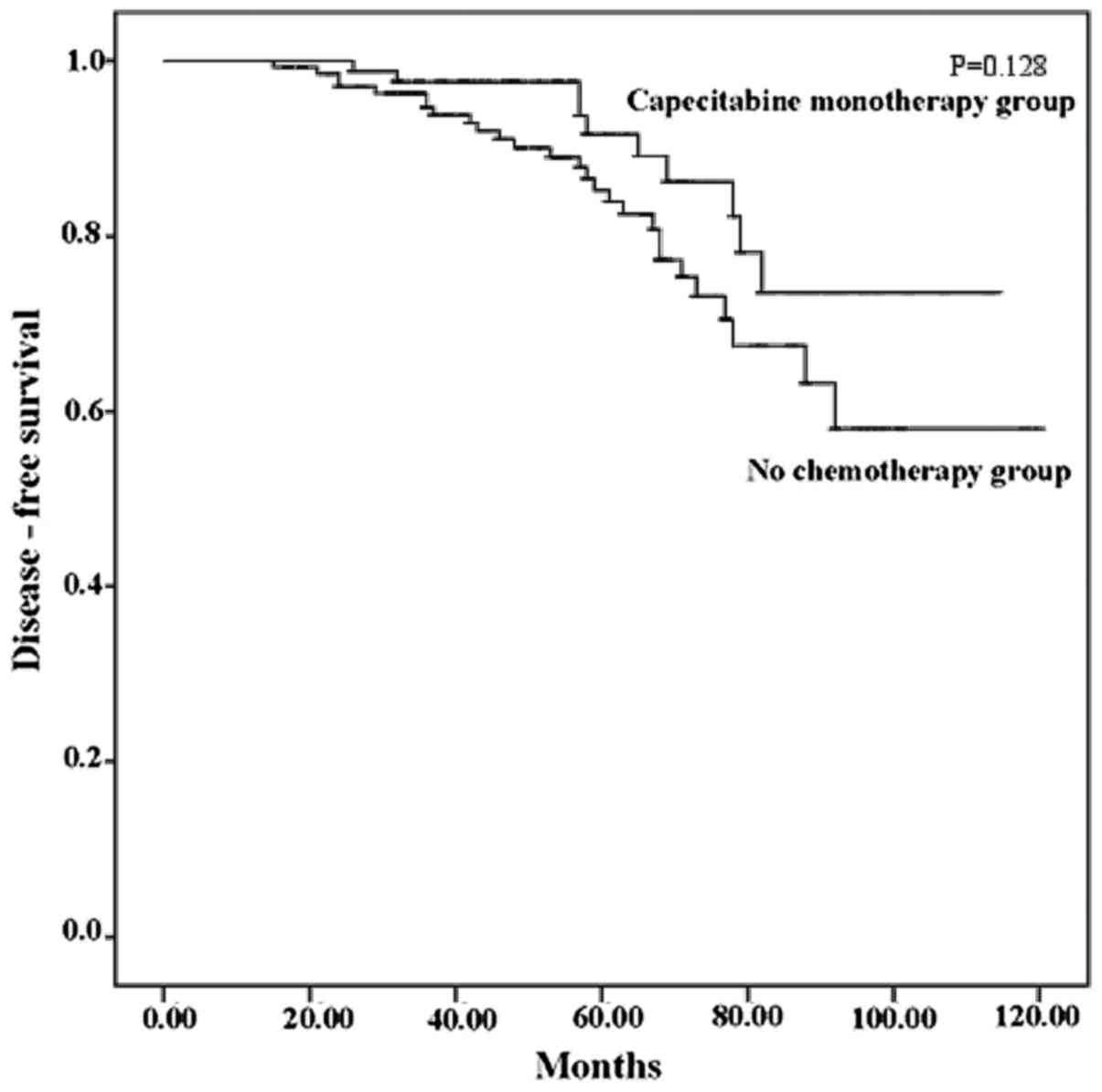
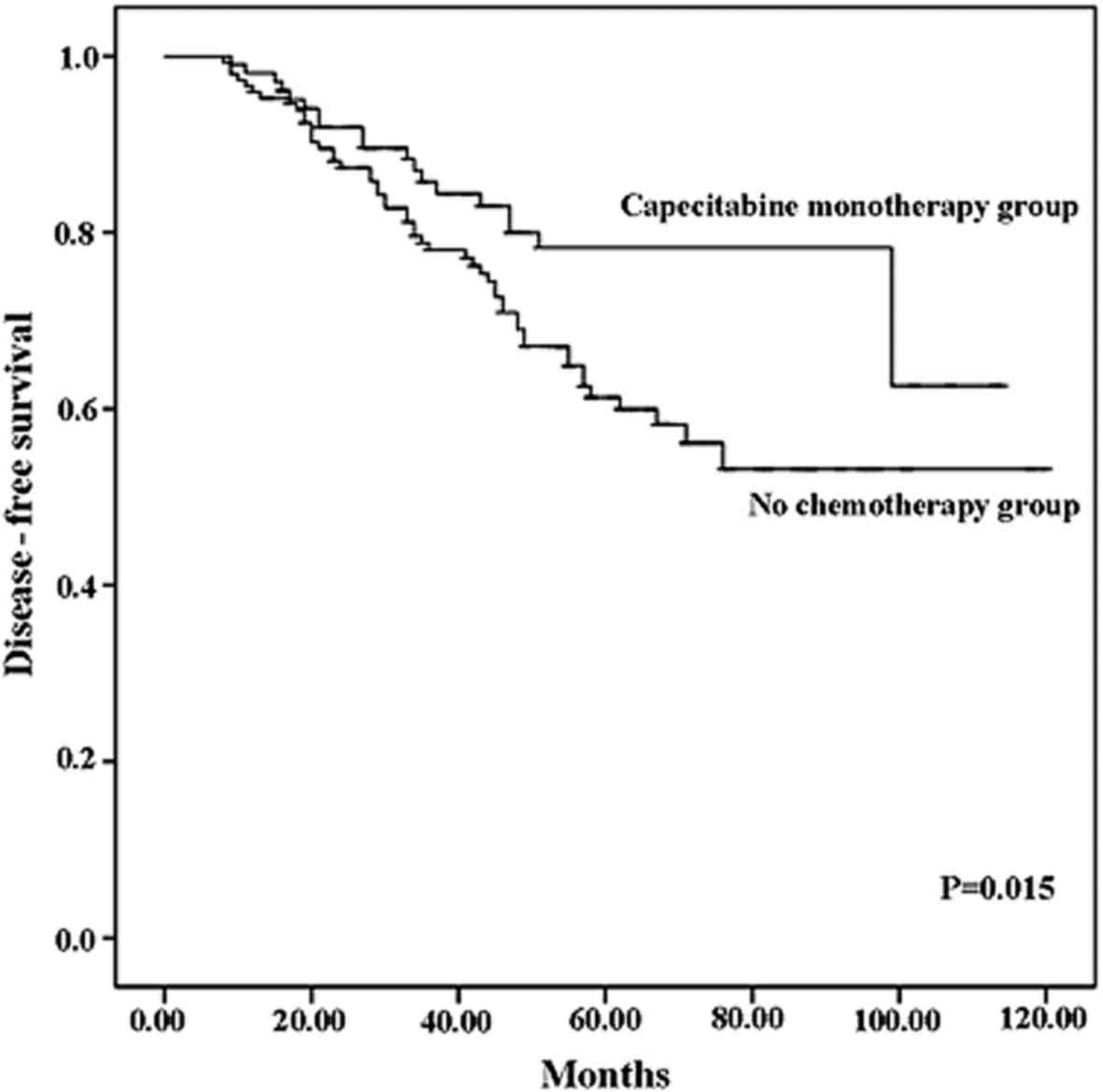
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Joensuu H: Systemic chemotherapy for
cancer: From weapon to treatment. Lancet Oncol. 9:3042008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar
|
|
4
|
Berry DA, Cronin KA, Plevritis SK, Fryback
DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JD and
Feuer EJ: Cancer Intervention and Surveillance Modeling Network
(CISNET) Collaborators: Effect of screening and adjuvant therapy on
mortality from breast cancer. N Engl J Med. 353:1784–1792. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mikhail SE, Sun JF and Marshall JL: Safety
of capecitabine: A review. Expert Opin Drug Saf. 9:831–841. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blum JL: The role of capecitabine, an
oral, enzymatically activated fluoropyrimidine, in the treatment of
metastatic breast cancer. Oncologist. 6:56–64. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blum JL, Jones SE, Buzdar AU, LoRusso PM,
Kuter I, Vogel C, Osterwalder B, Burger HU, Brown CS and Griffin T:
Multicenter phase II study of capecitabine in paclitaxel-refractory
metastatic breast cancer. J Clin Oncol. 17:485–493. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miller KD, Chap LI, Holmes FA, Cobleigh
MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD,
Sing AP, et al: Randomized phase III trial of capecitabine compared
with bevacizumab plus capecitabine in patients with previously
treated metastatic breast cancer. J Clin Oncol. 23:792–799. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Talbot DC, Moiseyenko V, Van Belle S,
O'Reilly SM, Alba Conejo E, Ackland S, Eisenberg P, Melnychuk D,
Pienkowski T, Burger HU, et al: Randomised, phase II trial
comparing oral capecitabine (Xeloda) with paclitaxel in patients
with metastatic/advanced breast cancer pretreated with
anthracyclines. Br J Cancer. 86:1367–1372. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schott AF, Barlow WE, Albain KS, Chew HK,
Wade JL III, Lanier KS, Lew DL, Hayes DF, Gralow JR, Livingston RB
and Hortobagyi GN: Phase II trial of simple oral therapy with
capecitabine and cyclophosphamide in patients with metastatic
breast cancer: SWOG S0430. Oncologist. 17:179–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stockler MR, Harvey VJ, Francis PA, Byrne
MJ, Ackland SP, Fitzharris B, Van Hazel G, Wilcken NR, Grimison PS,
Nowak AK, et al: Capecitabine versus classical cyclophosphamide,
methotrexate, and fluorouracil as first-line chemotherapy for
advanced breast cancer. J Clin Oncol. 29:4498–4504. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaufmann M, Maass N, Costa SD, Schneeweiss
A, Loibl S, Sütterlin MW, Schrader I, Gerber B, Bauer W, Wiest W,
et al: First-line therapy with moderate dose capecitabine in
metastatic breast cancer is safe and active: Results of the MONICA
trial. Eur J Cancer. 46:3184–3191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oshaughnessy JA, Blum J, Moiseyenko V,
Jones SE, Miles D, Bell D, Rosso R, Mauriac L, Osterwalder B,
Burger HU and Laws S: Randomized, open-label, phase II trial of
oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF
(cyclophosphamide, methotrexate and 5-fluorouracil) as first-line
therapy for advanced/metastatic breast cancer. Ann Oncol.
12:1247–1254. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blum JL, Barrios CH, Feldman N, Verma S,
McKenna EF, Lee LF, Scotto N and Gralow J: Pooled analysis of
individual patient data from capecitabine monotherapy clinical
trials in locally advanced or metastatic breast cancer. Breast
Cancer Res Treat. 136:777–788. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kamal AH, Camacho F, Anderson R, Wei W,
Balkrishnan R and Kimmick G: Similar survival with single-agent
capecitabine or taxane in first-line therapy for metastatic breast
cancer. Breast Cancer Res Treat. 134:371–378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arowolo OA, Njiaju UO, Ogundiran TO,
Abidoye O, Lawal OO, Obajimi M, Adetiloye AV, Im HK, Akinkuolie AA,
Oluwasola A, et al: Neo-adjuvant capecitabine chemotherapy in women
with newly diagnosed locally advanced breast cancer in a
resource-poor setting (Nigeria): Efficacy and safety in a phase II
feasibility study. Breast J. 19:470–477. 2013.PubMed/NCBI
|
|
17
|
Tolaney SM, Jeong J, Guo H, Brock J,
Morganstern D, Come SE, Golshan M, Bellon J, Winer EP and Krop IE:
A phase II study of preoperative capecitabine in women with
operable hormone receptor positive breast cancer. Cancer Med.
3:293–299. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Q, Jiang Y, Wei W, Yang H and Liu J:
Clinical efficacy of including capecitabine in neoadjuvant
chemotherapy for breast cancer: A systematic review and
meta-analysis of randomized controlled trials. PLoS One.
8:e534032013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang Y, Yin W, Zhou L, Yan T, Zhou Q, Du
Y, Shen Z, Shao Z and Lu J: First efficacy results of capecitabine
with anthracycline- and taxane-based adjuvant therapy in high-risk
early breast cancer: A meta-analysis. PLoS One. 7:e324742012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Joensuu H, Kellokumpu-Lehtinen PL,
Huovinen R, Jukkola-Vuorinen A, Tanner M, Kokko R, Ahlgren J,
Auvinen P, Paija O, Helle L, et al: Adjuvant capecitabine,
docetaxel, cyclophosphamide, and epirubicin for early breast
cancer: Final analysis of the randomized FinXX trial. J Clin Oncol.
30:11–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu W, Shi J, Sheng Y, Li L, Su D and Wang
CK: Clinical study of adjuvant capecitabine monotherapy in Chinese
elderly patients (aged 55–70) with stage IIa breast cancer.
Onkologie. 33:433–436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Muss HB, Berry DA, Cirrincione CT,
Theodoulou M, Mauer AM, Kornblith AB, Partridge AH, Dressler LG,
Cohen HJ, Becker HP, et al: Adjuvant chemotherapy in older women
with early-stage breast cancer. N Engl J Med. 360:2055–2065. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gianni L, Dafni U, Gelber RD, Azambuja E,
Muehlbauer S, Goldhirsch A, Untch M, Smith I, Baselga J, Jackisch
C, et al: Treatment with trastuzumab for 1 year after adjuvant
chemotherapy in patients with HER2-positive early breast cancer: A
4-year follow-up of a randomised controlled trial. Lancet Oncol.
12:236–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nole F, Catania C, Munzone E, Rocca A,
Verri E, Sanna G, Ascione G, Adamoli L, Zampino MG, Minchella I and
Goldhirsch A: Capecitabine/vinorelbine: An effective and
well-tolerated regimen for women with pretreated advanced-stage
breast cancer. Clin Breast Cancer. 6:518–524. 2006. View Article : Google Scholar : PubMed/NCBI
|