Introduction
Mature cystic teratomas (MCTs) are the most common
ovarian tumors, accounting for ~20% of all ovarian tumors, and are
composed of mature tissues derived from two to three germ layers
(ectoderm, mesoderm and endoderm) (1–4).
Patients present with tumor-related symptoms, such as abdominal
pain with or without tumor torsion, abdominal mass and abdominal
swelling (2–4). However, a considerable amount of MCTs
are asymptomatic and are incidentally discovered by pelvic
examination, caesarean section or surgery for other diseases
(2–4). Almost all MCTs have cystic spaces
surrounded by organized skin-like tissues composed of
ectoderm-derived epidermis, sebaceous glands and hair follicles and
mesoderm-derived dermal stromal elements, which together contribute
to the synonym ‘dermoid cysts’ (1–4). Other
commonly found tissues include adipose tissues, smooth muscle,
glia, cerebrum, peripheral nerve, cartilage, bone and respiratory
epithelium (1–4). Thyroid tissues, salivary glands and
ependymal tissues are occasionally present. Gastrointestinal (GI)
epithelium and organized GI tracts may be observed in MCTs
(5–12). To date, only two previous articles
(11,12) have described interstitial cells of
Cajal (ICC) within the GI tract-like muscular walls in three
sporadic cases of MCT. However, the frequency and histopathological
features of these muscular walls are not fully understood.
Therefore, the present study examined GI tract-like muscular walls
in surgically removed ovarian MCTs.
Patients and methods
Patients
A total of 149 ovarian MCTs removed from 126 females
were retrieved from the surgical pathology files of the Department
of Pathology, Japan Self-Defense Forces Central Hospital (Tokyo,
Japan), from between November 1983 and September 2016. The clinical
findings and macroscopic features were obtained from surgical
pathology request forms and patient medical charts. Patients ranged
in age from 14–79 years (mean, 35.1 years). Of the patients, 23 had
bilateral ovarian MCTs and the other 103 had unilateral MCTs. The
sizes of 144 MCTs were known, and ranged from 1–21 cm (mean, 6.6
cm). The present study was a retrospective study, which was
approved by the Medical Research Ethics Committee of Japan
Self-Defense Forces Central Hospital (approval number, 28–015).
MCT examination
All representative hematoxylin and eosin
(H&E)-stained sections were histologically examined for the
presence of MCT-related GI tract-like muscular walls with or
without other components. In the present study, the term ‘muscular
walls’ was used to describe muscularis propria-like layered
aggregations of smooth muscle cells, and did not include muscularis
mucosae (MM). To exclude respiratory, urogenital and uterine
cervical walls, muscular walls covered by ciliated columnar,
squamous and/or urothelial-like epithelium were not considered.
For all samples, 10–20% buffered formalin-fixed and
paraffin-embedded representative sections containing MCT-related
muscular walls were available. The fixation was performed at room
temperature, ranging in duration from 12–48 h. These sections were
serially cut (4–5 µm-thick), stained with H&E, and subjected to
immunohistochemical analysis by incubation with antibodies against
KIT (polyclonal; A4501; 1:100; Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA) for 60 min, cluster of differentiation (CD)
34 (NU-4A1; 413361; 1:100 dilution; Nichirei Biosciences, Inc.,
Tokyo, Japan) for 30 min, α-smooth muscle actin (SMA; 1A4; 412021;
prediluted; Nichirei Biosciences, Inc.) for 60 min, S-100 protein
(polyclonal; 422091; prediluted; Nichirei Biosciences, Inc.) for 60
min, synaptophysin (27G12; 413831; prediluted; Nichirei
Biosciences, Inc.) for 30 min, discovered on gastrointestinal
stromal tumors 1 (DOG1; K9; NCL-L-DOG-1; 1:100; Leica Biosystems,
Newcastle, UK) for 60 min, keratin 7 (K7; OV-TL 12/30; M7018;
1:100; Dako; Agilent Technologies, Inc.) for 30 min and keratin 20
(K20; Ks 20.8; M7019; 1:100; Dako; Agilent Technologies, Inc.) for
30 min. For secondary antibody incubation, Histofine Simple Stain
MAX PO (MULTI)® (424152; Nichirei Biosciences, Inc.) was
used for a duration of 30 min. Incubations with primary and
secondary antibodies were performed at room temperature. Endogenous
peroxidase activity and non-specific background staining was
blocked with 3% hydrogen peroxidase and the blocking solution,
Protein Block Serum-Free® (X0909; Dako; Agilent
Technologies, Inc.), respectively. Each blocking reagent was
incubated for 30 min at room temperature before incubation with the
primary and secondary antibodies.
Excluding distinct neural tissues and vessels
observed on H&E-based histology, KIT+,
CD34+, S-100+, synaptophysin+ and
DOG1+ spindle or stellate cells within muscular walls
were evaluated in each case, and were graded as follows: 0, no
positive cells; 1, <1% positive muscular wall spindle and/or
stellate cells; 2, 1–5% positive muscular wall spindle and/or
stellate cells; and 3, cells >5% positive muscular wall spindle
and/or stellate cells.
Statistical analysis
Clinicopathological findings were analyzed using the
appropriate Spearman's rank correlation test and Fisher's exact
test. Data were analyzed on a personal computer using a statistical
software package (StatMate® IV for Windows v. 4.01; ATMS
Corp., Ltd., Tokyo, Japan). P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinicopathological features
GI tract-like muscular walls were hemi-laterally
identified in 9 (7.1%) of 126 cases of MCT (4 left and 5 right
MCTs). Table I summarizes the main
clinicopathological findings in these 9 patients. These walls were
accompanied by mucosae in 5 cases, MM-like structures in 5 cases,
serosa-like features in 5 cases, and all of these components were
present in 3 cases. Patients ranged in age from 19–52 years (mean,
34.3 years), and 4 patients had bilateral MCTs. The sizes of MCTs
with these muscular walls ranged from 1.4–11.0 cm (mean, 4.8 cm).
The largest dimension of GI tract-like structures in each case
ranged from 0.2–1.5 cm (mean, 0.6 cm). GI tract-like muscular walls
partially or totally demonstrated incomplete features, such as
indistinct two-layered structures (all cases), thinning (7 cases)
and adhesion to MM-like structures (1 case). In all muscular walls,
neural tissues of varying size were observed between two
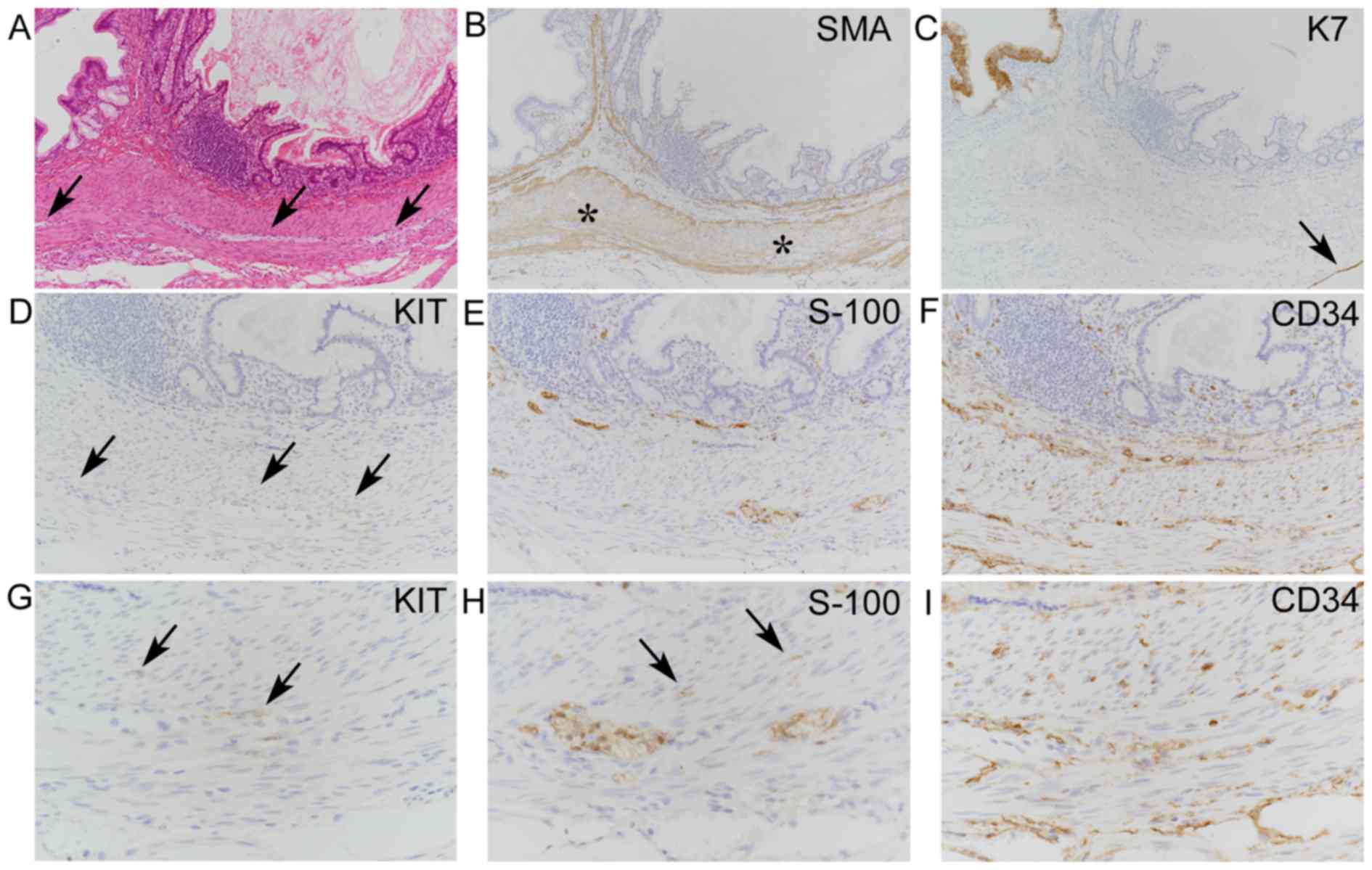
conspicuous or inconspicuous muscular layers (Figs. 1 and 2), partially exhibiting Auerbach's
plexus-like features, and also within the muscular layers (Fig. 2A). Gastric and intestinal mucosae
were found in 2 and 4 cases, respectively. In 1 case, partially
atrophic, gastric pyloric mucosa and small intestine-like mucosa
were surrounded by mutual muscular walls (Fig. 1A). In another case, gastric body
mucosa merged with marked flattened mucosa was observed. In 3 other
cases, intestinal mucosae were difficult to distinctly divide into
small and large intestinal types. Neoplastic lesions arising in GI
tract-like structures, such as gastrointestinal stromal tumor
(GIST), leiomyoma, glandular neoplasia and neuroendocrine tumor,
were not observed.
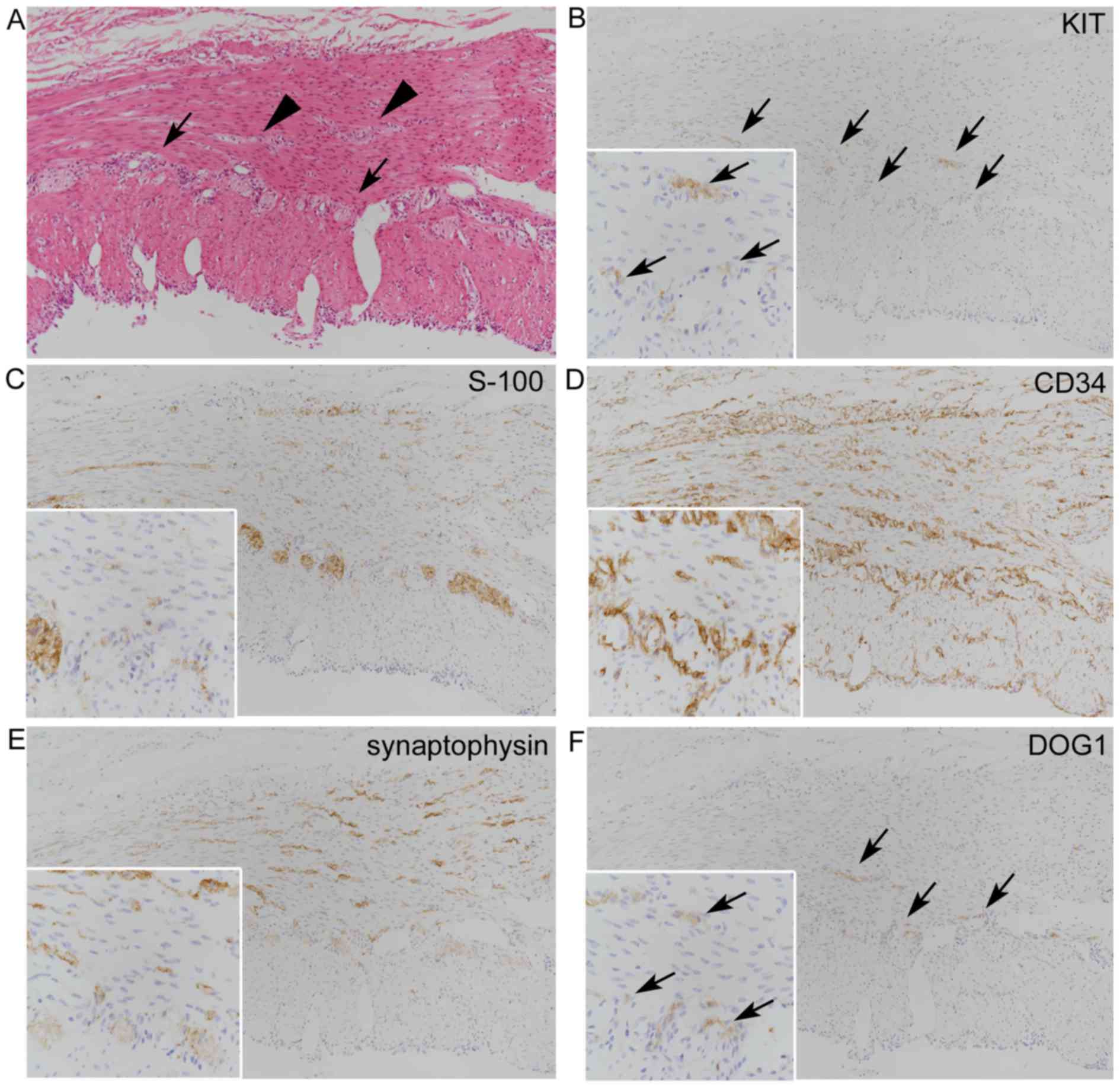
 | Figure 1.Histopathological analysis of
gastrointestinal tract-like structures in a teratoma (case 4). (A)
Hematoxylin and eosin-stained intestinal mucosa and atrophic
gastric mucosa surrounded by a muscular wall with scattered neural
tissues (arrows) inconspicuously observed between two muscular
layers. (B) Immunohistochemical staining of SMA in the muscular
wall (asterisks) revealed partially decreased expression. (C)
K7+ immunostaining in gastric mucosa and serosa (arrow)
(A-C magnification, ×100). (D-F) Moderate-power views
(magnification, ×200) of immunostaining for (D) KIT (E) S-100
protein and (F) CD34. (G-I) High-power (magnification, ×400) views
of (D), (E) and (F), respectively. (D) A few KIT+
spindle or stellate cells graded as 1, chiefly between two muscular
layers (arrows), and a (G) high-power view suggesting their close
proximity to S-100 protein+ and CD34+ cells
shown in (H) and (I), respectively. (E) S-100 protein+
neural cells within the muscular layer itself, graded as 2,
together with S-100 protein+ distinct neural tissues,
and a (H) high-power view of intramuscular S-100
protein+ spindle cells (arrows). (F) Increased
intramuscular CD34+ cells in the muscular wall, graded
as 3, and a (I) high-power view. The distributions of intramuscular
S-100 protein+ cells and CD34+ cells were
similar. SMA, smooth muscle actin; K7, keratin 7; CD34, cluster of
differentiation 34. |
 | Table I.Clinicopathological findings of
gastrointestinal tract-like muscular walls in ovarian MCT. |
Table I.
Clinicopathological findings of
gastrointestinal tract-like muscular walls in ovarian MCT.
| Case | Age, years | Site of MCT
containing GI tract-like muscular wallsa | Size of MCT
containing tract-like muscular wallsa, cm | Bilateral MCTs | Size of GI tract-like
structures, cm | Mucosa | Muscularis
mucosae | Adhesion between
muscularis mucosae and muscular wallsa | Serosa-like
features |
|---|
| 1 | 19 | Left | 3.5 | Present | 0.4 | Present
(Ib) | Present | None | Present |
| 2 | 52 | Right | 6.0 | Unilateral | 0.4 | None | Present | None | Present |
| 3 | 43 | Right |
6.2 | Present | 0.7 | Present
(Ib) | Present | Present | None |
| 4 | 20 | Right |
1.5 | Present | 0.4 | Present
(G+I)c | Present | None | Present |
| 5 | 52 | Left | 11.0 | Unilateral | 0.7 | None | None | – | None |
| 6 | 39 | Left |
5.0 | Unilateral | 1.2 | None | None | – | Present |
| 7 | 30 | Left |
4.5 | Unilateral | 0.3 | None | None | – | None |
| 8 | 29 | Right |
3.7 | Unilateral | 1.5 | Present (G) | None | – | Present |
| 9 | 25 | Right |
1.4 | Present | 0.2 | Present
(Ib) | Present | None | None |
Immunohistochemical features
Table II summarizes
the immunohistochemical features of GI tract-like structures. In
case 2, neither CD34 nor synaptophysin positivity was observed
within muscular walls or the inner controls (vascular endothelium
and neural cells, respectively). Hence, these findings were not
included in the results. Muscular walls and MM-like structures were
highlighted by SMA positivity, but the SMA positivity of the former
was relatively weak in 3 cases (Fig.
1B). A few weakly KIT+ spindle/stellate cells (not
including KIT+ mast cells) were observed chiefly nearby
neural tissues between muscular layers in 7 cases (Figs. 1D and 2B), and were graded 1 and 2 in 5 cases and
2 cases, respectively. S-100+ spindle or stellate cells
were scattered within muscular layers and within the distinct
neural tissues recognizable on H&E-stained histology in all
cases (Figs. 1E and 2C). These intramuscular S-100+
cells were considered to be richly distributed neural cells or
networks, and were graded 1, 2 and 3 in 3 cases each.
CD34+ spindle or stellate cells were scattered near the
boundary zones of two muscular layers and within muscular layers in
8 cases (Figs. 1F and 2D) along with or without prominent
CD34+ microvessels, and were graded 1, 2 and 3 in 3, 1
and 4 cases, respectively. Other than in the context of distinct
neural tissue positivity, intramuscular synaptophysin+
spindle or stellate cells were observed in 7 cases (Fig. 2E) and were graded 1, 2 and 3 in 3, 2
and 2 cases, respectively. The intramuscular distributions of
KIT+, CD34+, S-100+ and
synaptophysin+ spindle or stellate cells were similar. A
few DOG1+ spindle or stellate cells were observed near
the Auerbach's plexus-like neural tissues in only 1 case (case 6;
Fig. 2F), possibly corresponding
with KIT+ cells. Accompanying gastric mucosae contained
only K7+/K20− cells in 1 case, and both
K7+/K20− and K7−/K20+
cells in 1 case. Intestinal mucosa specimens were composed of only
K7−/K20+ cells in 2 cases, and were admixed
with K7+/K20− cells and
K7−/K20+ cells in 2 cases. Serosa-like
structures were positive for K7 (Fig.
1C).
 | Table II.Immunohistochemical features of
gastrointestinal tract-like structures in ovarian MCTs. |
Table II.
Immunohistochemical features of
gastrointestinal tract-like structures in ovarian MCTs.
|
|
|
| Grading
scoree of muscular
wallsf |
|---|
|
|
|
|
|
|---|
| Case | Keratin pattern of
mucosa | Diminished SMA
staining of muscular walls | KIT | CD34 | S-100 | Synaptophysin | DOG1 |
|---|
| 1 |
K7+/K20− >
K7−/K20+a | None | 1 | 1 | 1 | 0 | 0 |
| 2 | – | Present | 2 | –g | 3 | –g | 0 |
| 3 |
K7+/K20− <<
K7−/K20+b | None | 1 | 3 | 2 | 2 | 0 |
| 4 |
K7+/K20−(G)c,
K7−/K20+ (I)c | Present | 1 | 3 | 2 | 2 | 0 |
| 5 | – | None | 0 | 1 | 1 | 1 | 0 |
| 6 | – | Present | 2 | 3 | 3 | 3 | 1 |
| 7 | – | None | 1 | 3 | 3 | 3 | 0 |
| 8 |
K7+/K20− >>
K7+/K20+d | None | 1 | 2 | 2 | 1 | 0 |
| 9 |
K7−/K20+ only | None | 0 | 1 | 1 | 1 | 0 |
Association between various
histopathological and immunohistochemical features
The presence of GI tract-like muscular walls was not
statistically associated with age (P=0.855), MCT size (P=0.082) or
bilateral presence of MCTs (P=0.096; Table III). Spearman's rank correlation
analysis demonstrated significant correlations between grading
scores of KIT+ and S-100+ cells (P=0.014),
CD34+ and S-100+ cells (P=0.003),
CD34+ and synaptophysin+ cells (P=0.009) and
S-100+ and synaptophysin+ cells (P=0.002);
however, no significant correlations were observed between other
combinations (Table IV). Fisher's
exact test (data not shown) revealed significant relationships
between CD34+ and S-100+ cells (P=0.018) and
between S-100+ and synaptophysin+ cells
(P=0.048); however, it revealed no significant associations between
KIT+ and S-100+ cells (P=0.500) or between
CD34+ and synaptophysin+ cells (P=0.143). No
statistical associations were observed between decreased SMA
positivity of muscular walls and other immunostaining results.
 | Table III.Relationship between MCT-related
gastrointestinal tract-like muscular walls and clinicopathological
variables. |
Table III.
Relationship between MCT-related
gastrointestinal tract-like muscular walls and clinicopathological
variables.
|
| Presence of
MCT-related gastrointestinal tract-like muscular wallsa |
|
|---|
|
|
|
|
|---|
| Variable | Yes (n=9) | No (n=117) | P-value |
|---|
| Age, years
(mean) | 19–52 (34.3) | 14–79 (35.1) | 0.855 |
| Size of MCT
containing muscular walls, cm (mean) | 1.5–11.0 (4.8) | 1.0–21.0 (4.0)
(n=135)b | 0.082 |
| Patients with
bilateral MCTs, n | 4 | 19 | 0.096 |
 | Table IV.Spearman's rank correlation analysis
of immumohistochemical grading scores of mature cystic
teratoma-related gastrointestinal tract-like muscular walls. |
Table IV.
Spearman's rank correlation analysis
of immumohistochemical grading scores of mature cystic
teratoma-related gastrointestinal tract-like muscular walls.
| Correlation of
immunohistochemistry | P-value | rs |
|---|
| KIT vs. CD34
(n=8)a | 0.130 | 0.58 |
| KIT vs. S-100
protein | 0.014 | 0.78 |
| KIT vs.
synaptophysin (n=8)a | 0.753 | 0.12 |
| KIT vs. DOG1 | 0.094 | 0.63 |
| CD34 vs. S-100
protein (n=8)a | 0.003 | 0.89 |
| CD34 vs.
synaptophysin (n=8)a | 0.009 | 0.84 |
| CD34 vs. DOG1
(n=8)a | 0.426 | 0.33 |
| S-100 protein vs.
synaptophysin (n=8)a | 0.002 | 0.91 |
| S-100 protein vs.
DOG1 | 0.129 | 0.54 |
| Synaptophysin vs.
DOG1 (n=8)a | 0.184 | 0.52 |
Discussion
In the present study, GI tract-like muscular walls
with or without other components were observed in 7.1% of MCT
cases. This incidence is consistent with the occurrence of GI-type
epithelium with or without other components in MCTs, which has been
reported to be 7–13% (6,13). Totally organized GI tracts within
ovarian MCTs are rare, with <20 previously reported cases
(8–12). The present study revealed GI
tract-like structures containing mucosa, MM, muscular walls and
serosa in 3 (2.4%) of 126 MCT cases, and muscular walls without
mucosa in 4 (3.2%) cases.
The detected MCT-related GI tract-like structures
were relatively small and histologically differed somewhat from
those of actual GI tracts. Accompanying intestinal epithelia were
frequently difficult to distinguish as small vs. large intestine,
and gastric mucosae were partially atrophic or prominently
flattened. Muscular walls commonly demonstrated inconspicuous
two-layered structures, thinning and partially diminished SMA
positivity. These findings underscore the disorganization of the GI
tracts, which may influence the development of ICCs. However, a
high percentage (78%) of the present cases had KIT+
spindle or stellate cells chiefly nearby the neural tissues between
muscular layers, which is consistent with those of ICCs of actual
GI tracts (14). KIT and S-100
grading scores were also significantly correlated with each other.
Although DOG1 is also known to be a marker of ICCs (15,16),
DOG1 co-expression in KIT+ ICCs was observed in only 1
case.
A study by Agaimy et al (11) first described KIT+ ICCs
within GI tract-like muscular derivatives in 2 MCTs, and a case of
hyperplastic ICCs was reported later (12). In the present study, an increased
number of KIT+ cells were not observed; however, there
appeared to be more intramuscular CD34+ spindle or
stellate cells compared to KIT+ cells. As CD34 is also a
marker of ICCs (14), these findings
suggest the possibility of ICC hyperplasia. However,
CD34+ cells are also detectable in normal endoneural
fibroblasts (14) and in peripheral
nerve sheath tumors (17).
Furthermore, in the present study, the distribution of
intramuscular CD34+ cells resembled that of
intramuscular S-100+ neural cells, and their grading
scores were closely correlated with each other. Therefore,
CD34+ cells are expected to be neuron-related cells
rather than ICCs. Similarly, intramuscular
synaptophysin+ cells are expected to represent
intramuscular neural cells rather than neuroendocrine cells because
synaptophysin positivity may be found in synaptic vesicles of
neural cells (18) and the
neuroendocrine system of GI tracts is usually present in the mucosa
and submucosa, and is unknown within the muscularis propria
(14). We believe that ICC
hyperplasia is a rare event in MCT-related GI tract-like
structures.
GISTs are considered to arise from ICCs or their
precursor cells (3,17,19);
however, they were previously misinterpreted as smooth muscle
tumors in the ‘pre-KIT era’ (12).
Our previous review of the literature identified a case report
describing a ‘spindle cell nodule’ in a MCT-related gastric wall
(7). However, based on the
description and the photomicrographs in this article (7), this spindle cell nodule did not
demonstrate a well-demarcated GIST-like configuration, thus was
likely a reactive stromal reaction associated with an ulcer.
Therefore, to the best of our knowledge, no distinct cases of GISTs
arising in MCTs have been reported previously, regardless of their
incidence in normal GI tract tissue (3,15,17,19)
and the presently demonstrated frequent KIT+ ICCs within
MCT-related GI tract-like structures. Agaimy et al (11) reported that no previous cases of
MCT-related GISTs have contributed to unknown underlying pathogenic
factors other than the rarity of somatic secondary tumors in MCTs.
Based on the present results, this may be partially due to the
rarity of ICC hyperplasia.
In conclusion, the present study revealed GI
tract-like muscular walls in 7.1% of ovarian MCT cases, which
frequently contained KIT+ ICCs but did not demonstrate
any hyperplastic features. The present study included a limited
number of cases. Therefore, to elucidate true incidence of
KIT+ ICC in MCT, further study using a larger number of
cases is required.
Glossary
Abbreviations
Abbreviations:
|
DOG1
|
discovered on gastrointestinal stromal
tumors 1
|
|
GI
|
gastrointestinal
|
|
GIST
|
gastrointestinal stromal tumor
|
|
H&E
|
hematoxylin and eosin
|
|
ICC
|
interstitial cell of Cajal
|
|
K7
|
keratin 7
|
|
K20
|
keratin 20
|
|
MCT
|
mature cystic teratoma
|
|
MM
|
muscularis mucosae
|
|
SMA
|
α-smooth muscle action
|
References
|
1
|
Scully RE, Young RH and Clement PB: Tumors
of the ovary, maldeveloped gonads, fallopian tube and broad
ligament. In: Atlas of Tumor Pathology. 3rd edition. fascicle
23Rosai J and Sobin LH: American Registry of Pathology. Washington:
1998
|
|
2
|
Russel P and Bannatyne P: Surgical
Pathology of the Ovaries. Churchill Livingstone; New York, NY: pp.
416–452. 1989
|
|
3
|
Rosai J: Rosai and Ackerman's Surgical
Pathology. 10th. Mosby/Elsevier; Philadelphia, PA: 2011, View Article : Google Scholar
|
|
4
|
Kurman RJ, Carcangiu ML, Herrington CS and
Young RH: WHO Classification of Tumours of Female Reproductive
Organs. 4th. IARC Press; Lyon: pp. 60–61. 2014
|
|
5
|
Gundersen JH and Greene RR: Vermiform
appendix in benign cystic teratoid tumor of the ovary. Am J Obstet
Gynecol. 89:534–535. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Woodfield B, Kats DA, Cantrell CJ and
Bogard PJ: A benign cystic teratoma with gastrointestinal tract
development. Am J Clin Pathol. 83:236–240. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sahin AA, Ro JY, Chen J and Ayala AG:
Spindle cell nodule and peptic ulcer arising in a fully developed
gastric wall in a mature cystic teratoma. Arch Pathol Lab Med.
114:529–531. 1990.PubMed/NCBI
|
|
8
|
Fujiwara K, Ginzan S and Silverberg SG:
Mature cystic teratomas of the ovary with intestinal wall
structures harboring intestinal-type epithelial neoplasms. Gynecol
Oncol. 56:97–101. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang P, Soukkary S and Kahn E: Mature
cystic teratoma of the ovary associated with complete colonic wall
and mucinous cystadenoma. Ann Clin Lab Sci. 33:465–470.
2003.PubMed/NCBI
|
|
10
|
Ki EY, Jang DG, Jeong DJ, Kim CJ and Lee
SJ: Rare case of complete colon structure in a mature cystic
teratoma of the ovary in menopausal woman: A case report. BMC
Women's Health. 16:702016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Agaimy A, Lindner M and Wuensch PH:
Interstitial cells of Cajal (ICC) in mature cystic teratoma of the
ovary. Histopathology. 48:208–209. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Agaimy A and Wünsch PH: Coexistence of
interstitial cell of Cajal hyperplasia and microcarcinoidosis in a
mature cystic teratoma. Histopathology. 52:260–262. 2008.PubMed/NCBI
|
|
13
|
Blackwell WJ, Dockerty MB, Masson JC and
Mussey RD: Dermoid cysts of the ovary: Their clinical and
pathologic significance. Am J Obstet Gynecol. 51:151–172. 1946.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mills SE: Histology for pathologists. 4th.
Lippincott Williams & Wilkins; Philadelphia PA: pp. 605–708,
pp1255-1276. 2012
|
|
15
|
Espinosa I, Lee CH, Kim MK, Rouse BT,
Subramanian S, Montgomery K, Varma S, Corless CL, Heinrich MC,
Smith KS, et al: A novel monoclonal antibody against DOG1 is a
sensitive and specific marker for gastrointestinal tumors. Am J
Surg Pathol. 32:210–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miettinen M, Wang ZF and Lasota J: DOG1
antibody in the differential diagnosis of gastrointestinal stromal
tumors. A study of 1840 cases. Am J Surg Pathol. 33:1401–1408.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fletcher CDM, Bridge JA, Hogendoorn PCW
and Mertens F: WHO Classification of Tumours of Soft tissues and
Bone. 4th. IARC Press; Lyon: pp. 170–186. 2013
|
|
18
|
Dabbs: Diagnostic immunohistochemistry.
2nd. Churchill Livingston/Elsevier; Philadelphia, PA: pp.
2632006
|
|
19
|
Hirota S and Isozaki K: Pathology of
gastrointestinal stromal tumors. Pathol Int. 56:1–9. 2006.
View Article : Google Scholar : PubMed/NCBI
|