metastatic renal cell carcinoma. Eur J Cancer.
51:2580–2586. 2015. View Article : Google Scholar : PubMed/NCBI
|