Introduction
Mucinous breast carcinoma (MBC) of the breast is a
special type of breast cancer that is characterized by the presence
of carcinoma cells surrounded by large amounts of extracellular
mucin (1). MBC may be encountered in
all age groups, and the median age at presentation is 50–57 years
(2–4). MBC comprises approximately <10% of
all invasive breast cancers. This type of tumor has an overall
better prognosis and a higher incidence among peri- and
post-menopausal patients. Pathologically, there are two main
subtypes of MBC, namely pure type MBC (PMBC) and mixed type MBC
(MMBC) (1). PMBC in particular is
known to have a favorable prognosis compared with invasive ductal
carcinoma (IDC) (4–9). We herein report a case of a giant MBC
causing rupture of the skin and bleeding due to tumor pressure, and
review the characteristics and palliative management of these
tumors, particularly in elderly patients.
Case report
A 81-year-old Japanese woman presented to the
outpatient clinic of the Department of Breast Surgery of the Shiga
Medical Center for Adults (Moriyama, Japan) in January 2016 with a
history of rapid enlargement of a right breast mass. The patient
had noticed a mass 10 years prior. There was no family history of
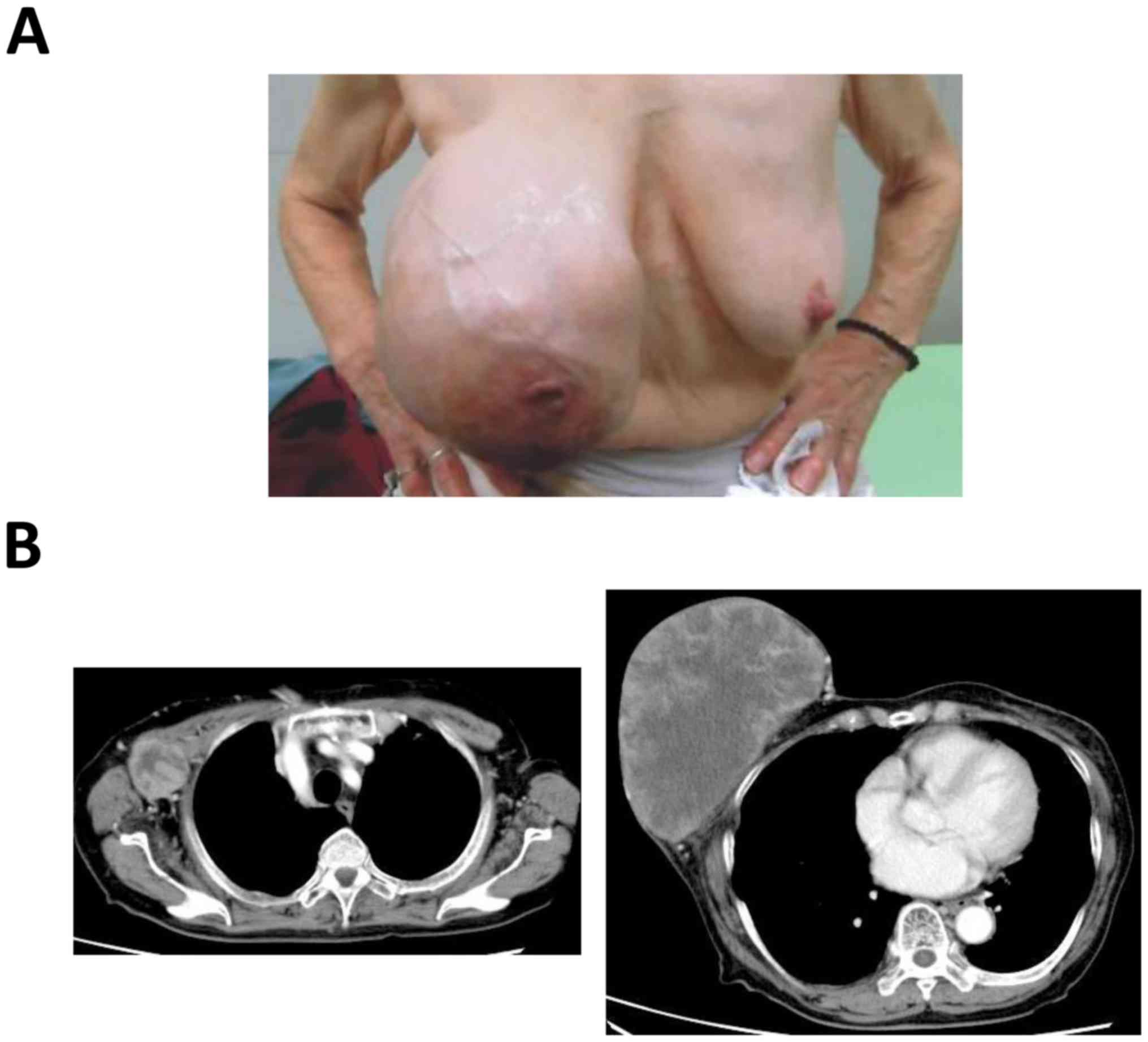
breast cancer. On physical examination, a large nodular mass was
identified, measuring 18×15 cm, involving the entire right breast
(Fig. 1A). There were also palpable
right axillary lymph nodes, with the largest measuring 4×3 cm. The
left breast was normal. A computed tomography (CT) scan revealed a
large heterogeneous solid mass with axillary lymph node metastases
(Fig. 1B); however, there was no
evidence of distant metastasis.
PMBC with axillary lymph nodes metastases was
diagnosed by core needle biopsy and fine-needle aspiration
cytology. The patient received letrozole endocrine therapy as a
primary systemic therapy, as she declined surgery. Soon after
endocrine therapy initiation, the patient visited our emergency
room due to continuous bleeding from lacerated skin caused by tumor
pressure. The patient was found to be anemic (red blood cells
2.98×1012/µl, hemoglobin 8.4 g/dl), due to tumor
neovascularization and intratumoral bleeding.
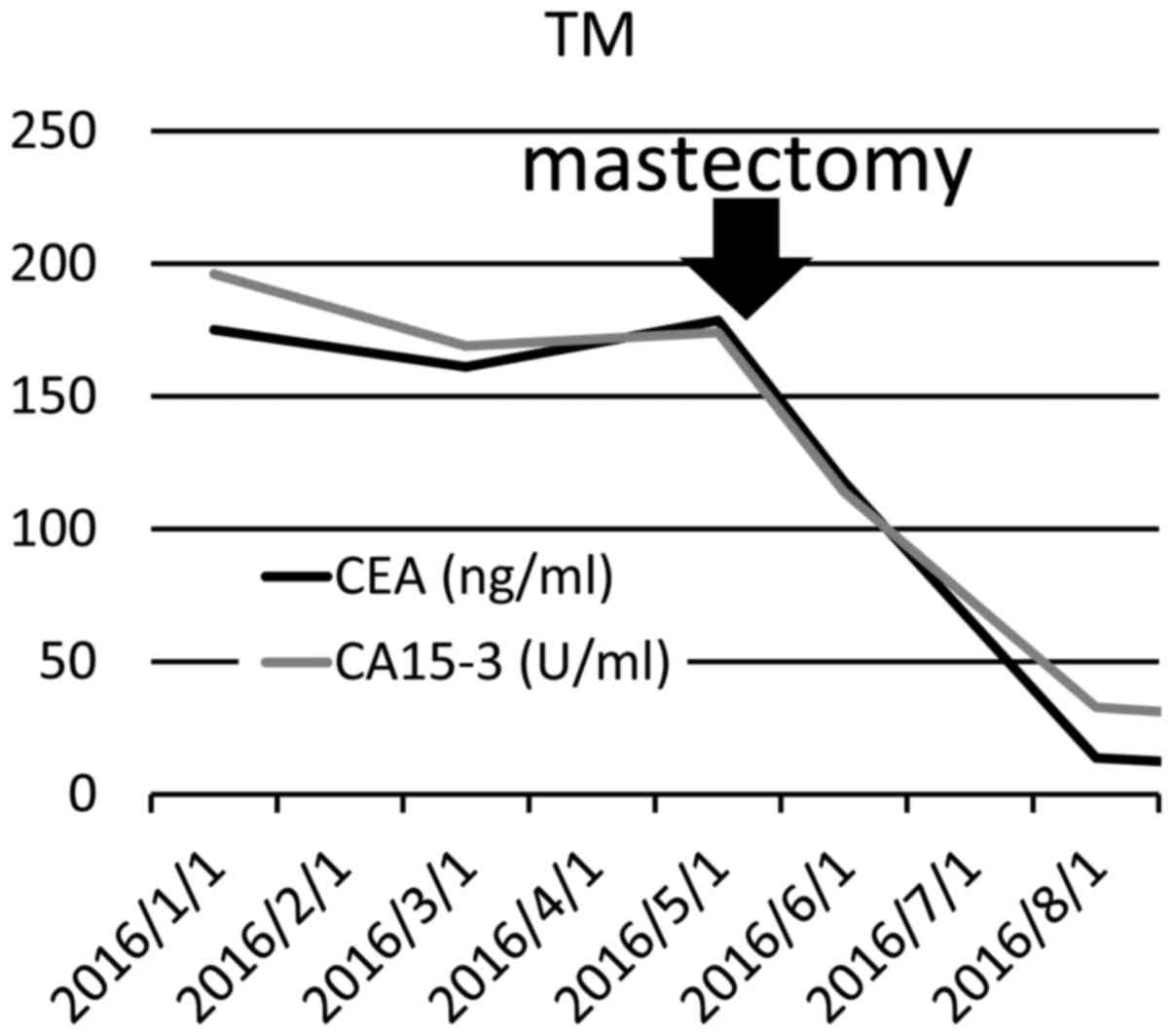
Laboratory data revealed marked elevation of the
serum tumor markers carcinoembryonic antigen (CEA) and carbohydrate
antigen 15–3 (CA15-3) to 175.1 ng/ml and 196.0 U/ml,
respectively.
After 4 months, the tumor had increased in size, and
the patient was started on exemestane and tegafur plus uracil
(UFT®; Taiho Pharma, Tokyo, Japan), as the tumor was
considered difficult to control by endocrine therapy alone. The
patient's advanced age was taken into consideration when selecting
a chemotherapy regimen, in order to preserve her quality of life
(QOL). A large clinical trial of UFT-based postoperative
chemotherapy conducted in Japan (NSASBC-01 trial) demonstrated that
UFT is useful for the treatment of intermediate-risk patients
(10). At 4 weeks after the patient
was started on second-line therapy, there was no significant
reduction in the breast tumor. Finally, the patient consented to
receive simple mastectomy. Despite the lymph node metastases, the
axillary nodes were not removed, as this was a palliative surgery
aiming to remove the bleeding source. The main purpose of the
treatment for this patient was not to completely remove the
macroscopically visible tumor, but maintain the patient's QOL and
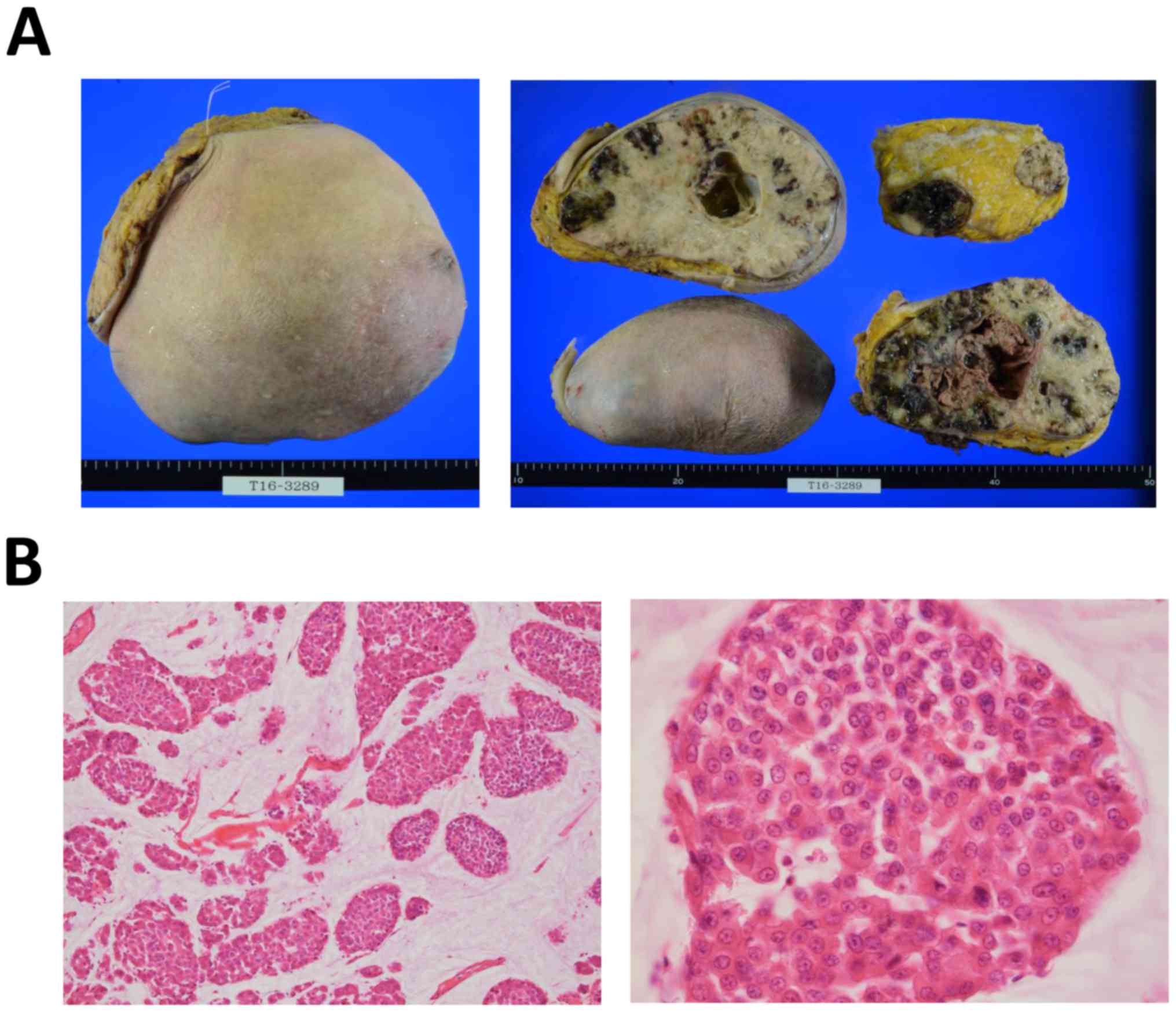
avoid breast cancer-related death. The surgically resected tumor
was 1.96 kg in weight and 17.8 cm in diameter (Fig. 2A). The surgical margin of the breast
was clear, but metastatic lymph nodes remained in the axilla. On
histological analysis, the tumor was classified as a PMBC with
mucinous differentiation in the histologically high-grade
intraductal component, as determined by the accumulation of
intraductal mucin (Fig. 2B).
The patient's anemia subsided after the bleeding
source was removed (red blood cells 4.14×1012/µl,
hemoglobin 12.5 g/dl). In addition, the tumor marker levels
significantly decreased (CEA from 175.1 to 13.6 ng/ml, and CA15-3
from 196.0 U/ml to 32.7 U/ml) following mastectomy (Fig. 3). The patient remained well without
evidence of distant metastasis after the surgery and, despite the
metastatic axillary lymph nodes, lymphedema or neuropathy of the
right arm has not appeared thus far (last follow-up, May 2017). The
patient provided consent regarding the publication of the case
details and associated images.
The medical records of 55 MBC patients were
retrospectively reviewed. Between 1989 and 2016, all the patients
who underwent breast surgery at the Shiga Medical Center for Adults
were investigated and χ2 tests were used to analyze
qualitative data. Overall survival (OS) was defined as the period
from the date of diagnosis to the date of the last follow-up or
death from any cause, and disease-free survival (DFS) was defined
as the period from the date of diagnosis to occurrence of any
event, such as disease progression, relapse, recurrence or death.
Kaplan-Meier estimates were used to calculate OS and DFS using Stat
Mate V for Win & Mac Hybrid software (ATMS, Tokyo, Japan).
Discussion
MBC represents 1–7% of all breast cancers (1,3,4) and is classified by the World Health
Organization into two subtypes: i) PMBC if the non-mucinous
component is <10% and ii) MMBC if the non-mucinous component
comprises 10–49% of the tumor. PMBC may be subtyped into a
hypocellular variant (PMBC-A), exhibiting a tubular, cribriform,
cord-like, micropapillary or papillary growth pattern, and a
hypercellular variant (PMBC-B), growing in solid nests (1). It is generally accepted that PMBC has a
favorable prognosis compared with IDC (2–9).
MMBC is mainly associated with lobular or ductal
neoplasia (in situ or invasive), and a proportion of these
tumors exhibit neuroendocrine differentiation. However, a specific
percentage has not been clearly established for the diagnosis of
MMBC. Due to the distinct clinicopathological characteristics of
PMBC and MMBC, there may be a prognostic difference between the two
types.
Locally advanced MBC is relatively rare. However, it
was previously reported that there is no correlation between tumor
size or subtype and prognosis in PMBC-A or PMBC-B. However, MMBC is
known to have a prognosis similar to that of IDC. It has also been
reported that breast-conserving surgery is effective for MBC due to
their low local recurrence rate (3,7,11).
In order to select the optimal therapy in rare
cases, such as elderly patients with locally advanced MBC, our
experience with this disease and the previous related literature
was reviewed.
Between 1989 and 2016, 55 patients underwent breast
surgery for mucinous carcinoma at the Shiga Medical Center for
Adults. The mean patient age was 63 years (range, 37–85 years).
Following surgery, the patients received therapies administered
according to the National Comprehensive Cancer Network guidelines,
version 1.2015 (12).
Prior to 2,000, selective estrogen receptor
modulator (SERM) therapy was selected for pre- and post-menopausal
patients with estrogen receptor (ER)-or progesterone receptor
(PgR)-positive cancer. Endocrine therapy was mainly administered
for 2 years. However, in patients with high risk of recurrence,
such as node-positive patients or those with tumors sized >5 cm,
5-year endocrine therapy with 2 years of UFT was used prior to
1995, after which time 5-year hormonal therapy became the standard.
Since 2000, aromatase inhibitors were used in post-menopausal
hormonal receptor (HR) -positive patients for 5 years after
surgery. Intravenous chemotherapy was administered in HR-negative
patients and highly node-positive patients.
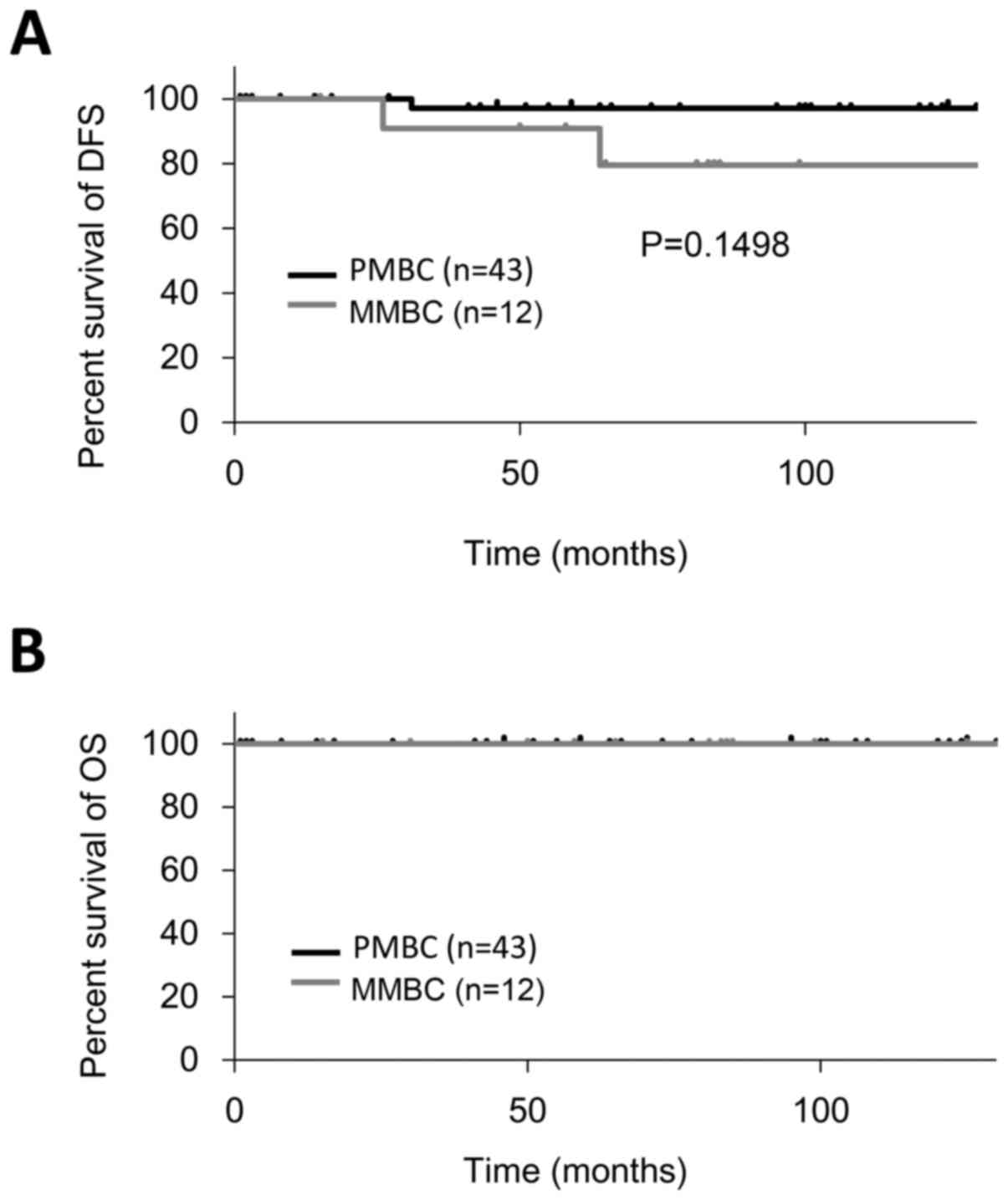
The 10-year DFS and OS were 94.5 and 100.0%,
respectively (Fig. 4). The 10-year
DFS of PMBC and MMBC was 97.7 and 83.3%, respectively. The
clinicopathological characteristics are summarized in Table I. Statistically significant
differences were not observed between the characteristics of PMBC
and MMBC. Of note, a male patient was included in the data.
Although male MBC is rare, it has been previously reported
(13–15).
 | Table I.Clinicopathological variables of
patients with mucinous breast carcinoma (n=55). |
Table I.
Clinicopathological variables of
patients with mucinous breast carcinoma (n=55).
|
| MBC, no. (%) |
|---|
|
|
|
|---|
| Variables | PMBC (n=43) | MMBC (n=12) |
|---|
| Age (years, mean ±
SD) | 59.19±14.12 | 59.25±15.24 |
| Tumor size (mm, mean
± SD) | 26.97±28.70 | 32.00±19.64 |
| Number of positive LN
(mean ± SD) | 0.22±0.58 | 1.25±3.14 |
| Sex |
|
|
|
Female | 42 (97.7) | 12 (100.0) |
| Male | 1 (2.3) | 0 (0.0) |
| Axillary LN
status |
|
|
|
Negative | 36 (83.7) | 8 (66.7) |
|
Positive | 6 (14.0) | 4 (33.3) |
|
Unknown | 1 (2.3) | 0 (0.0) |
| TNM stage |
|
|
| I | 19 (44.2) | 4 (33.3) |
| II | 22 (51.2) | 6 (50.0) |
| III | 2 (4.6) | 2 (16.7) |
| ER status |
|
|
|
Positive | 27 (62.8) | 9 (75.0) |
|
Negative | 6 (14.0) | 1 (8.3) |
|
Unknown | 10 (23.2) | 2 (16.7) |
| PgR status |
|
|
|
Positive | 22 (51.2) | 7 (58.3) |
|
Negative | 11 (25.6) | 3 (25.0) |
|
Unknown | 10 (23.2) | 2 (16.7) |
| HER2 status |
|
|
|
Positive | 1 (2.3) | 1 (8.3) |
|
Negative | 18 (41.9) | 7 (58.3) |
|
Unknown | 24 (55.8) | 4 (33.4) |
| Ki-67 expression,
% |
|
|
|
<20 | 16 (37.2) | 5 (41.7) |
| ≥20 | 2 (4.7) | 0 (0.0) |
|
Unknown | 25 (58.1) | 7 (58.3) |
| Breast surgery |
|
|
|
Mastectomy | 19 (44.2) | 7 (58.3) |
|
Breast-conserving | 24 (55.8) | 5 (41.7) |
| Axillary surgery |
|
|
| Sentinel
lymph node biopsy | 11 (25.6) | 3 (25.0) |
| Axillary
lymph node dissection | 28 (65.1) | 8 (66.7) |
| No
axillary surgery | 4 (9.3) | 1 (8.3) |
| Chemotherapy |
|
|
| No | 32 (74.4) | 7 (58.3) |
| Yes | 8 (18.6) | 4 (33.4) |
|
Unknown | 3 (7.0) | 1 (8.3) |
| Radiotherapy |
|
|
| No | 18 (41.8) | 6 (50.0) |
| Yes | 23 (53.5) | 2 (16.7) |
|
Unknown | 2 (4.7) | 4 (33.3) |
| Anti-HER2 target
therapy |
|
|
| No | 42 (97.7) | 11 (91.7) |
| Yes | 1 (2.3) | 1 (8.3) |
| Endocrine
therapy |
|
|
| No | 11 (25.6) | 2 (16.7) |
| Yes | 29 (67.4) | 9 (75.0) |
|
Unknown | 3 (7.0) | 1 (8.3) |
| Arm lymphedema |
|
|
| No | 39 (90.7) | 12 (100.0) |
|
Yes | 1 (2.3) | 0 (0.0) |
|
Unknown | 3 (7.0) | 0 (0.0) |
Of the 55 patients, 3 developed recurrence. One
patient had stage I,
ER+/PgR+/HER2− PMBC, and developed
intramammary local recurrence 2 years and 7 months after
breast-conserving surgery, followed by bone metastases at 8 years
and 9 months after the operation. Second-line letrozole with
zoledronic acid were continued, and the patient maintained stable
disease at the last follow-up visit (May 2017). The remaining 2
patients experienced lung metastases: One patient had stage I
ER+/PgR−/HER2− MMBC, and developed
multiple lung metastases 5 years and 4 months after the surgery. At
the last follow-up visit (June 2017) the patient had progressive
disease, controlled by 8th-line weekly paclitaxel. The
second patient had stage IIIA (pt3n1M0)
ER+/PgR+/HER2− MMBC, and developed
lung and parasternal lymph node metastases 2 years and 2 months
after the surgery. Surprisingly, at the last follow-up visit (May
2017) the patient had achieved clinically complete response by
multidisciplinary therapy.
One of the PMBC patients suffered from arm
lymphedema following axillary lymph node dissection. In the data
presented herein, the incidence rate of patients who underwent
axillary lymph node dissection was 2.8%, which is relatively lower
compared with previous reports (5.3–54.0%) (14,16,17). The
incidence rate of postoperative complications such as pain,
lymphedema, numbness or motility disorders differs between sentinel
node biopsy and axillary lymph node dissection (16,17). It
has been reported that chemotherapy with taxanes and radiation
covering the breast and supraclavicular region were independent
risk factors for lymphedema (18,19).
Generally, MBC is potentially resistant to chemotherapy or
radiotherapy (1,2,4). The
combination of chemotherapy and radiation therapy with axillary
dissection is infrequently selected, even in locally advanced MBC,
and we recommend that it is avoided.
Consistent with previous reports, the postoperative
recurrence rate was higher in MMBC compared with PMBC (5,7–9). Since distant metastasis is rare,
particularly in PMBC, some reports observed no association between
tumor size (2,5,8) or lymph
node status (8) and prognosis. It is
considered that relapse develops after a long-term disease-free
period, indicating that elderly PMBC patients do not always require
aggressive chemotherapy or radical surgery. Even in patients with
axillary lymph node metastasis, it may be considered a viable
option to obtain sufficient symptom improvement by palliative
rather than radical surgery.
If axillary lymph node status is not correlated with
prognosis in PMBC, the main purpose of axillary surgery is staging
and dissection may be omitted.
As in the present case, axillary lymph node
dissection is not always considered necessary, as there was no
complaint of arm edema or pain due to tumor pressure, and the
patient continues systemic therapy after surgery. The patient's
severe anemia improved immediately after the removal of the main
tumor. Given the characteristics of PMBC, it is less likely to lead
to immediate tumor-related death, and the possibility of arm edema
due to direct vascular invasion from axillary lymph nodes is
low.
In conclusion, palliative surgery may be a viable
option, particularly for elderly patients with locally advanced
PMBC, in order to maintain their QOL.
Glossary
Abbreviations
Abbreviations:
|
MBC
|
mucinous breast carcinoma
|
|
PMBC
|
pure type mucinous breast
carcinoma
|
|
MMBC
|
mixed type mucinous breast
carcinoma
|
|
IDC
|
invasive ductal carcinoma
|
|
OS
|
overall survival
|
|
DFS
|
disease-free survival
|
References
|
1
|
Rosen PP: Mucinous, Carcinoma Rosen's
Breast Pathology. 3rd. Lippincott and Wiliams & Wilkins;
Philadelphia: pp. 515–177. 2009
|
|
2
|
Bae SY, Choi MY, Cho DH, Lee JE, Nam SJ
and Yang JH: Mucinous carcinoma of the breast in comparison with
invasive ductal carcinoma: Clinicopathologic characteristics and
prognosis. J Breast Cancer. 14:308–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Di Saverio S, Gutierrez J and Avisar E: A
retrospective review with long term follow up of 11,400 cases of
pure mucinous breast carcinoma. Breast Cancer Res Treat.
111:541–547. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Diab SG, Clark GM, Osborne CK, Libby A,
Allred DC and Elledge RM: Tumor characteristics and clinical
outcome of tubular and mucinous breast carcinomas. J Clin Oncol.
17:1442–1448. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Komenaka IK, El-Tamer MB, Troxel A,
Hamele-Bena D, Joseph KA, Horowitz E, Ditkoff BA and Schnabel FR:
Pure mucinous carcinoma of the breast. Am J Surg. 187:528–532.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lacroix-Triki M, Suarez PH, MacKay A,
Lambros MB, Natrajan R, Savage K, Geyer FC, Weigelt B, Ashworth A
and Reis-Filho JS: Mucinous carcinoma of the breast is genomically
distinct from invasive ductal carcinomas of no special type. J
Pathol. 222:282–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan B, Yao R, Shi J, Xu QQ, Zhou YD, Mao
F, Lin Y, Guan JH, Wang XJ, Zhang YN, et al: Prognosis of subtypes
of the mucinous breast carcinoma in Chinese women: A
population-based study of 32-year experience (1983-2014).
Oncotarget. 7:38864–38875. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paramo JC, Wilson C, Velarde D, Giraldo J,
Poppiti RJ and Mesko TW: Pure mucinous carcinoma of the breast: Is
axillary staging necessary? Ann Surg Oncol. 9:161–164. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang M, Teng XD, Guo XX, Zhao JS and Li
ZG: Clinicopathological characteristics and prognosis of mucinous
breast carcinoma. J Cancer Res Clin Oncol. 140:265–269. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Watanabe T, Sano M, Takashima S, Kitaya T,
Tokuda Y, Yoshimoto M, Kohno N, Nakagami K, Iwata H, Shimozuma K,
et al: Oral uracil and tegafur compared with classic
cyclophosphamide, methotrexate, fluorouracil as postoperative
chemotherapy in patients with node-negative, high-risk breast
cancer: National Surgical Adjuvant Study for Breast Cancer 01
Trial. J Clin Oncol. 27:1368–1374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anan K, Mitsuyama S, Tamae K, Nishihara K,
Iwashita T, Abe Y, Ihara T, Nakahara S, Katsumoto F and Toyoshima
S: Pathological features of mucinous carcinoma of the breast are
favourable for breast-conserving therapy. Eur J Surg Oncol.
27:459–463. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
National Comprehensive Cancer Network
Guidelines Version 1. 2015 Panel Members. https://www.nccn.orgAccessed. Aug 21–2017.
|
|
13
|
Gupta K, Sharma S, Kudva R and Kumar S:
Mixed mucinous and infiltrating carcinoma occurring in male breast-
study of clinico-pathological features: A rare case report. J Clin
Diagn Res. 9:ED07–ED08. 2015.PubMed/NCBI
|
|
14
|
Pawar PS, Poflee SV, Pande NP and
Shrikhande AV: Preoperative cytological diagnosis of mucinous
carcinoma (MC) of male breast. J Cytol. 33:58–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ishida M, Umeda T, Kawai Y, Mori T, Kubota
Y, Abe H, Iwai M, Yoshida K, Kagotani A, Tani T and Okabe H:
Mucinous carcinoma occurring in the male breast. Oncol Lett.
7:378–380. 2014.PubMed/NCBI
|
|
16
|
Haid A, Kuehn T, Konstantiniuk P,
Köberle-Wührer R, Knauer M, Kreienberg R and Zimmermann G:
Shoulder-arm morbidity following axillary dissection and sentinel
node only biopsy for breast cancer. Eur J Surg Oncol. 28:705–710.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schrenk P, Rieger R, Shamiyeh A and Wayand
W: Morbidity following sentinel lymph node biopsy versus axillary
lymph node dissection for patients with breast carcinoma. Cancer.
88:608–614. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim M, Shin KH, Jung SY, Lee S, Kang HS,
Lee ES, Chung SH, Kim YJ, Kim TH and Cho KH: Identification of
prognostic risk factors for transient and persistent lymphedema
after multimodal treatment for breast cancer. Cancer Res Treat.
48:1330–1337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Swaroop MN, Ferguson CM, Horick NK, Skolny
MN, Miller CL, Jammallo LS, Brunelle CL, O'Toole JA, Isakoff SJ,
Specht MC and Taghian AG: Impact of adjuvant taxane-based
chemotherapy on development of breast cancer-related lymphedema:
Results from a large prospective cohort. Breast Cancer Res Treat.
151:393–403. 2015.PubMed/NCBI
|