Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second
most common primary liver cancer after hepatocellular carcinoma
(HCC) (1–3). ICC has been categorized as peripheral
and perihilar types based on location (1,4), and as
mass-forming, periductular infiltrating and intraductal growth
types, based on the growth pattern classification of ICC by the
Liver Cancer Study Group of Japan (5). An increasing number of studies suggest
that surgical resection usually offers the possibility of long-term
survival to patients with this disease (2,6,7). Although there has been a worldwide
increase in the incidence and mortality of ICC in recent years
(2,3), ICC has not been investigated as
extensively as HCC (1).
Previous studies suggested that hepatitis B virus
(HBV) infection and cirrhosis, which are well-documented pathogenic
factors in the development of HCC (8–11), may
also be associated with an increased risk of ICC (12–15).
Cirrhosis is common among HCC patients (8–10), and
has been proven to be a poor prognostic factor following surgical
treatment of HCC (10,16,17). A
significant proportion of ICC patients are also cirrhotic; however,
the prognostic role of this finding has not been extensively
investigated. Although HBV infection has been reported to be a
favorable prognostic factor for ICC patients and the
clinicopathological characteristics differ between patients with
and those without HBV infection (13,18,19), the
role of cirrhosis in the prognosis of ICC patients has not been
fully elucidated due to the limited number of related studies.
Cirrhosis has been found to be a favorable prognostic factor for
ICC patients in our former study (20); however, the opposite result was
reported by another previous study (21). The aim of the present study was to
determine the effect of cirrhosis on the prognosis of ICC patients
and the mechanism underlying this effect through comparing
clinicopathological characteristics and survival data in large
series of ICC patients with and without cirrhosis.
Patients and methods
Patients recruiting and grouping
A retrospective study was undertaken, including all
consecutive patients with ICC who were admitted to the Eastern
Hepatobiliary Surgery Hospital (Shanghai, China) for initial
surgical treatment between January 2007 and December 2011. The
inclusion criteria were as follows: No history of previous
anticancer therapy and no history of other malignancies; no severe
comorbidities that may affect survival; potentially resectable ICC
on preoperative imaging; and no general contraindications to
surgery. The exclusion criteria were as follows: Hilar or
extrahepatic cholangiocarcinoma; combined HCC and
cholangiocarcinoma; periductular infiltrating type and intraductal
growth pattern of ICC; Child's C liver function; hepatitis C virus
infection; definitive distant metastasis beyond the abdomen; and
incomplete survival data. The patients were identified through
computerized hospital databases. Subsequently, demographic data
were collected for each patient, including age, gender, symptoms,
underlying liver diseases, imaging findings, laboratory tests and
pathological results. The patients were divided into two groups
according to the presence or absence of cirrhosis, which was
defined as widespread disruption of normal liver structure by the
formation of pseudolobules or Scheuer stage 4 fibrosis in
pathological findings (22). The
protocol of the present study was approved by the local Ethics
Committee.
Preoperative workup
The preoperative workup included abdominal
ultrasonography, computed tomography (CT) and/or magnetic resonance
imaging (MRI), cardiac and pulmonary function testing, endoscopic
examination and laboratory tests. For patients with local or
complete biliary obstruction, endoscopic retrograde
cholangiopancreatography or magnetic resonance
cholangiopancreatography was performed. In patients with suspected
metastasis, positron emission tomography (PET) and CT (PET-CT) was
performed.
Surgery
Patients underwent R0 (curative) or R1 (microscopic
infiltration of the resection margin) liver resection, apart from
cases where distant metastases, peritoneal carcinomatosis,
extensive vascular involvement and/or multiple intrahepatic
metastases were identified intraoperatively. R2 (palliative)
resection and exploratory laparotomy with biopsy intention prior to
surgery were not recommended, except when the abovementioned
unfavorable findings during intraoperative exploration were beyond
the preoperative evaluation of ICC and R0/R1 resection could not be
performed. The majority of the liver resections were performed
under vascular control, and anatomical or non-anatomical
hepatectomy was determined depending on the size and location of
tumor, as well as on the background of chronic liver disease. The
types of hepatic resection performed included segmentectomy or
local resection, bisegmentectomy, right or left hemihepatectomy,
and extended hemihepatecomy, according to the 2000 Brisbane
Classification of the International Hepato-Pancreato-Biliary
Association (23). Additional
procedures included cholecystectomy, resection of the biliary
confluence and extrahepatic bile duct with Roux-en-Y
hepatojejunostomy, portal vein cancerous thrombectomy, and vascular
reconstruction. In patients with R0/R1 liver resection and
suspected lymph node (LN) metastasis, LN dissection was performed
when possible. In other patients with R0/R1 tumor resection and
without evidence of macroscopic LN enlargement, preventive
skeletonization of the hepatoduodenal ligament was performed to
confirm the stage.
Pathological and immunohistochemical
methods
All the resected and bioptic specimens were
pathologically examined, including tumor size and number, capsule
formation, LN metastasis, vascular invasion, perineural invasion
and tumor cell differentiation. Each tumor was staged according to
the 7th edition of the American Joint Committee on Cancer (AJCC)
staging system for ICC (24). The
surgical margins were examined for the presence of residual tumor
and were classified according to the R classification as R0 (no
residual tumor and resection margin >0 mm), R1 (microscopic
residual tumor or null-margin resection) or R2 (macroscopic
residual tumor) (25). Curative
resection was defined a negative resection margin on
histopathological examination.
Follow-up
All the patients were followed up postoperatively by
X-ray of the chest, ultrasound scan of the liver, liver function
tests and serum levels of carbohydrate antigen (CA) 19-9,
carcinoembryonic antigen (CEA) and α-fetoprotein (AFP) at an
interval of 1-3 months. When recurrence or metastasis were
suspected, a CT or MRI scan was performed to confirm the diagnosis.
Treatments for recurrent disease included surgery, transarterial
chemoembolization, radiotherapy and supportive therapy. Survival
was evaluated from the date of surgery; the patients were followed
up for survival until death or until the study deadline date of
September 30, 2014.
Statistical analysis
Continuous variables are presented as the mean ±
standard deviation or as median values and range. Categorical
variables are presented as total and percentage. Comparisons were
performed using the unpaired t-test for continuous variables and
the Chi-squared or Wilcoxon test for categorical variables. Overall
survival (OS) rates were calculated using the Kaplan-Meier method.
The statistically significant prognostic factors were analyzed by
univariate analysis, evaluated using the Kaplan-Meier method and
compared with the log-rank test. The multivariate analysis was
performed using the Cox proportional hazards model to identify the
independent prognostic factors for survival. Statistical analysis
was performed using the SPSS 19.0 software for Windows (SPSS Inc.,
Chicago, IL). Differences with P-values of <0.05 were considered
statistically significant.
Results
Clinical characteristics
A total of 1,312 patients with ICC were recruited,
with a male predominance (896 patients; 68.3%) and a median age of
54 years (range, 18–82 years). The patients included 302 (23.0%)
with and 1,010 (77.0%) without cirrhosis. The differences in
clinical characteristics between the two groups of patients are
listed in Table I. Compared with
patients without cirrhosis, those with cirrhosis were younger,
included a higher percentage of men, and fewer had symptoms,
elevated serum levels of CA19-9 and/or CEA or other concurrent
liver diseases, such as schistosomiasis and cholelithiasis;
however, a higher percentage of cirrhotic patients had elevated
serum levels of AFP.
 | Table I.Comparison of clinical
characteristics between intrahepatic cholangiocarcinoma patients
with and without cirrhosis. |
Table I.
Comparison of clinical
characteristics between intrahepatic cholangiocarcinoma patients
with and without cirrhosis.
|
Characteristics | With cirrhosis, n
(%) (n=302) | Without cirrhosis,
n (%) (n=1,010) | P-value |
|---|
| Age (years) |
|
| <0.001 |
| Mean ±
standard deviation | 51.65±10.05 | 54.80±11.07 |
|
| Gender |
|
| <0.001 |
|
Male | 270 (89.4) | 626 (62.0) |
|
|
Female | 32 (10.6) | 384 (38.0) |
|
| Symptoms |
|
| <0.001 |
| No | 155 (51.3) | 344 (34.1) |
|
|
Yes | 147 (48.7) | 666 (65.9) |
|
| HBsAg
positivity | 272 (90.1) | 326 (32.3) | <0.001 |
| Alcoholic | 59 (19.5) | 131 (13.0) | 0.004 |
|
Schistosomiasis | 7
(2.3) | 64 (6.3) | 0.007 |
| Cholelithiasis | 30 (9.9) | 224 (22.2) | <0.001 |
| Elevated AFP
level | 119 (39.4) | 129 (12.8) | <0.001 |
| Elevated CA19-9
and/or CEA level | 143 (47.4) | 633 (62.7) | <0.001 |
| Albumin (g/l) |
|
| 0.164 |
| Mean ±
standard deviation | 41.53±3.97 | 41.91±4.29 |
|
| Bilirubin
(µmol/l) |
|
|
0.016 |
|
≤20 | 243 (80.5) | 870 (86.1) |
|
|
>20 | 59 (19.5) | 140 (13.9) |
|
| ALT(U/l) |
|
| 0.001 |
|
≤42 | 197 (65.2) | 759 (75.1) |
|
|
>42 | 105 (34.8) | 251 (24.9) |
|
Pathological characteristics
On pathological examination, more patients without
cirrhosis had well/moderately differentiated tumors, while more
patients with cirrhosis had tumors with capsule formation (Table II). Patients with cirrhosis had
relatively smaller tumors, with a lower likelihood of LN metastasis
and perineural invasion, but with a higher likelihood of vascular
invasion when compared with patients without cirrhosis. According
to the 7th edition of the AJCC staging system, 506 cases (38.6%)
were stage I, 323 (24.5%) were stage II, 103 (7.9%) were stage III
and 380 (29.0%) were stage IV; more patients with cirrhosis had
tumors at an earlier stage compared with those without cirrhosis
(Table II).
 | Table II.Comparison of pathological
characteristics between intrahepatic cholangiocarcinoma patients
with and without cirrhosis. |
Table II.
Comparison of pathological
characteristics between intrahepatic cholangiocarcinoma patients
with and without cirrhosis.
|
Characteristics | With cirrhosis, n
(%) (n=302) | Without cirrhosis,
n (%) (n=1,010) | P-value |
|---|
| Tumor size
(cm) |
|
| <0.001 |
| Mean ± standard
deviation | 6.39±3.69 | 7.29±3.53 |
|
| Tumor number |
|
|
0.225 |
|
Single | 202 (66.9) | 637 (63.1) |
|
|
Multiple | 100 (33.1) | 373 (36.9) |
|
| Capsule
formation | 40
(13.2) | 36 (3.6) | <0.001 |
|
Differentiation |
|
|
|
| High or
moderate | 269 (89.1) | 944 (93.5) |
0.011 |
|
Poor | 33
(10.9) | 66 (6.5) |
|
| Lymphatic
metastasis | 52
(17.2) | 318 (31.5) | <0.001 |
| Vascular
invasion | 92
(30.5) | 111 (11.0) | <0.001 |
| Perineural
invasion | 5
(1.7) | 91 (9.0) | <0.001 |
| Stage |
|
| <0.001 |
|
I–II | 225 (74.5) | 604 (59.8) |
|
|
III–IV | 77
(25.5) | 406 (40.2) |
|
Surgical results
Of the 1,312 ICC patients undergoing surgery, 1,260
received tumor resection (overall resectability rate, 96.0%), among
whom 296 (98.0%) were cirrhotic and 964 (95.4%) were non-cirrhotic.
The types of liver resection included extended right or left
hemihepatectomy in 69 (5.3%), right or left hemihepatectomy in 424
(32.3%), bisegmentectomy in 517 (39.4%), and segmentectomy or local
resection in 250 (19.1%) patients; in patients with multiple
tumors, different types of liver resections were used in
combination, depending on the location and number of the tumors.
The distribution of different types of liver resection in patients
with and without cirrhosis is shown in Table III. Compared with cirrhotic
patients, a wider resection range was more common among
non-cirrhotic patients.
 | Table III.Distribution of different types of
liver resection in intrahepatic cholangiocarcinoma patients with
and without cirrhosis. |
Table III.
Distribution of different types of
liver resection in intrahepatic cholangiocarcinoma patients with
and without cirrhosis.
| Types of liver
resection | With cirrhosis, n
(%) | Without cirrhosis,
n (%) | P-value |
|---|
| Surgical range | 296 | 964 |
|
|
Segmentectomy or local
resection | 90 (30.4) | 160 (16.6) | <0.001 |
|
Bisegmentectomy | 129 (43.6) | 388 (40.2) |
0.308 |
|
Hemihepatectomy | 63 (21.3) | 361 (37.4) | <0.001 |
|
Extended hemihepatectomy | 14 (4.7) | 55 (5.7) |
0.519 |
| Surgical margin
status | 302 | 1,010 |
|
| R0
resection | 146 (48.3) | 308 (30.5) | <0.001 |
| R1
resection | 113 (37.4) | 478 (47.3) |
0.002 |
| R2
resection | 37 (12.3) | 178 (17.6) |
0.027 |
|
Exploratory laparotomy | 6 (2.0) | 46 (4.6) |
0.045 |
R0, R1 and R2 resection was performed in 454
(34.6%), 591 (45.0%) and 215 (16.4%) patients, respectively; the
remaining 52 (4.0%) patients only underwent exploratory laparotomy
with biopsy due to unresectable disease (e.g., extensive
intrahepatic metastases or peritoneal seeding). Compared with
patients without cirrhosis, a significantly higher rate of R0
resection was achieved in patients with cirrhosis (Table III).
Survival of the entire cohort
The duration of survival was defined as the time
from surgery to the date of death or the last follow-up, and the
median follow-up period was 47 months (range, 1–93 months). The 1-,
3- and 5-year OS rates for the entire cohort were 57.0, 19.9 and
13.3%, respectively, with a median survival time (MST) of 14.0
months.
Survival of patients with and without
cirrhosis
A significant difference in survival rates was
observed between patients with and those without cirrhosis; the 1-,
3- and 5-year OS rates for patients with and without cirrhosis were
62.3, 24.1 and 14.7% (MST, 16.0 months), and 55.4, 18.7 and 13.0%
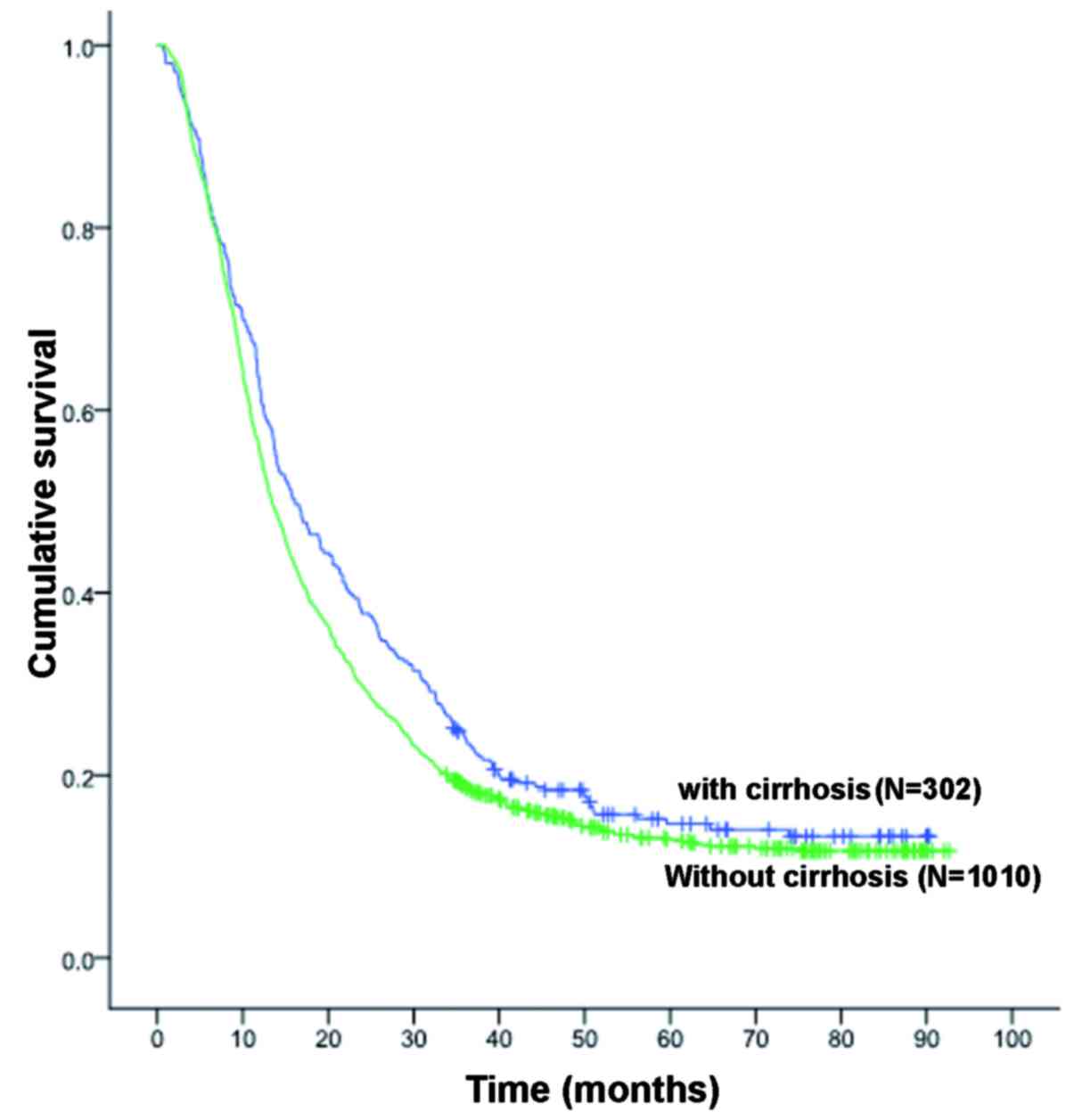
(MST, 13.0 months), respectively (P<0.038, Fig. 1).
Univariate and multivariate
analyses
The univariate analysis demonstrated that certain
variables, including HBV infection, cirrhosis, presence of
cholelithiasis, serum level of CA19-9 and/or CEA, tumor size, tumor
number, capsule formation, LN metastasis, vascular invasion,
perineural invasion and surgical margin status, were statistically
significant prognostic factors affecting the survival of ICC
patients (Table IV). Cox's
regression multivariate analysis identified HBV infection and
cirrhosis as independent favorable prognostic factors, while the
presence of cholelithiasis, elevated CA19-9 and CEA levels,
multiple tumors, lymphatic metastasis, vascular invasion and
positive surgical margin status were independent unfavorable
prognostic factors, with hazard ratios of 1.330, 1.726, 1.380,
1.297, 1.193 and 1.788, respectively (Table IV).
 | Table IV.Univariate and multivariate analyses
of variables associated with overall survival after surgery in
1,312 patients with intrahepatic cholangiocarcinoma. |
Table IV.
Univariate and multivariate analyses
of variables associated with overall survival after surgery in
1,312 patients with intrahepatic cholangiocarcinoma.
|
|
|
|
| Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Variables | N (%) | Median survival
(months) | Univariate analysis
(P-value) | P-value | HR (95% CI) |
|---|
| Age (years) |
|
| 0.795 | – | – |
|
≤60 | 920 (70.1) | 14 |
|
|
|
|
>60 | 392 (29.9) | 14 |
|
|
|
| Gender |
|
| 0.736 | – | – |
|
Male | 896 (68.3) | 14 |
|
|
|
|
Female | 416 (31.7) | 13 |
|
|
|
| HBsAg |
|
| <0.001 | <0.001 | 1.435
(1.248–1.649) |
|
(−) | 714 (54.4) | 12 |
|
|
|
|
(+) | 598 (45.6) | 19 |
|
|
|
| Cirrhosis |
|
| 0.038 | <0.001 | 1.367
(1.161–1.609) |
| No | 1,010 (77.0) | 13 |
|
|
|
|
Yes | 302 (23.0) | 16 |
|
|
|
| Alcoholic |
|
| 0.473 | – |
|
| No | 1,122 (85.5) | 14 |
|
|
|
|
Yes | 190 (14.5) | 15 |
|
|
|
|
Schistosomiasis |
|
| 0.762 | – |
|
| No | 1,241 (94.6) | 14 |
|
|
|
|
Yes | 71 (5.4) | 13 |
|
|
|
| Cholelithiasis |
|
| <0.001 | <0.001 | 1.330
(1.145–1.545) |
| No | 1,058 (80.6) | 15 |
|
|
|
|
Yes | 254 (19.4) | 10 |
|
|
|
| AFP elevation |
|
| 0.675 |
|
|
| No | 1,064 (81.1) | 14 |
|
|
|
|
Yes | 248 (19.0) | 14 |
|
|
|
| CA19-9 and/or CEA
elevation |
|
| <0.001 | <0.001 | 1.726
(1.520–1.959) |
| No | 536 (40.9) | 23 |
|
|
|
|
Yes | 776 (59.1) | 11 |
|
|
|
| Tumor size
(cm) |
|
| <0.001 |
0.105 | – |
| ≤5 | 464 (35.4) | 20 |
|
|
|
|
>5 | 848 (64.6) | 12 |
|
|
|
| Tumor number |
|
| <0.001 | <0.001 | 1.380
(1.212–1.571) |
|
Single | 839 (63.9) | 18 |
|
|
|
|
Multiple | 473 (36.1) | 10 |
|
|
|
| Capsule
formation |
|
| <0.001 |
0.141 | – |
| No | 1,236 (94.2) | 14 |
|
|
|
|
Yes | 76 (5.8) | 25 |
|
|
|
| Lymph node
metastasis |
|
| <0.001 |
0.001 | 1.297
(1.110–1.515) |
| No | 942 (71.8) | 18 |
|
|
|
|
Yes | 370 (28.2) | 8 |
|
|
|
| Vascular
invasion |
|
| 0.010 |
0.036 | 1.193
(1.012–1.407) |
| No | 1,109 (84.5) | 14 |
|
|
|
|
Yes | 203 (15.5) | 13 |
|
|
|
| Perineural
invasion |
|
|
0.001 | 0.235 | – |
| No | 1,216 (92.7) | 14 |
|
|
|
|
Yes | 96 (7.3) | 11 |
|
|
|
|
Differentiation |
|
| 0.836 | – | – |
| High or
moderate | 1,213 (92.5) | 14 |
|
|
|
|
Poor | 99 (7.5) | 13 |
|
|
|
| Surgical margin
status |
|
| <0.001 | <0.001 | 1.788
(1.612–1.984) |
| R0
resection | 454 (34.6) | 24 |
|
|
|
| R1
resection | 591 (45.0) | 14 |
|
|
|
| R2
resection | 215 (16.4) | 6 |
|
|
|
|
Exploratory laparotomy | 52 (4.0) | 4 |
|
|
|
Discussion
ICC is a heterogeneous group of tumors, with
different risk factors, biological behavior and clinicopathological
characteristics and, consequently, different prognosis (1,2,6,15). HBV
infection and cirrhosis are established risk factors for HCC
(8,11), and several recent studies have
suggested HBV infection may also be associated with the occurrence
of ICC (12,13,18,19);
however, the association between cirrhosis and the
pathogenesis/prognosis of ICC remains unknown. The present study
confirmed the earlier observation that cirrhosis is prevalent among
patients with ICC in highly endemic areas (6), as it was observed in 23.0% of our
patients, which is a markedly higher percentage compared with
Western countries (15). Cirrhosis
is most likely associated with HBV infection in China (26), and 90.1% of cases with cirrhosis in
the present series were seropositive for hepatitis B surface
antigen, indicating HBV-related cirrhosis. Although the prevalence
in our study may not prove a causal association between cirrhosis
and ICC, as is the case with HCC, it indicates a correlation
between the two. Patients with cirrhosis have a ~16-fold higher
risk of HCC compared with inactive carriers (8). Therefore, future investigation should
examine whether cirrhosis plays a synergistic role in ICC
development in patients with HBV infection.
It is generally hypothesized that the prognosis of
ICC is worse compared with that of HCC following surgical treatment
(2,5,27).
Complete surgical resection is the only curative treatment for ICC;
however, similar to previous reports (2,6,7,25), the
OS of this entire cohort indicated that the prognosis of ICC is
dismal following surgical management, with an MST of only 14.0
months, as the disease is usually advanced at the time of
diagnosis. There are several known factors affecting the prognosis
of ICC after surgery, including surgical margin status, multiple
tumors, LN metastasis and vascular invasion (2,6,7,25,28). In
earlier studies on ICC, cirrhosis (21), unlike HBV infection (13,18,19), was
shown to be an independent unfavorable prognostic factor for
survival, but little is known on the underlying mechanism. However,
the multivariate analysis in this series and our former study
(20) revealed that cirrhosis was an
independent favorable prognostic factor for survival of ICC
patients following surgery. Different sample sizes may be the
reason for the different results reported by these studies
regarding the role of cirrhosis in ICC prognosis.
In the present study, all the patients with
underlying liver disease had well-compensated liver function (Child
A), which, in non-cirrhotic cases, should not significantly affect
the extent of hepatectomy or postoperative morbidity and mortality.
However, as reported by an earlier study on ICC (21), cirrhosis exerts a negative effect on
major hepatectomy, and cirrhotic patients in our study tended to
have a smaller resection range (Table
III). Non-cirrhotic patients may be more amenable to resection
due to a relatively better preserved liver function, as in HCC
tumors (16,17). However, the R0 resection rate was
significantly higher among cirrhotic patients (P<0.001), while
the rates of non-curative resection [R1 (P=0.002) and R2 (P=0.027)]
and exploratory laparotomy (P=0.045) were significantly higher
among non-cirrhotic patients, which may be one of the reasons for
the superior survival of ICC patients with cirrhosis. ICCs in
non-cirrhotic patients tended to be larger, with a lower incidence
of capsule formation, and at a more advanced stage at diagnosis
compared with those in cirrhotic patients (Table II), which may be the reason for the
better surgical margin status and survival in ICC patients with
cirrhosis. ICC patients with cirrhosis exhibited a significantly
better survival compared with those without cirrhosis (Fig. 1), which may not be attributed to
early tumor detection. Although our data demonstrated that patients
with cirrhosis had significantly smaller tumors compared with those
without cirrhosis (Table I), tumor
size was not found to be an independent prognostic factor for ICC
patients. In the present study, the presence of cholelithiasis, HBV
infection, elevated CA19-9 and CEA levels, surgical margin status
and certain pathological characteristics, such as multiple tumors,
capsule formation, lymphatic metastasis and vascular invasion, were
significantly associated with the presence of cirrhosis, but the
associations were not causative, and multivariate analysis
demonstrated that these factors together with cirrhosis were all
independent prognostic factors for ICC (Table IV). The differences in the
clinicopathological characteristics between ICC patients with and
those without cirrhosis may be due to different underlying
pathogenic mechanisms in the two groups of patients.
According to previous studies, the development of
HCC in cirrhotic and non-cirrhotic livers may be underlined by
distinct mechanisms (8,9), which has not been proven in ICC
patients. In the present study, in the clinical setting, ICC
patients with cirrhosis exhibited different and unique
characteristics compared with patients without cirrhosis. The
findings of this study suggested that ICC associated with cirrhosis
may display a biological behavior similar to that of HCC and, thus,
have a better prognosis. Although the etiology of ICC remains
unclear, there is growing evidence suggesting that ICC associated
with cirrhosis may be derived from the same hepatic progenitor
cells as HCC (13,14,18,19) and,
thus, behaves more like HCC, which is generally considered to have
a more favorable prognosis compared with ICC (2,5,27,29). The
observations of the present study indicate that ICC occurring in
patients with cirrhosis may share a common carcinogenic process
with HCC. Compared with non-cirrhotic patients, cirrhotic ICC
patients were more likely younger and male, a profile resembling
that of HCC patients (18,19). The formation of vascular tumor
thrombi, one of pathological characteristics of HCC, was observed
more often among ICC patients with cirrhosis compared with those
without cirrhosis. In contrast to a previous study (21), LN metastasis and perineural invasion,
which are typical pathological characteristics of adenocarcinoma,
were less often found in ICC patients with cirrhosis compared with
those without cirrhosis. AFP is often used as a tumor marker for
HCC and, in the present study, a significantly higher number of
cirrhotic ICC patients exhibited elevated serum AFP levels compared
with non-cirrhotic patients, suggesting that the ICC cells may
exhibit hepatocellular differentiation. These findings also suggest
that ICC with cirrhosis and HCC may share a common carcinogenic
process.
The present study had several limitations. First, a
small number of patients with mild fibrosis or steatosis were
included, which may have affected the findings; however, none of
these patients had true cirrhosis and, therefore, were considered
eligible for inclusion in the cohort of non-cirrhotic patients.
Patients with HCV infection, a known inciting factor of
hepatocarcinogenesis (12), were not
included in the present study due to the small case series of HCV
infection. A number of patients received non-radical resection and
a considerable percentage of non-anatomical hepatectomies were
included in this study, due to the advanced tumor stage at the time
of diagnosis and the high incidence of chronic liver disease, such
as HBV infection and cirrhosis, prevalent in China. Furthermore,
although it included the largest case series of ICC patients, this
study was retrospective in nature, which may be associated with
certain limitations with regards to data selection.
In conclusion, cirrhosis is an independent favorable
prognostic factor for survival of ICC patients, due to the distinct
biological characteristics as well as the different pathogenic
mechanism in this subgroup of patients. More emphasis should be
placed on aggressive surgical treatment for ICC patients with
cirrhosis, considering safety and better survival in this group.
Non-cirrhotic patients may lack the typical ‘field-defect’ of a
cirrhotic liver; however, these patients may harbor a molecular
field defect that differs from that of a cirrhotic liver, leading
to higher-risk pathological characteristics, lower resection rates
and worse survival. Further investigation should be focused on the
genomic profile of livers with and without cirrhosis in order to
elucidate the different pathogenic mechanisms underlying the
development of ICC, in order to design novel targeted treatments to
improve the survival of ICC patients.
Glossary
Abbreviations
Abbreviations:
|
AFP
|
α-fetoprotein
|
|
CA19-9
|
carbohydrate antigen 19-9
|
|
CEA
|
carcinoembryonic antigen
|
|
CT
|
computed tomography
|
|
HBV
|
hepatitis B virus
|
|
HCC
|
hepatocellular carcinoma
|
|
ICC
|
intrahepatic cholangiocarcinoma
|
|
LN
|
lymph node
|
|
MRI
|
magnetic resonance imaging
|
|
MST
|
median survival time
|
|
OS
|
overall survival
|
References
|
1
|
Sempoux C, Jibara G, Ward SC, Fan C, Qin
L, Roayaie S, Fiel MI, Schwartz M and Thung SN: Intrahepatic
cholangiocarcinoma: New insights in pathology. Semin Liver Dis.
31:49–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Endo I, Gonen M, Yopp AC, Dalal KM, Zhou
Q, Klimstra D, D'Angelica M, DeMatteo RP, Fong Y, Schwartz L, et
al: Intrahepatic cholangiocarcinoma: Rising frequency, improved
survival, and determinants of outcome after resection. Ann Surg.
248:84–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khan SA, Taylor-Robinson SD, Toledano MB,
Beck A, Elliott P and Thomas HC: Changing international trends in
mortality rates for liver, biliary and pancreatic tumours. J
Hepatol. 37:806–813. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakanuma Y, Sasaki M, Ikeda H, Sato Y, Zen
Y, Kosaka K and Harada K: Pathology of peripheral intrahepatic
cholangiocarcinoma with reference to tumorigenesis. Hepatol Res.
38:325–334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
The general rules for the clinical and
pathological study of primary liver cancer. Liver cancer study
group of Japan. Jpn J Surg. 19:98–129. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohtsuka M, Ito H, Kimura F, Shimizu H,
Togawa A, Yoshidome H and Miyazaki M: Results of surgical treatment
for intrahepatic cholangiocarcinoma and clinicopathological factors
influencing survival. Br J Surg. 89:1525–1531. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu ZF, Zhang HB, Yang N, Zhao WC, Fu Y and
Yang GS: Postoperative adjuvant transcatheter arterial
chemoembolisation improves survival of intrahepatic
cholangiocarcinoma patients with poor prognostic factors: Results
of a large monocentric series. Eur J Surg Oncol. 38:602–610. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fattovich G, Stroffolini T, Zagni I and
Donato F: Hepatocellular carcinoma in cirrhosis: Incidence and risk
factors. Gastroenterology. 127:S35–S50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tretiakova MS, Shabani-Rad MT, Guggisberg
K, Hart J, Anders RA and Gao ZH: Genomic and immunophenotypical
differences between hepatocellular carcinoma with and without
cirrhosis. Histopathology. 56:683–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paquet KJ, Gad HA, Lazar A, Koussouris P,
Mercado MA, Heine WD, Jachman-Jahn V and Ruppert W: Analysis of
factors affecting outcomes after hepatectomy of patients with liver
cirrhosis and small hepatocellular carcinoma. Eur J Surg.
164:513–519. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bréchot C: Pathogenesis of hepatitis B
virus-related hepatocellular carcinoma: Old and new paradigms.
Gastroenterology. 127 (5 Suppl 1):S56–S61. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perumal V, Wang J, Thuluvath P, Choti M
and Torbenson M: Hepatitis C and hepatitis B nucleic acids are
present in intrahepatic cholangiocarcinomas from the United States.
Hum Pathol. 37:1211–1216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee CH, Chang CJ, Lin YJ, Yeh CN, Chen MF
and Hsieh SY: Viral hepatitis-associated intrahepatic
cholangiocarcinoma shares common disease processes with
hepatocellular carcinoma. Br J Cancer. 100:1765–1770. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Palmer WC and Patel T: Are common factors
involved in the pathogenesis of primary liver cancers? A
meta-analysis of risk factors for intrahepatic cholangiocarcinoma.
J Hepatol. 57:69–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shaib YH, El-Serag HB, Davila JA, Morgan R
and McGlynn KA: Risk factors of intrahepatic cholangiocarcinoma in
the United States: A case-control study. Gastroenterology.
128:620–626. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pandey D, Lee KH, Wai CT, Wagholikar G and
Tan KC: Long term outcome and prognostic factors for large
hepatocellular carcinoma (10 cm or more) after surgical resection.
Ann Surg Oncol. 14:2817–2823. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Poon RT, Fan ST, Lo CM, Liu CL, Ng IO and
Wong J: Long-term prognosis after resection of hepatocellular
carcinoma associated with hepatitis B-related cirrhosis. J Clin
Oncol. 18:1094–1101. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng NF, Li LQ, Qin X, Guo Y, Peng T, Xiao
KY, Chen XG, Yang YF, Su ZX, Chen B, et al: Evaluation of risk
factors and clinicopathologic features for intrahepatic
cholangiocarcinoma in Southern China: A possible role of hepatitis
B virus. Ann Surg Oncol. 18:1258–1266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Cai JQ, Zhao JJ, Bi XY, Tan XG,
Yan T, Li C and Zhao P: Impact of hepatitis B virus infection on
outcome following resection for intrahepatic cholangiocarcinoma. J
Surg Oncol. 101:233–238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo X, Yuan L, Wang Y, Ge R, Sun Y and Wei
G: Survival outcomes and prognostic factors of surgical therapy for
all potentially resectable intrahepatic cholangiocarcinoma: A large
single-center cohort study. J Gastrointest Surg. 18:562–572. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li YY, Li H, Lv P, Liu G, Li XR, Tian BN
and Chen DJ: Prognostic value of cirrhosis for intrahepatic
cholangiocarcinoma after surgical treatment. J Gastrointest Surg.
15:608–613. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scheuer PJ: Classification of chronic
viral hepatitis: A need for reassessment. J Hepatol. 13:372–374.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Strasberg SM: Nomenclature of hepatic
anatomy and resections: A review of the Brisbane 2000 system. J
Hepatobiliary Pancreat Surg. 12:351–355. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Farges O, Fuks D, Boleslawski E, Le Treut
YP, Castaing D, Laurent A, Ducerf C, Rivoire M, Bachellier P,
Chiche L, et al: Influence of surgical margins on outcome in
patients with intrahepatic cholangiocarcinoma: A multicenter study
by the AFC-IHCC-2009 study group. Ann Surg. 254:824–830. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hou J, Liu Z and Gu F: Epidemiology and
prevention of hepatitis B virus infection. Int J Med Sci. 2:50–57.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou XD, Tang ZY, Fan J, Zhou J, Wu ZQ,
Qin LX, Ma ZC, Sun HC, Qiu SJ, Yu Y, et al: Intrahepatic
cholangiocarcinoma: Report of 272 patients compared with 5,829
patients with hepatocellular carcinoma. J Cancer Res Clin Oncol.
135:1073–1080. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Li J, Xia Y, Gong R, Wang K, Yan
Z, Wan X, Liu G, Wu D, Shi L, et al: Prognostic nomogram for
intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin
Oncol. 31:1188–1195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu J, Igarashi S, Sasaki M, Matsubara T,
Yoneda N, Kozaka K, Ikeda H, Kim J, Yu E, Matsui O and Nakanuma Y:
Intrahepatic cholangiocarcinomas in cirrhosis are hypervascular in
comparison with those in normal livers. Liver Int. 32:1156–1164.
2012. View Article : Google Scholar : PubMed/NCBI
|