Introduction
Platinum-based chemotherapy plus cetuximab
represents the first-line treatment for recurrent or metastatic
squamous cell carcinoma (SCC) of the head and neck (1). In the European Phase III EXTREME trial
of patients with recurrent or metastatic SCC of the head and neck,
the addition of cetuximab to platinum/5-fluorouracil (5-FU) in the
first-line setting significantly improved the rates of overall
survival, progression-free survival and overall response compared
with platinum/5-FU alone (1).
However, cetuximab treatment is associated with certain adverse
events, the most common of which are infusion reactions and skin
reactions. Additionally, a risk of venous thromboembolic events
(VTEs) has recently been reported in association with cetuximab
(1). It has been suggested that the
association between cetuximab and risk of thrombosis may be due to
the antiangiogenic effects of cetuximab (2).
VTEs are considered to be under-diagnosed, as
patients are typically asymptomatic at presentation and are often
only diagnosed incidentally (2). A
previous study reported that the majority of VTEs (92%) were
identified during radiological examinations scheduled for tumor
reevaluation (3).
The present study reports the case of a 79-year-old
man who presented with lung and liver metastases from a tongue SCC,
and suffered rapid growth of a left ventricular (LV) thrombus
following 1 cycle of platinum-based chemotherapy plus cetuximab.
Anticoagulant therapy was administered to treat the LV thrombus,
which resolved within 1 week. The current report highlights the
potential association between platinum-based chemotherapy plus
cetuximab and the increased risk of embolic thrombus.
Case report
A 77-year old male patient initially presented to
Department of Oral and Maxillofacial Reconstructive Surgery,
Okayama University Hospital (Okayama, Japan) in October 2012 with a
chief complaint of a painless tumor in the left tongue accompanied
by ulceration. The tumor was elastic and hard, and measured 13×11
mm. Tongue SCC (cT1N0M0) was diagnosed from a biopsy specimen. The
patient's history included an old myocardial infarction, for which
he was undergoing treatment with antiplatelet drugs
(acetylsalicylic acid, 100 mg/day; ticlopidine hydrochloride, 100
mg/day). Glossectomy was performed in November 2012.
However, 8 months later, left submandibular lymph
node metastasis was detected. A left radical neck dissection was
performed in May 2013, followed by adjuvant radiotherapy (total
dose, 63 Gy).
After a further 18 months, fluorodeoxyglucose
(FDG)-positron emission tomography (PET) examination revealed lung
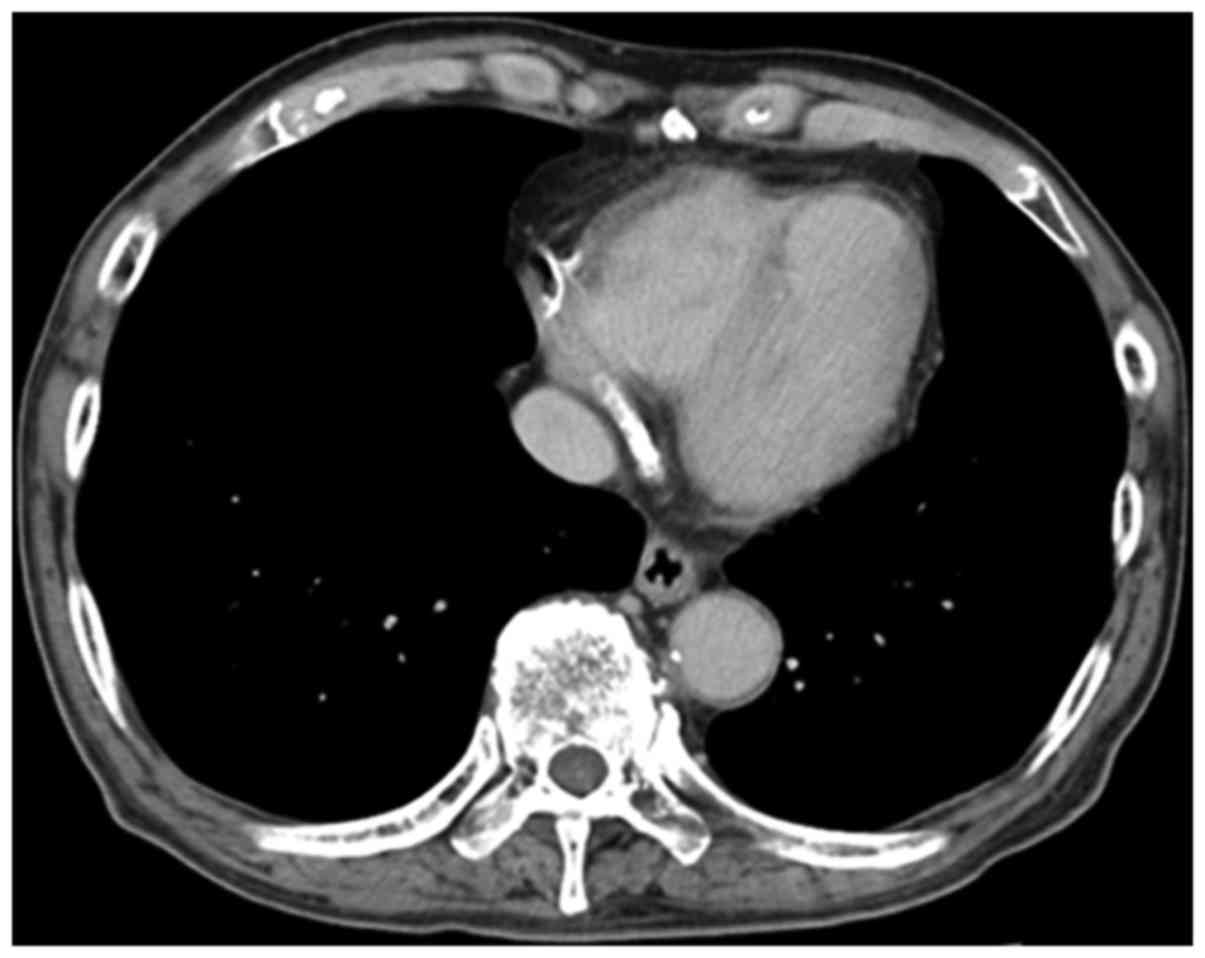
and liver metastases. Computed tomography (CT) scans performed at
the same time showed no LV thrombus (Fig. 1) and no reduction in ejection
fraction [EF; 57% (normal range, >55%)]. Platinum-based
chemotherapy plus cetuximab was initiated to treat the metastases.
Due to the patient's age and decreased renal function, the regimen
consisted of carboplatin (area under the curve, 5 mg/ml/min; 1-h
intravenous infusion on day 1), 5-FU (dose, 1,000
mg/m2/day for 4 days), and cetuximab (initial dose, 400
mg/m2; followed by subsequent weekly doses of 250
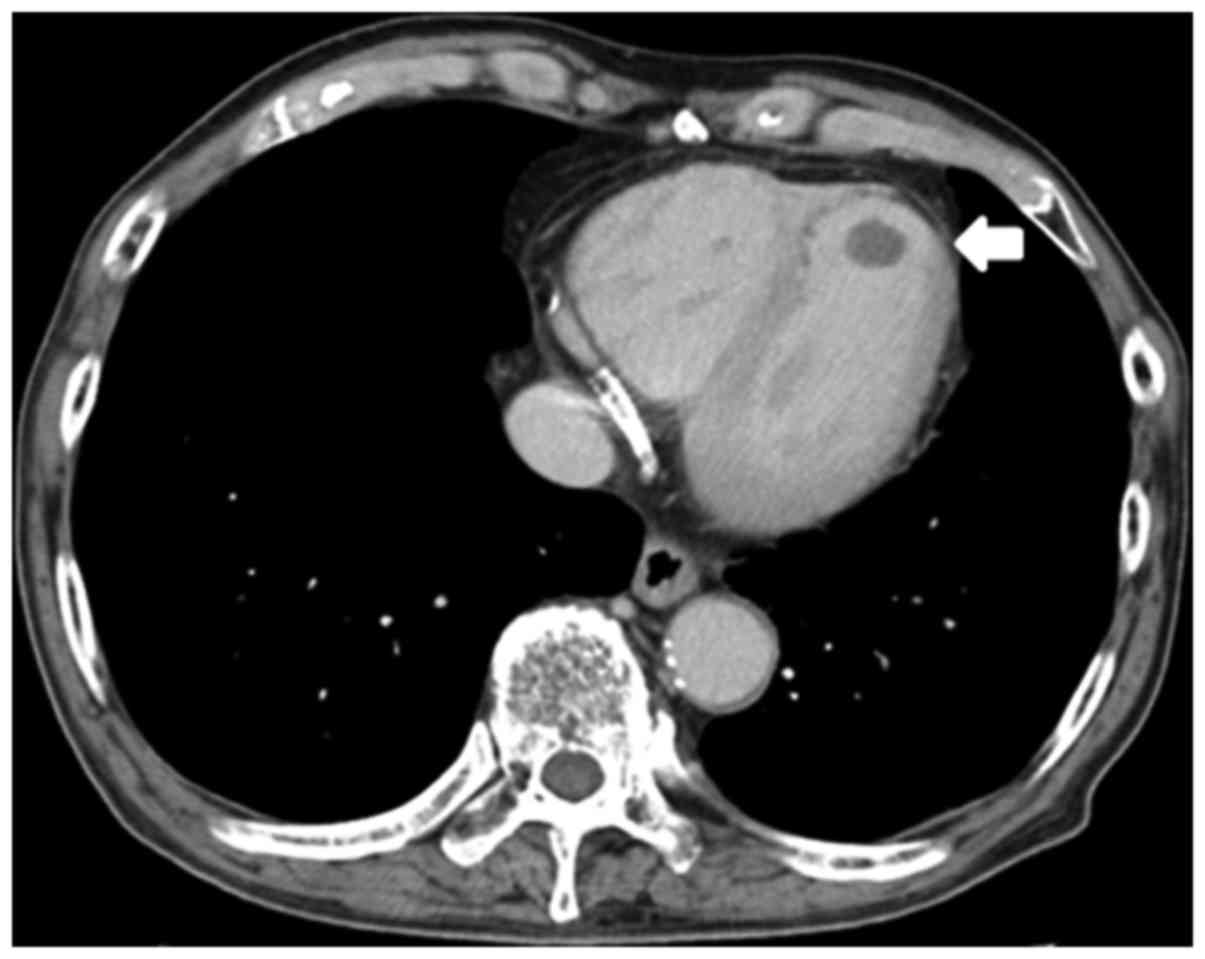
mg/m2). After 1 cycle, CT revealed a ball-like, movable
LV thrombus, measuring 13×10 mm (Fig.
2). The patient had a good general status, normal results on
electrocardiography, no reduction in EF (58%), no chest pain, and
no dyspnea. Laboratory data revealed a D-dimer level of 6.1 µg/ml,
a platelet count of 86×103/µl, and a level of fibrin
degradation products of 9.5 µg/ml. The cause of the LV thrombus was
unclear. To treat the LV thrombus, anticoagulant therapy (heparin,
14,000 U/day) was initiated. One day later, acute arterial
thrombosis was detected in the radial artery. However, the LV and
radial artery thrombi had completely disappeared after 1 week. The
LV thrombus showed no recurrence.
Chemotherapy was then restarted and continued for a
total of 6 cycles. Subsequent FDG-PET and CT scans revealed a
reduction in the size of lung metastases; however, the size and
number of liver metastases were increased, and the development of
bone metastases was detected. The patient eventually succumbed to
respiratory failure 6 months after commencing chemotherapy.
Informed consent for participation and for the
publication of the results of present study was obtained from the
patient.
Discussion
Cetuximab treatment is most commonly associated with
infusion reactions and skin reactions, and an increased risk of
VTEs has also been reported recently (1,2).
However, due to the typical lack of symptoms with VTEs, they are
often only diagnosed incidentally (2,3). In the
present case, CT scans that were performed to evaluate the
effectiveness of chemotherapy incidentally revealed LV thrombus.
For detecting deep vein thrombosis (DVT), D-dimer has been reported
to be extremely an useful marker (4), offering 84% sensitivity, 96%
specificity, and 90% accuracy when using a cutoff value of ≥3.0
µg/ml (4). In the present case, the
D-dimer level was 6.1 µg/ml; DVT was not detected, but LV thrombus
was revealed.
Transthoracic echocardiography is the initial
imaging technique (5,6) performed for detecting and diagnosing
cardiac masses (5). It is able to
reliably indicate the anatomical and functional features of a
detected cardiac mass; however, it has a limited ability to
characterize tissue. In this respect, cardiac magnetic resonance
imaging is superior at present, and should thus be the standard
diagnostic tool for the assessment of cardiac masses (5). It is easy to diagnose an LV thrombus if
any clinical and diagnostic findings are present, including
subjective symptoms, wall motion abnormalities, and changes on
electrocardiography. Currently, routine VTE prophylaxis during
chemotherapy in outpatient settings is not recommended by
international guidelines; however, certain studies have suggested
that the risk of VTE can be decreased by prophylactic
low-molecular-weight heparin in patients with metastatic or locally
advanced cancer who are receiving chemotherapy (7).
A large retrospective analysis reported that
cisplatin-based chemotherapy carried a high risk of venous and
arterial thromboembolic events (TEEs), with 88% of TEEs occurring
within 100 days of commencing cisplatin (8). TEEs included DVT alone in 49.7% of
cases, pulmonary embolism alone in 25.4%, DVT plus pulmonary
embolism in 13.6%, arterial TEEs alone in 8.3%, and DVT plus
arterial TEEs in 3.0% of cases. However, arterial TEEs did not
include LV thrombus. In the same observation period, a mortality
rate of 3.3% was noted, and it was considered that there was a
strong probability of TEEs contributing to or causing the majority
of these mortalities (8).
Additionally, the incidence rate of TEE was higher in cases of
metastatic disease (21.6%) than in cases of early-stage (16.7%) or
locally advanced disease (15.2%) (8). Cisplatin-associated vascular toxicities
may include hypomagnesemia, increased levels of von Willebrand
factor, and damage to endothelial cells via increased formation of
procoagulant endothelial microparticles (9), and it is likely that their pathogenesis
involves a pathway that affects arterial and venous systems
(8). Although it has been suggested
that the risk of VTEs increases when a patient is treated with
combined cetuximab and platinum-based chemotherapy (2), no reports have precisely described what
this risk level is. In a previous study, patients with malignancy
showed a 4.1-fold increased risk of VTEs compared with those
without malignancy, and the addition of chemotherapy increased that
risk to 6.5-fold (10). Cardiac
events, which represent a special category of adverse events,
include five conditions: Arrest, arrhythmia, congestive heart
failure, ischemia or infarction, and sudden death. In a previous
study (1), in patients receiving
platinum/5-FU plus cetuximab or platinum/5-FU alone, the
predominant grade 3–4 cardiac events were congestive heart failure
(4 patients and 1 patient, respectively), infarction and ischemia
(7 and 2 patients, respectively), and sudden death (3 patients and
1 patient, respectively). In that study (1), the reason for sudden death was not
clear; one possibility was the occurrence of an LV thrombus or a
suspended thromboembolism.
Although LV thrombus is an uncommon primary disease,
it is a common complication following acute myocardial infarction,
and is associated with a risk of systemic embolism (6,11). A
movable LV thrombus is more likely to be associated with
embolization compared with an immovable LV thrombus (11). Similarly, a thrombus that protrudes
into the LV cavity (ball-like thrombus) is more likely to be
associated with embolization compared with a flat thrombus
(mural-type thrombus) (5,11).
The treatment of LV thrombi remains controversial.
Surgery is recommended if the general condition of the patient is
sufficient and the thrombus is markedly protruding or is a
floating-type thrombus. As fibrinolytic therapy may cause fresh LV
thrombi to become fragmented and subsequently form emboli,
anticoagulant therapy is used frequently (6). In the current case, anticoagulation
therapy was selected. Acute arterial thrombosis of the radial
artery occurred 1 day after the commencement of anticoagulant
therapy; however, the LV and radial artery thrombi had completely
disappeared after 1 week.
It has been reported that the ability of tumor cells
to activate the coagulation system can lead to a hypercoagulable or
prothrombotic state in cases of malignancy (12). This hypercoagulable state is
associated with interactions between different mechanisms related
to the activation of various hemostatic components, such as the
coagulation and fibrinolytic pathways, vascular endothelium,
monocytes, and platelets (12).
Furthermore, functional DNA polymorphisms in genes encoding
thrombosis-related factors have been associated with increased risk
of oral SCC, and a number of single-nucleotide polymorphisms
associated with thrombosis may serve as primary predictors for oral
SCC risk (13).
A previous case report described the occurrence of
an LV thrombus in a patient who had a medical history of cutaneous
T-cell lymphoma and hypereosinophilia as well as a recent
Mycoplasma pneumoniae infection (5). The authors hypothesized that there was
an association between the Mycoplasma pneumoniae infection
and the occurrence of arterial and venous thrombi. However, the
underlying pathogenesis remained unclear, and the thrombus
formation may have resulted from a hypercoagulable state induced by
one or more of the patient's underlying diseases.
In the present case, no LV thrombus was apparent
prior to chemotherapy. Despite the patient taking an antiplatelet
agent, an LV thrombus suddenly arose within the first cycle of
chemotherapy. The patient had no subjective symptoms, cardiac
hypofunction, or acute myocardial infarction, and the LV thrombus
disappeared rapidly. Neoplastic thrombi are often reported
(5,10,14). In
the present case, it is possible that LV thrombus was caused by a
neoplastic thrombus; however, the LV thrombus occurred rapidly
following the initiation of chemotherapy. Additionally, although LV
thrombi are rare and are most often caused by acute myocardial
infarction (6,11,15), a
reduction of cardiac function was not detected in the present case.
Thus, the reason for the occurrence of the LV thrombus was not
clear. We hypothesize that the LV thrombus may be attributed to
both the presence of a neoplastic thrombus and chemotherapy.
In the present case, the LV thrombus was identified
early and was successfully cured. If chemotherapy had continued,
serious thromboembolism may have occurred. The risks of
thromboembolic events associated with cetuximab (2) and cisplatin-based chemotherapy
(8) have been reported previously;
however, it is possible that platinum-based chemotherapy plus
cetuximab, as in the present case, may carry a higher risk of
embolic thrombosis compared with cetuximab or platinum-based agents
administered individually.
Glossary
Abbreviations
Abbreviations:
|
SCC
|
squamous cell carcinoma
|
|
5-FU
|
5-fluorouracil
|
|
VTE
|
venous thromboembolic event
|
|
LV
|
left ventricular
|
|
FDG
|
fluorodeoxyglucose
|
|
PET
|
positron emission tomography
|
|
CT
|
computed tomography
|
|
EF
|
ejection fraction
|
|
DVT
|
deep vein thrombosis
|
|
TEE
|
venous and arterial thromboembolic
event
|
References
|
1
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petrelli F, Cabiddu M, Borgonovo K and
Barni S: Risk of venous and arterial thromboembolic events
associated with anti-EGFR agents: A meta-analysis of randomized
clinical trials. Ann Oncol. 23:1672–1679. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mandala M, Barni S, Floriani I, Isa L,
Fornarini G, Marangolo M, Mosconi S, Corsi D, Rulli E, Frontini L,
et al: Incidence and clinical implications of venous
thromboembolism in advanced colorectal cancer patients: The
‘GISCAD-alternating schedule’ study findings. Eur J Cancer.
45:65–73. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sassa H, Sone T, Tsuboi H, Kondo J and
Yabashi T: Diagnostic significance of thrombin-antithrombin III
complex (TAT) and D-dimer in patients with deep venous thrombosis.
Jpn Circ J. 60:201–206. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oeser C, Andreas M, Rath C, Habertheuer A
and Kocher A: Left ventricular thrombus in a patient with cutaneous
T-cell lymphoma, hypereosinophilia and Mycoplasma pneumoniae
infection-a challenging diagnosis: A case report. J Cardiothorac
Surg. 10:212015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iga K, Kondo H, Tamura T, Izumi C, Inoko
M, Kitaguchi S, Hirozane T, Himura Y, Gen H and Konishi T: Clinical
characteristics of patients with fresh left ventricular thrombus.
Jpn Circ J. 64:254–256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Agnelli G, Gussoni G, Bianchini C, Verso
M, Mandalà M, Cavanna L, Barni S, Labianca R, Buzzi F, Scambia G,
et al: Nadroparin for the prevention of thromboembolic events in
ambulatory patients with metastatic or locally advanced solid
cancer receiving chemotherapy: A randomised, placebo-controlled,
double-blind study. Lancet Oncol. 10:943–949. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moore RA, Adel N, Riedel E, Bhutani M,
Feldman DR, Tabbara NE, Soff G, Parameswaran R and Hassoun H: High
incidence of thromboembolic events in patients treated with
cisplatin-based chemotherapy: A large retrospective analysis. J
Clin Oncol. 29:3466–3473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lechner D, Kollars M, Gleiss A, Kyrle PA
and Weltermann A: Chemotherapy-induced thrombin generation via
procoagulant endothelial microparticles is independent of tissue
factor activity. J Thromb Haemost. 5:2445–2452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heit JA, Silverstein MD, Mohr DN,
Petterson TM, O'Fallon WM and Melton LJ III: Risk factors for deep
vein thrombosis and pulmonary embolism: A population-based
case-control study. Arch Intern Med. 160:809–815. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Haugland JM, Asinger RW, Mikell FL,
Elsperger J and Hodges M: Embolic potential of left ventricular
thrombi detected by two-dimensional echocardiography. Circulation.
70:588–598. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Caine GJ, Stonelake PS, Lip GY and Kehoe
ST: The hypercoagulable state of malignancy: Pathogenesis and
current debate. Neoplasia. 4:465–473. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vylliotis A, Yapijakis C, Nkenke E,
Nisyrios T, Avgoustidis D, Adamopoulou M, Ragos V, Vassiliou S,
Koronellos N and Vairaktaris E: Effect of thrombosis-related gene
polymorphisms upon oral cancer: A regression analysis. Anticancer
Res. 33:4033–4039. 2013.PubMed/NCBI
|
|
14
|
Cogo A, Bernardi E, Prandoni P, Girolami
B, Noventa F, Simioni P and Girolami A: Acquired risk factors for
deep-vein thrombosis in symptomatic outpatients. Arch Intern Med.
154:164–168. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Asinger RW, Mikell FL, Elsperger J and
Hodges M: Incidence of left-ventricular thrombosis after acute
transmural myocardial infarction. Serial evaluation by
two-dimensional echocardiography. N Engl J Med. 305:297–302. 1981.
View Article : Google Scholar : PubMed/NCBI
|