Introduction
Cancer is among the leading causes of morbidity and
mortality worldwide, with an estimated 14 million new cases and 8.2
million cancer-related deaths annually. Over 60% of the cases
globally occur in developing countries, which account for ~70% of
cancer-related mortality worldwide (1). Given their poor overall condition,
patients with late-stage cancer are mostly not eligible for
conventional anticancer treatments such as surgery, chemotherapy,
or radiotherapy, and best supportive care (BSC) is currently
considered as the only option for patients with a relatively poor
prognosis. Several efforts have been made to improve the survival
of patients with advanced cancer (2). However, the results thus far have been
unsatisfactory. Therefore, further efforts must be made to improve
the current therapeutic modalities and to explore novel therapies
for advanced cancer, in order to improve patient care and prolong
survival.
Immunotherapy has been shown to be effective in the
treatment of a number of malignant tumors, with adoptive cellular
immunotherapy being considered a promising and effective modality
(3–8). Several types of immune cells, such as
lymphokine-activated killer cells (9), tumor-infiltrating lymphocytes (10) and anti-CD3 monoclonal
antibody-induced killer cells (11)
have shown efficacy in advanced cancers. Among these cells,
cytokine-induced killer (CIK) cells have several advantages
compared with traditional immune cells, such as proliferating
rapidly in vitro, exhibiting intensified antitumor activity
and a broader spectrum of targeted tumors, and being associated
with less severe side effects, which qualifies them as one of the
most promising treatments, particularly for patients with advanced
cancer (6,12–17). It
is noteworthy that the antitumor activity of CIK cells may be
activated and enhanced by dendritic cells (DCs), which are the main
antigen-presenting cells (18,19). DCs
present tumor antigens through MHCII molecules, preventing the
immune escape of tumor cells. CIK cells are also able to recognize
DCs in a T-cell receptor-independent manner, and the DC-CIK
interaction stimulates the proliferation and antitumor activity of
CIK cells through secreting interleukin (IL)-12, interferon
(IFN)-γ, and other cytokines (20).
DCs plus CIK cells not only enhance the antitumor effect, but also
regulate and improve the immune function in cancer patients
(20–22). Lately, a report from the
International Registry on CIK cells found that adjuvant
immunotherapy with CIK cells may prevent recurrence and improve the
quality of life and progression-free survival rates in cancer
patients (6). Our previous study
also indicated that DC-CIK treatment may be able to recover
cellular immunity and improve the Eastern Cooperative Oncology
Group (ECOG) performance status and quality of life in patients
with advanced cancer (23,24).
Current data from clinical studies on the antitumor
effects and prognostic benefits of DC-CIK cells are limited,
particularly for patients with advanced cancer. The aim of the
present study was to evaluate the clinical efficacy of DC-CIK cell
treatment in patients with advanced cancer.
Patients and methods
Patients
A paired study was performed to compare the clinical
outcomes of advanced cancer patients received either autologous
DC-CIK immunotherapy or BSC alone. Between June 2012 and January
2014, a total of 90 patients with advanced cancer were recruited in
the present study from the Beijing Shijitan Hospital Cancer Center
(Capital Medical University, Beijing, China). A total of 57
patients underwent DC-CIK immunotherapy (DC-CIK group) and 33
patients were administered BSC alone (BSC group). All the patients
had a definitive histological or cytological diagnosis and were
unresponsive or intolerant to conventional anticancer treatments,
such as surgery, chemotherapy or radiotherapy. The characteristics
of the patients are summarized in Table
I. The criteria for patient selection were as follows: i)
Patient aged 18–85 years; ii) chemoradiotherapy-free for ≥3 months;
iii) expected survival duration of >3 months; iv) ECOG
performance status of 0–2; v) white blood cell (WBC) count
>3,500/µl; vi) hemoglobin level >80 g/dl; vii) platelet count
>100,000/µl; viii) serum aspartate aminotransferase
(AST)/alanine aminotransferase (ALT) <2.0 the upper limit of
normal; ix) no cardiac arrhythmias, congestive heart failure or
severe coronary artery disease; x) no active autoimmune disease or
T-cell lymphoma; and xi) no pregnancy or lactation. The subjects in
the two groups were matched for sex, age, stage, pathology, tumor
size and metastasis at the first visit.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
Characteristics | DC-CIK group | BSC group | P-value |
|---|
| No. of
patients | 57 | 33 |
|
| Sex (n) | 0.230 |
|
|
|
Male | 27 | 20 |
|
|
Female | 30 | 13 |
|
| Age (years) | 0.874 |
|
|
| Mean ±
standard deviation | 60.00±16.30 | 60.52±11.64 |
|
| <60
(n) | 31 | 16 |
|
| ≥60
(n) | 26 | 17 |
|
| Tumor types
(n) | 0.450 |
|
|
|
Digestive system cancer | 19 | 11 |
|
| Lung
cancer and mesothelioma | 12 | 11 |
|
| Breast
cancer | 8 | 3 |
|
| Head
and neck cancer | 4 | 2 |
|
| Male
genitourinary system cancer | 4 | 1 |
|
| Female
genitourinary system cancer | 3 | 0 |
|
|
Lymphoma | 3 | 5 |
|
|
Sarcoma | 2 | 0 |
|
|
Glioblastoma | 1 | 0 |
|
|
Melanoma | 1 | 0 |
|
| Stage (n) |
|
| 0.105 |
| IV | 40 | 19 |
|
|
III | 15 | 11 |
|
| II | 2 | 3 |
|
| DC-CIK treatment
cycles (n) |
| 1 | 33 | – |
|
| 2 | 11 | – |
|
| 3 | 7 | – |
|
| ≥4 | 6 | – |
|
Informed consent was obtained from all the patients.
After enrollment, a complete medical history was taken and physical
examination was conducted by professional oncologists for each
patient. This study was approved by the Ethics Committee of the
Beijing Shijitan Hospital.
Treatment
Patients in the control group received BSC alone,
which included active symptom control, pain management, and the
multiprofessional attention to the individual's overall physical,
psychosocial, spiritual and cultural needs. Patients in the DC-CIK
group received autologous DC-CIK cells with an interval of 1 month
in addition to BSC. For each cycle of treatment, the patients
received three intravenous infusions of DC-CIK cells with 1-day
intervals. Patients without disease progression were eligible for
maintenance treatment. For each cycle of treatment, the patients
received a median of 6.47×108 (range,
5.35×107-2.98×109) of autologous DC cells and
7.35×109 (range, 3.00–17.25×109) of CIK
cells. The median number of CIK cell immunotherapy cycles was 2
(range, 1–13 cycles). All the patients were seen biweekly or
monthly by oncology specialists, and clinical examinations were
performed monthly, including physical examination, T-cell subsets,
routine blood count, serum AST and ALT, blood urea nitrogen and
creatinine, and electrocardiogram. Additional care was provided if
needed.
Clinical assessment of response and
toxicity
All the patients were followed up at the outpatient
clinic or the oncology ward from the date of initial treatment to
March 31, 2016, or to the date of death. Clinical response was
determined according to the National Cancer Institute's Response
Evaluation Criteria in Solid Tumors, version 1.1 (https://ctep.cancer.gov/protocoldevelopment/docs/recist_guideline.pdf).
Patients were assessed by oncologists after each cycle of
treatment, including color Doppler ultrasound, computed tomography
scan, magnetic resonance imaging and technetium bone scan. Overall
survival (OS) was calculated from the time of treatment initiation
until death, and patients who remained alive were censored at the
time of the last follow-up. Adverse events were evaluated according
to the World Health Organization (WHO) criteria (25).
Preparation of DC and CIK cells
CIK cells were prepared as previously described
(23). Briefly, peripheral blood
mononuclear cells (PBMCs) were mobilized by granulocyte
colony-stimulating factor (G-CSF) until WBC ≥10,000/µl, lymphocytes
+ monocytes ≥15%. Apheresis was performed from the patients using
the COBE Spectra cell separator (COBE BCT, Lakewood, CO, USA) and
repeated until ≥4.5×106/kg CD34+ cells were
collected. PBMCs were separated by the Ficoll-Hypaque
centrifugation method and incubated for 2 h, and the adherent cells
were cultured in vitro to generate autologous DCs in the
presence of IL-4, tumor necrosis factor-α and
granulocyte-macrophage (GM)-CSF (Boehringer, Mannheim, Germany).
For the culture of autologous CIKs, PBMCs were cultured in AIM-V
medium containing the recombinant cytokines IL-2, IFN-γ and
monoclonal anti-human CD3 antibody (50 ng/ml; IM1650, Boehringer).
The cells were incubated in a humidified atmosphere with 5%
CO2 at 37°C. IL-2 and IFN-γ were added to the medium
every 5 days.
Cell growth was observed under the microscope, and
DC phenotypes were determined by flow cytometry of CD80, CD86,
HLA-DR, CD1a and CD11c (Beckman-Coulter, Shanghai, China). The DC
suspension contained >80% of CD80+/CD86+
cells prior to infusion. The CIKs expressed CD3 and CD56
(Beckman-Coulter). After culture in vitro for 7–10 days, DCs
and CIKs were harvested and administered intravenously 3 times with
1-day intervals.
Detecting the phenotype of DCs, CIK
cells and T-cell subsets
The phenotype of DCs and CIK cells was determined
prior to infusion. T-cell subsets were assessed at the beginning of
the first treatment and redetected monthly. Briefly, 2 ml of
heparinized peripheral blood was drawn from each patient and PBMCs
were separated by the Ficoll-Hypaque centrifugation method. A total
of 100 µl PBMCs were incubated in the dark with primary antibody at
4°C for 15 min. After hemolysis for 10 min, the samples were
centrifuged for 10 min at 450 × g at room temperature, and then
washed twice in phosphate-buffered saline and subjected to flow
cytometric analysis (Becton-Dickinson, Franklin Lakes, NJ). The
primary antibodies included: CD4-FITC (A07550), CD8-PE (IM1650),
CD3-PerCP (A07749), CD25-PE (A07774), CD28-FITC (IM1236U), CD3-FITC
(A07746) and CD56-PE (IM2073U) (Beckman-Coulter). All the
antibodies were mouse anti-human monoclonal antibodies, with 1:10
dilution.
The cell phenotypes were analyzed by flow cytometry
(FC500, Beckman-Coulter) and CXP analysis software
(Beckman-Coulter) was used. Lymphocyte subset levels were reported
as percentages of the total population.
Statistical analysis
Data were analyzed using the SPSS 16.0 software
package (SPSS Inc., Chicago, IL, USA). T-cell subsets were
expressed as mean ± standard deviation. The independent samples
t-test and paired t-test were used to compare the changes in T-cell
populations between the two groups. The OS rate and survival curves
were calculated by the Kaplan-Meier method. Associations between OS
and potential prognostic factors were estimated using the log-rank
test in univariate analyses. The significant variables were further
analyzed by the Cox hazard proportional regression model with
adjustments for age, sex and tumor type. All tests were two-sided
and the significance level was set at 0.05.
Results
Patient characteristics
Of the 57 patients in the DC-CIK group, 27 were male
and 30 were female; the mean patient age was 60 years (range, 6–81
years). The patient characteristics are summarized in Table I. For each cycle of treatment, the
patients received three intravenous infusions of DC-CIK cells with
1-day intervals. The median number of CIK cell immunotherapy cycles
was 2 (range, 1–13 cycles).
Of the 33 patients in the BSC group, 20 were male
and 13 were female; the mean patient age was 61 years (range, 42–85
years). The patients were administered BSC at the Department of
Oncology of our hospital between June 2012 and January 2014.
The patient characteristics, such as sex, age, tumor
type and clinical stage, were comparable between the two groups
(Table I).
Comparison of T-cell subsets before
and after DC-CIK infusion
To investigate the immunomodulatory effects of
DC-CIK cell treatment, the T-cell subsets in the 57 patients in the
DC-CIK group were analyzed prior to and 1 month after DC-CIK cell
infusion. The CD3+, CD3+/CD4+,
CD3+/CD8+, CD8+/CD28+
and CD3+/CD56+ T-cell subsets were
significantly increased following DC-CIK treatment (P<0.05).
Conversely, the CD4+/CD25+,
CD8+/CD28− subsets were significantly
decreased following DC-CIK immunotherapy (P<0.05) (Table II).
 | Table II.T-cell subsets in the peripheral
blood before and after DC-CIK cell treatment. |
Table II.
T-cell subsets in the peripheral
blood before and after DC-CIK cell treatment.
| T-cell subsets | Before treatment
(%) | After treatment
(%) | P-value |
|---|
|
CD3+ |
68.67±11.23 |
82.55±12.59 | 0.000 |
|
CD3+/CD4+ |
34.23±11.97 |
40.22±17.09 | 0.005 |
|
CD3+/CD8+ |
32.27±12.21 |
42.42±17.83 | 0.000 |
|
CD4+/CD25+ |
4.48±3.05 |
3.23±2.65 | 0.024 |
|
CD8+/CD28− |
23.39±10.09 |
17.97±9.18 | 0.000 |
|
CD8+/CD28+ |
14.11±8.46 |
27.00±15.10 | 0.000 |
|
CD3+/CD56+ |
10.88±7.76 |
15.56±16.24 | 0.047 |
Comparison of the changes in T-cell
subsets between the DC-CIK and BSC groups
Next, the changes in the T-cell subsets in
peripheral blood between the DC-CIK and BSC groups we observed. The
T-cell subsets were analyzed prior to and 1 month after the first
treatment in patients from the two groups. Although no significant
difference in the T-cell subsets were observed between the two
groups at the beginning of the first treatment (data not shown),
the CD3+, CD3+/CD8+,
CD8+/CD28+ and
CD3+/CD56+ T-cell subsets were significantly
increased in the DC-CIK group compared with the BSC group, while
the CD8+/CD28− subset decreased
significantly. No significant differences in the
CD3+/CD4+ and
CD4+/CD25+ subsets were observed between the
two groups before and after treatment (Table III). Thus, these data indicated
that DC-CIK cell treatment improved cellular immune function in
advanced cancer patients.
 | Table III.Comparison of the changes in T-cell
subsets between the BSC and DC-CIK groups. |
Table III.
Comparison of the changes in T-cell
subsets between the BSC and DC-CIK groups.
| T cell subsets | BSC group
(%)a | DC-CIK group
(%)a | P-value |
|---|
|
CD3+ |
2.09±10.89 |
13.88±13.72 | 0.000 |
|
CD3+/CD4+ |
0.85±10.54 |
5.99±15.55 | 0.367 |
|
CD3+/CD8+ |
0.97±7.38 |
10.15±18.00 | 0.006 |
|
CD4+/CD25+ |
−0.79±4.55 |
−1.25±4.07 | 0.247 |
|
CD8+/CD28− |
1.96±8.68 |
−5.42±7.85 | 0.011 |
|
CD8+/CD28+ |
−0.22±5.20 |
12.89±14.76 | 0.000 |
|
CD3+/CD56+ |
−1.88±5.17 |
4.67±17.37 |
0.018 |
Association between cycles of DC-CIK
infusion and T-cell subset changes
The effect of the frequency of DC-CIK infusion on
the changes in T-cell subsets was further evaluated, and it was
observed that the T-cell subsets changed after 1 cycle of DC-CIK
immunotherapy. CD3+, CD3+/CD4+,
CD3+/CD8+, CD8+/CD28+
and CD3+/CD56+ subsets were significantly
increased, while CD4+/CD25+ and
CD8+/CD28− were significantly decreased.
However, no obvious changes in T-cell subsets were observed before
or after >2 cycles of infusion (Table IV).
 | Table IV.Associations between cycles of DC-CIK
infusion and T-cell subset changes. |
Table IV.
Associations between cycles of DC-CIK
infusion and T-cell subset changes.
|
| Before
treatment | 1 cycle | 2 cycles | 3 cycles | ≥4 cycles |
|---|
| Cases, n |
| 57 | 26 | 13 | 6 |
|
CD3+ |
68.67±11.23 |
82.55±12.59b |
80.87±15.01a |
83.59±13.30 |
86.20±12.54a |
|
CD3+/CD4+ |
34.23±11.97 |
40.22±17.09b |
41.17±18.71 |
29.73±15.75 |
41.25±8.38a |
|
CD3+/CD8+ |
32.27±12.21 |
42.42±17.83b |
29.49±14.27 |
44.79±9.32 |
35.88±15.38 |
|
CD4+/CD25+ |
4.48±3.05 |
3.23±2.65a |
2.35±1.49a |
2.61±1.35 |
3.07±2.42 |
|
CD8+/CD28− |
23.39±10.09 |
17.97±9.18b |
16.98±6.92 |
22.07±10.95a |
15.72±6.58a |
|
CD8+/CD28+ |
14.11±8.46 |
27.00±15.10b |
25.94±17.11 |
18.47±11.32 |
32.05±12.77a |
|
CD3+/CD56+ |
10.88±7.76 |
15.56±16.24a |
24.25±21.96 |
9.50±5.55 |
5.73±2.42 |
Association between OS and T-cell
subset changes
To investigate the factors that affect the OS of
patients with advanced cancer, a univariate analysis was conducted,
demonstrating that a lower CD8+/CD28− and a
higher of CD8+/CD28+ ratio may be associated
with longer OS. In addition, DC-CIK treatment administration, age
(>60 vs. <60 years), clinical stage and the frequency of CIK
treatment significantly affected the OS of patients in the DC-CIK
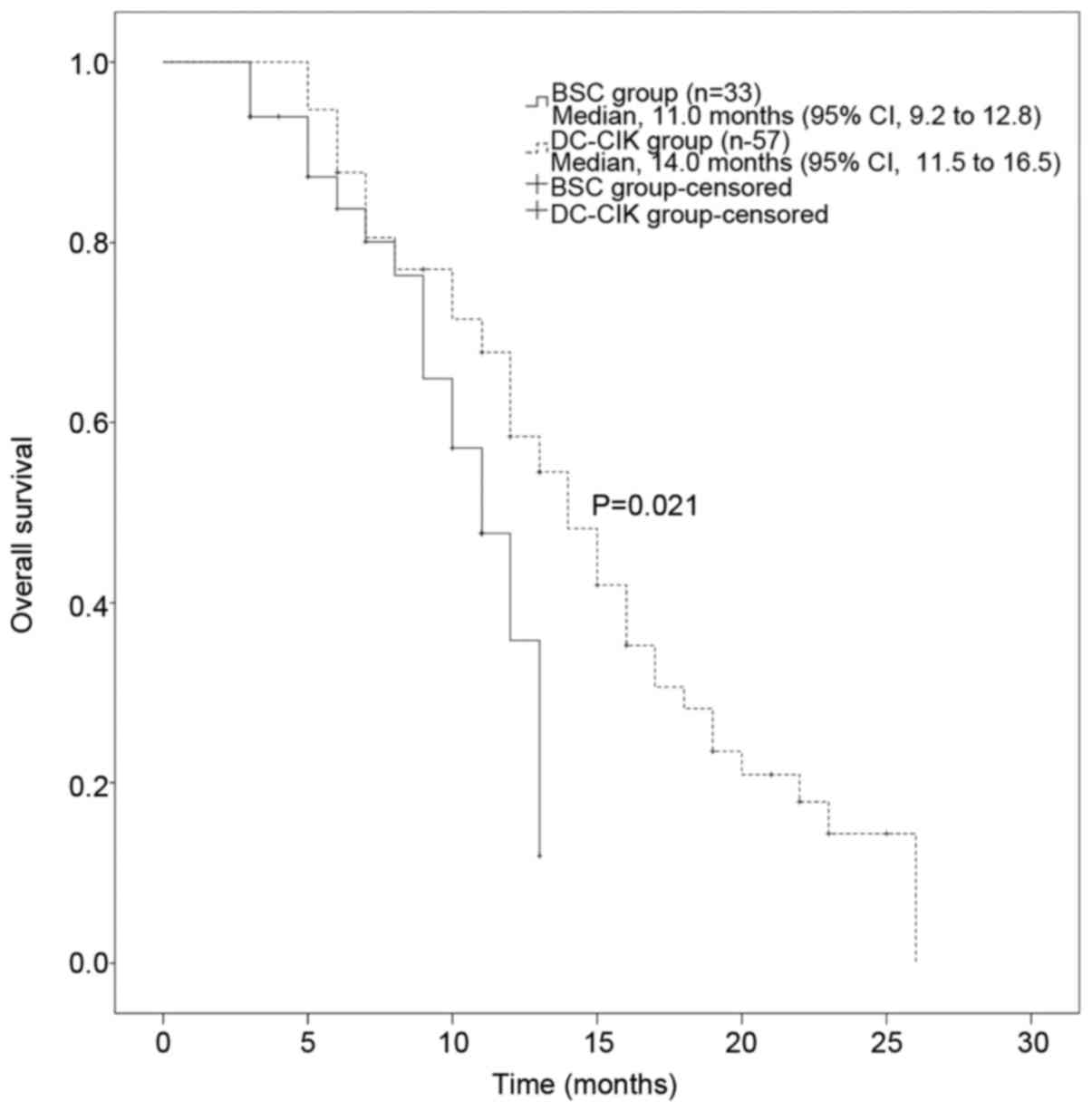
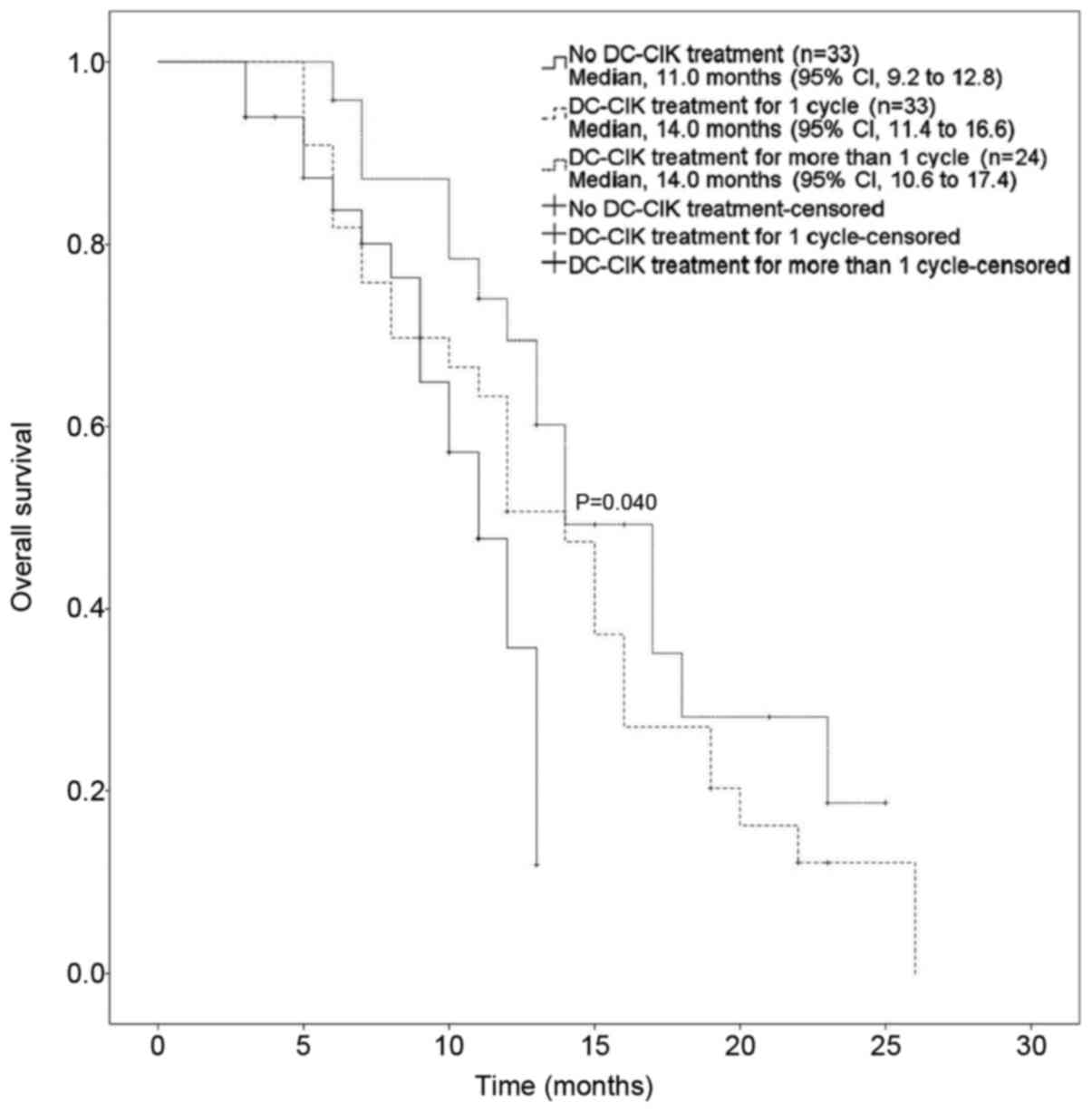
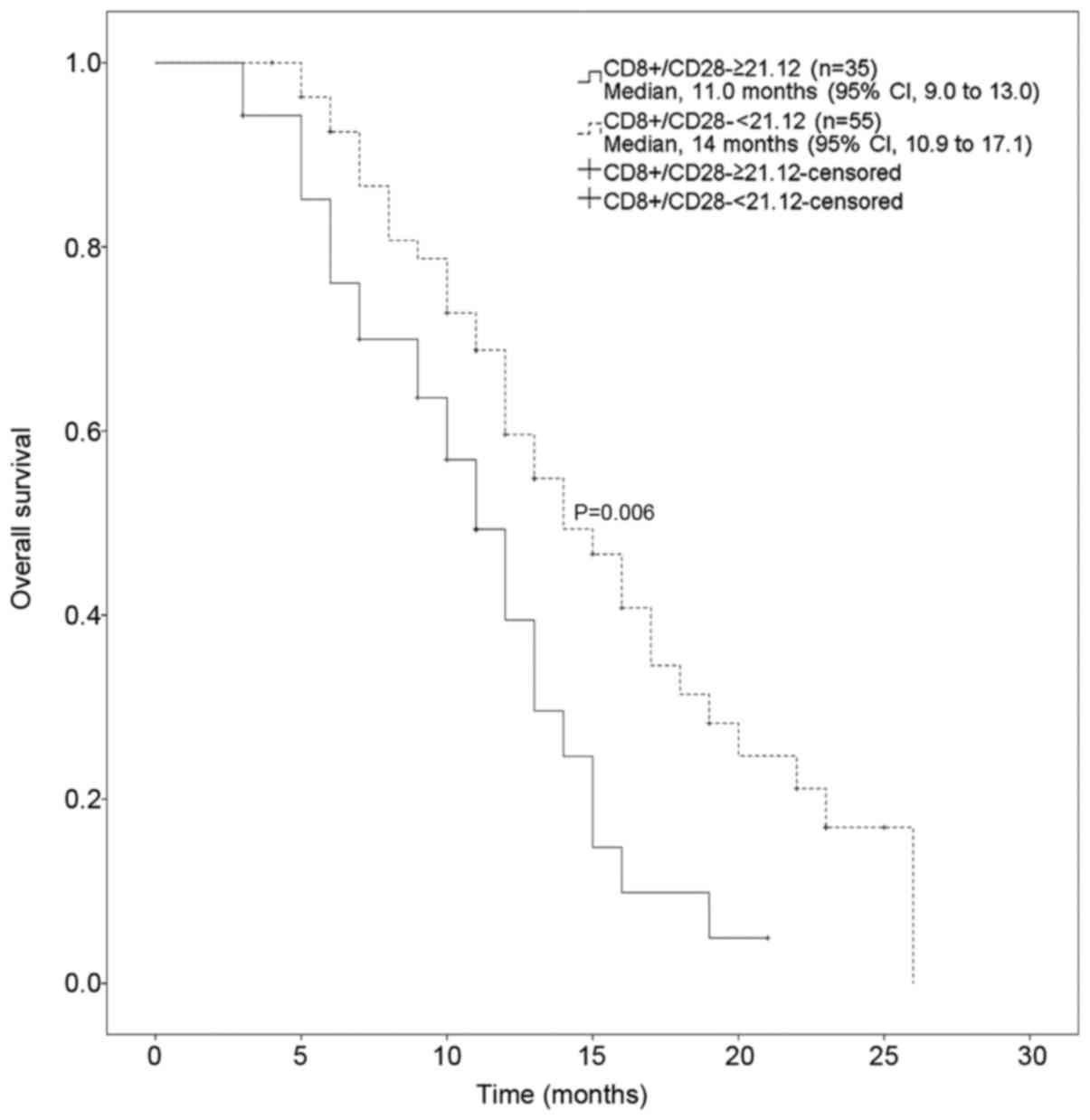
group (Table V, Figs. 1 and 2).
 | Table V.Univariate analysis of the patient's
clinical characteristics and overall survival. |
Table V.
Univariate analysis of the patient's
clinical characteristics and overall survival.
| Variables | Median OS
(months) | Log-rank Relative
risk (95% CI) | P-value |
|---|
|
CD8+/CD28− |
|
| 0.006 |
|
≥21.12 | 11.00 | 8.96–13.04 |
|
|
<21.12 | 14.00 | 10.88–17.12 |
|
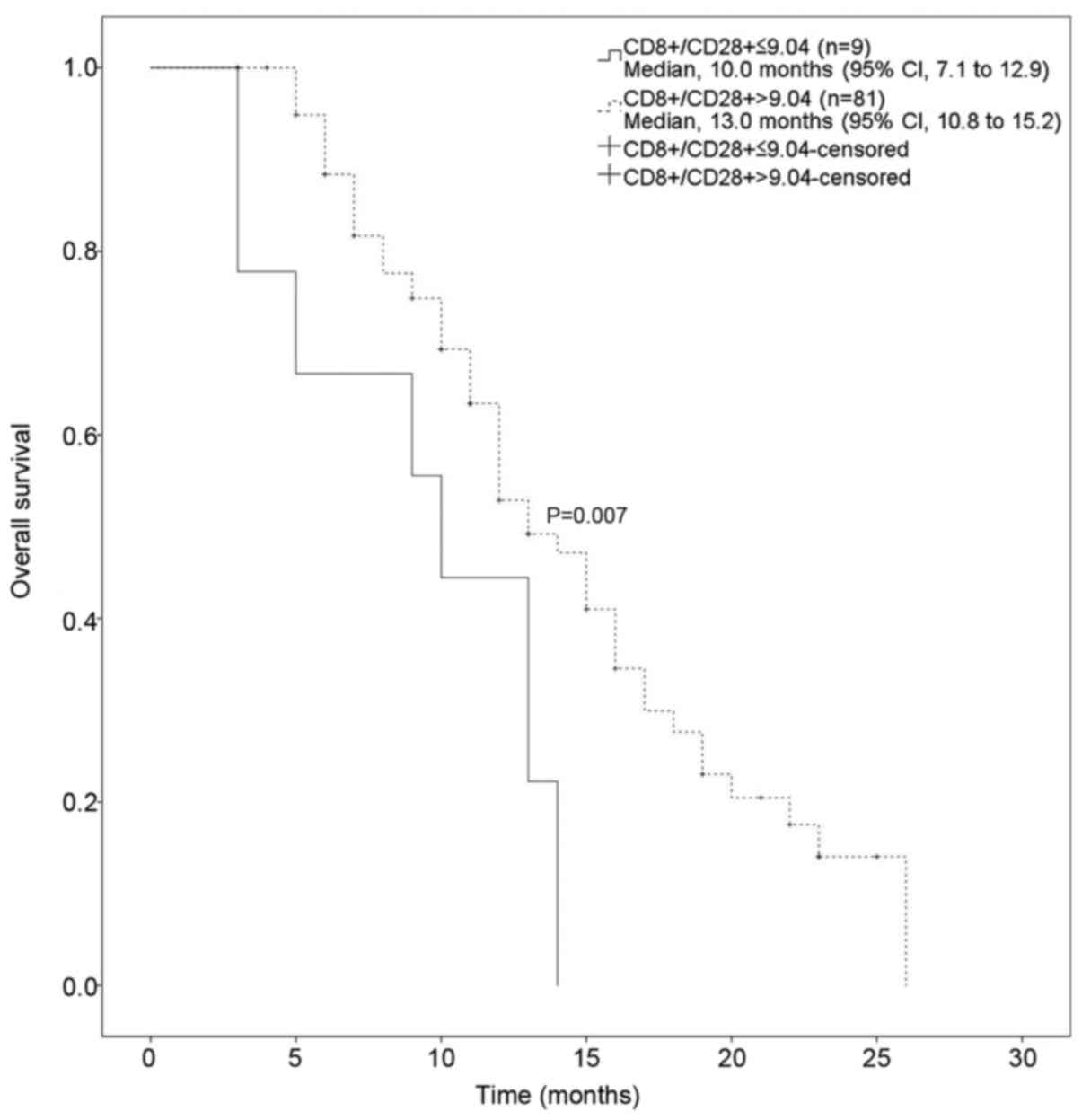
|
CD8+/CD28+ |
|
| 0.007 |
|
≤9.04 | 10.00 | 7.08–12.92 |
|
|
>9.04 | 13.00 | 10.82–15.18 |
|
| DC-CIK therapy |
|
| 0.021 |
|
Yes | 11.00 | 9.17–12.83 |
|
| No | 14.00 | 11.54–16.46 |
|
| Age (years) |
|
| 0.050 |
|
<60 | 15.00 | 10.54–19.46 |
|
|
≥60 | 12.00 | 10.51–13.49 |
|
| Clinical stage |
|
| 0.032 |
| II | – | 12.00–28.00 |
|
|
III | 19.00 | 13.34–20.54 |
|
| IV | 12.00 | 11.05–15.44 |
|
| DC-CIK cycles
(n) |
|
| 0.040 |
| 0 | 11.00 | 9.17–12.83 |
|
| 1 | 14.00 | 11.35–16.65 |
|
| ≥2 | 14.00 | 10.56–17.45 |
|
The parameters that affected the prognosis of
advanced cancer patients in this study were further analyzed
through the Cox hazard proportional regression model with
adjustments for age, sex and tumor type. A multivariate analysis
demonstrated that a CD8+/CD28− ratio
<21.12 decreased the hazard ratio (HR) of OS to 0.50 [95%
confidence interval (CI): 0.29–0.87] and a
CD8+/CD28+ ratio >9.04 decreased the HR of
OS to 0.45 (95% CI: 0.21–0.98) (Table
VI, Figs. 3 and 4). Thus, taken together, these results
demonstrated that T-cell populations may be associated with the OS
of patients with advanced cancer, and DC-CIK immunotherapy may
improve OS.
 | Table VI.Multivariable analysis of the
patients' clinical characteristics and overall survival. |
Table VI.
Multivariable analysis of the
patients' clinical characteristics and overall survival.
| Parameters | Hazard ratio | 95% CI |
|---|
|
CD8+/CD28−
<21.12 | 0.500 | 0.288–0.866 |
|
CD8+/CD28+
>9.04 | 0.435 | 0.209–0.907 |
Side effects
No severe side effects were observed during DC-CIK
cell treatment. Three patients in the DC-CIK group developed fever
(<38.5°C) that was spontaneously relieved 2 h after the
infusion. No other serious adverse events, such as high fever,
chills, rash or hemolytic anemia, were reported in patients
receiving DC-CIK cell treatment.
Discussion
Accumulating evidence supports cellular
immunotherapy, which directly or indirectly regulates the
biological interaction between the host and the tumor (26), as a potential strategy for the
improvement of cancer treatment, with CIK cells representing a
promising cellular immunotherapy associated with several
advantages, such as MHC-unrestricted cytotoxic activity, increase
in cytokine secretion, improvement of immune function and induction
of apoptosis of cancer cells (27),
and may thus be beneficial to patients with advanced cancer.
An increasing amount of studies demonstrated that
CIK cell-based immunotherapy is a promising new treatment modality
with the potential of improving the prognosis of cancer patients
(18–24,26,27). A
study from the International Registry reported the results of 11
CIK cell treatment trials for a variety of cancers, including
hepatocellular carcinoma, gastric cancer and Hodgkin or non-Hodgkin
lymphoma, and demonstrated a total response rate of 91/384 reported
patients; 161 patients had stable disease and 129 patients had
progressive disease. The disease-free survival rates were
significantly higher in patients treated with CIK cells compared
with those in the control group without CIK treatment (6). The present study demonstrated that the
OS favored the DC-CIK arm compared with the BSC arm. The results
indicated a 3-month prolongation in the median OS in favor of the
DC-CIK treatment compared with the BSC alone arm (14.00 vs. 11.00
months, respectively). Thus, DC-CIK cell immunotherapy prolonged
the OS of patients with advanced cancer.
The association between the T-cell subsets and the
clinical outcome of advanced cancer was also investigated, as any
successful host immune response against a tumor requires a
well-balanced positive and negative regulation of lymphocytes.
Imbalance of the host cellular immunity may trigger cancer
progression disease progression and treatment failure (28). Previous studies have demonstrated
significant changes of peripheral blood lymphocyte cell subsets in
patients with different malignant lesions and poor prognosis
(29–32). Consequently, we also investigated the
T-cell subsets before and after treatment in both groups, which
indicated that DC-CIK infusions altered the ratios of T-cell
subsets, increasing the T-helper and cytotoxic T lymphocyte (CTL)
subsets, while decreasing regulatory T lymphocyte (Treg) subsets,
particularly the CD8+/CD28− subset.
Emerging evidence supports the hypothesis that the
improvement of the host immune status, including the anti-PD-1
antibody and anti-CTLA4 antibody, may favor the clinical outcome of
cancer patients (33). Our previous
data also indicated that elevated levels of
CD8+/CD28− suppressor T lymphocytes represent
a novel independent predictor of progression-free survival in
patients with metastatic breast cancer during post-chemotherapy
follow-up (31). The present study
revealed that T-cell subsets significantly affected the OS of
patients with advanced cancer, and that DC-CIK treatment did not
only improve the imbalance in the immune status, but also prolonged
the OS in advanced cancer patients.
According to these results, there was a significant
difference in the OS among the three groups (no immunotherapy, 1
cycle and ≥2 cycles of DC-CIK infusion). One cycle of DC-CIK
infusion significantly altered the T-cell subsets, but no obvious
difference was observed among the groups receiving 2, 3 and ≥4
cycles of DC-CIK infusion, which was likely due to the small number
of cases. A larger randomized clinical trial will be conducted in
the near future to confirm the treatment benefit and the preferred
treatment courses of DC-CIK cells for patients with advanced
cancer.
No serious adverse events were observed in the
patients receiving DC-CIK cell immunotherapy, which was consistent
with the results of other studies (22). Therefore, DC-CIK cells are able to
eliminate tumor cells without severe injury to normal tissues,
which renders this treatment suitable for elderly and
advanced-stage cancer patients.
It should be noted that there were several
limitations to this study. First, the results were generated from a
retrospective observational study, and a prospective paired study
is required to confirm the clinical outcomes of DC-CIK cell
immunotherapy. Second, only 90 patients were considered eligible
for the present study. More cases are required and randomized
clinical studies according to different tumor types must be
performed to further analyze the treatment benefits of DC-CIK cells
for patients with advanced cancer. Third, it must be mentioned that
the CD4+/CD25+ T-cell subsets detected in
this study may not represent
CD4+/CD25+FoxP3+ Tregs, as not
only Tregs but different T-cell subsets were considered. Foxp3
expression will be included along with CD4 and CD25 to validate the
reduction of Tregs in a following study.
The data presented herein demonstrated that T-cell
subset changes were associated with the OS of advanced cancer
patients, while DC-CIK cell immunotherapy may regulate and enhance
the host's immune function, significantly improving the OS of
patients with advanced cancer. No severe side effects were recorded
during the immunotherapy process, indicating that this is a safe
treatment modality. A larger randomized, prospective clinical trial
is required to further validate the clinical efficacy of DC-CIK
cell therapy for patients with advanced cancer, and to elucidate
the detailed underlying mechanism.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81470139).
References
|
1
|
Stewart BW and Wild CP: World Cancer
Report 2014International Agency for Research on Cancer. Lyon: World
Health Organization; 2014, View Article : Google Scholar
|
|
2
|
Abrams J, Conley B, Mooney M, Zwiebel J,
Chen A, Welch JJ, Takebe N, Malik S, McShane L, Korn E, et al:
National cancer institute's precision medicine initiatives for the
new national clinical trials network. Am Soc Clin Oncol Educ Book.
2014:71–76. 2014. View Article : Google Scholar
|
|
3
|
Couzin-Frankel J: Breakthrough of the year
2013. Cancer immunotherapy. Science. 342:1432–1433. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bernstein R: Translational research:
Cancer killers. Nature. 508:139–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hontscha C, Borck Y, Zhou H, Messmer D and
Schmidt-Wolf IG: Clinical trials on CIK cells: First report of the
international registry on CIK cells (IRCC). J Cancer Res Clin
Oncol. 137:305–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stroncek D, Berlyne D, Fox B, Gee A,
Heimfeld S, Lindblad R, Loper K, McKenna D Jr, Rooney C, Sabatino
M, et al: Developments in clinical cell therapy. Cytotherapy.
12:425–428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang L, Wang J, Wei F, Wang K, Sun Q,
Yang F, Jin H, Zheng Y, Zhao H, Wang L, et al: Profiling the
dynamic expression of checkpoint molecules on cytokine-induced
killer cells from non-small-cell lung cancer patients. Oncotarget.
7:43604–43615. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rosenberg S: Lymphokine-activated killer
cells: A new approach to immunotherapy of cancer. J Natl Cancer
Inst. 75:595–603. 1985.PubMed/NCBI
|
|
10
|
Rosenberg SA, Spiess P and Lafreniere R: A
new approach to the adoptive immunotherapy of cancer with
tumor-infiltrating lymphocytes. Science. 233:1318–1321. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yun YS, Hargrove ME and Ting CC: In vivo
antitumor activity of anti-CD3-induced activated killer cells.
Cancer Res. 49:4770–4774. 1989.PubMed/NCBI
|
|
12
|
Yang L, Ren B, Li H, Yu J, Cao S, Hao X
and Ren X: Enhanced antitumor effects of DC-activated CIKs to
chemotherapy treatment in a single cohort of advanced
non-small-cell lung cancer patients. Cancer Immunol Immunother.
62:65–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu J, Li H, Cao S, Zhang X, Yu J, Qi J,
An X, Yu W and Ren X: Maintenance therapy with autologous
cytokine-induced killer cells in patients with advanced epithelial
ovarian cancer after first-line treatment. J Immunother.
37:115–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang X, Liu T, Zang X, Liu H, Wang D, Chen
H and Zhang B: Adoptive cellular immunotherapy in metastatic renal
cell carcinoma: A systematic review and meta-analysis. PLoS One.
8:e628472013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo H, Gong L, Zhu B, Huang Y, Tang C, Yu
S, Yang Z and Zhou X: Therapeutic outcomes of autologous CIK cells
as a maintenance therapy in the treatment of lung cancer patients:
A retrospective study. Biomed Pharmacother. 84:987–993. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kong DS, Nam DH, Kang SH, Lee JW, Chang
JH, Kim JH, Lim YJ, Koh YC, Chung YG, Kim JM and Kim CH: Phase III
randomized trial of autologous cytokine-induced killer cell
immunotherapy for newly diagnosed glioblastoma in korea.
Oncotarget. 8:7003–7013. 2017.PubMed/NCBI
|
|
17
|
Li X, Dai X, Shi L, Jiang Y, Chen X, Chen
L, Zhao J, Qiang W, Wu J, Ji M, et al: Phase II/III study of
radiofrequency ablation combined with cytokine-induced killer cells
treating colorectal liver metastases. Cell Physiol Biochem.
40:137–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leemhuis T, Wells S, Scheffold C, Edinger
M and Negrin RS: A phase I trial of autologous cytokine-induced
killer cells for the treatment of relapsed Hodgkin disease and
non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 11:181–187.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou C, Liu D, Li J, Sun H, Zheng X, Wang
S, Hong G, Mallampati S, Sun H, Zhou X, et al: Chemotherapy plus
dendritic cells co-cultured with cytokine-induced killer cells
versus chemotherapy alone to treat advanced non-small-cell lung
cancer: A meta-analysis. Oncotarget. 7:86500–86510. 2016.PubMed/NCBI
|
|
20
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gallimore A and Godkin A: Regulatory T
cells and tumour immunity-observations in mice and men. Immunology.
123:157–163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ,
Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW and Yoon JH: Adjuvant
immunotherapy with autologous cytokine-induced killer cells for
hepatocellular carcinoma. Gastroenterology. 148:1383–1391.e6. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao YJ, Jiang N, Song QK, Wu JP, Song YG,
Zhang HM, Chen F, Zhou L, Wang XL, Zhou XN, et al: Continuous
DC-CIK infusions restore CD8+ cellular immunity, physical activity
and improve clinical efficacy in advanced cancer patients
unresponsive to conventional treatments. Asian Pac J Cancer Prev.
16:2419–2423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li YC, Zhao L, Wu JP, Qu CX, Song QK and
Wang RB: Cytokine-induced killer cell infusion combined with
conventional treatments produced better prognosis for
hepatocellular carcinoma patients with barcelona clinic liver
cancer B or earlier stage: A systematic review and meta-analysis.
Cytotherapy. 18:1525–1531. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
World Health Organization, . WHO Handbook
for Reporting Results of Cancer Treatment. Geneva, Switzerland: WHO
Offset publication No. 48; 1979
|
|
26
|
Wu C, Jiang J, Shi L and Xu N: Prospective
study of chemotherapy in combination with cytokine-induced killer
cells in patients suffering from advanced non-small cell lung
cancer. Anticancer Res. 28:3997–4002. 2008.PubMed/NCBI
|
|
27
|
Sun S, Li XM, Li XD and Yang WS: Studies
on inducing apoptosis effects and mechanism of CIK cells for
MGC-803 gastric cancer cell lines. Cancer Biother Radiopharm.
20:173–180. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zou W: Regulatory T cells, tumor immunity
and immunotherapy. Nat Rev Immunol. 6:295–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen B, Liu L, Xu H, Yang Y, Zhang L and
Zhang F: Effectiveness of immune therapy combined with chemotherapy
on the immune function and recurrence rate of cervical cancer. Exp
Ther Med. 9:1063–1067. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang ZX, Cao JX, Wang M, Li D, Cui YX,
Zhang XY, Liu JL and Li JL: Adoptive cellular immunotherapy for the
treatment of patients with breast cancer: A meta-analysis.
Cytotherapy. 16:934–945. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song G, Wang X, Jia J, Yuan Y, Wan F, Zhou
X, Yang H, Ren J, Gu J and Lyerly HK: Elevated level of peripheral
CD8(+)CD28(−) T lymphocytes are an independent predictor of
progression-free survival in patients with metastatic breast cancer
during the course of chemotherapy. Cancer Immunol Immunother.
62:1123–1130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhong R, Han B and Zhong H: A prospective
study of the efficacy of a combination of autologous dendritic
cells, cytokine-induced killer cells, and chemotherapy in advanced
non-small cell lung cancer patients. Tumour Biol. 35:987–994. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Topalian SL, Drake CG and Pardoll DM:
Immune checkpoint blockade: A common denominator approach to cancer
therapy. Cancer Cell. 27:450–461. 2015. View Article : Google Scholar : PubMed/NCBI
|