Introduction
Prostate cancer is a common malignancy among men in
Western countries; however, a sharp increase in its morbidity and
mortality was recently observed in several Asian countries,
including China (1). Patients with
aggressive pathological characteristics are at increased risk for
tumor progression and metastasis, even following radical treatment.
Furthermore, these characteristics are commonly found in tumors
from patients who succumb to prostate cancer. Cholesterol
homeostasis is crucial for cell function and survival, whereas
dysregulation of cholesterol homeostasis is known to be associated
with multiple cancers, including prostate cancer (2), and altered lipid metabolism is
increasingly recognized as a hallmark of prostate cancer cells
(3). In fact, lethal prostate
cancers exhibit higher expression of squalene monooxygenase, which
is the second rate-limiting enzyme of cholesterol synthesis
(4). Moreover, low serum cholesterol
levels have been found in prostate cancer patients, suggesting that
cholesterol may accumulate in tumor tissue. Compared with normal
prostate cells, prostate tumor cells exhibit increased levels of
intracellular cholesterol precursors, with loss of ABCA1-mediated
cholesterol efflux (5).
ATP-binding cassette (ABC) transporters are
transmembrane proteins responsible for the transfer of various
substrates through extracellular and intracellular membranes. The
ABC-type transporters act as gatekeepers by allowing or limiting
the entrance of a wide variety of substrates across cellular
membranes (6). A total of 51 ABC
transporter genes have been identified and are grouped into seven
subfamilies, namely A-G, based on their phylogenetic distance
(7). The physiological importance of
ABCA subfamily proteins is underscored by their association with
various inherited diseases. Examples of ABC A-subfamily disorders
include Tangier disease (ABCA1), Alzheimer's disease (ABCA2/ABCA7),
Stargardt's disease (ABCR/ABCA4), harlequin-type ichthyosis
(ABCA12) and others (8) (Table I). ABCA1, an ABC subfamily A
exporter, mediates the cellular efflux of phospholipids and
cholesterol to the extracellular acceptor apolipoprotein A-I
(apoA-I) for generation of nascent high-density lipoprotein (HDL)
(9). The human ABCA1 has a length of
2,261 amino acids, and was found to contain two transmembrane
domains (TMDs) (10). Each TMD is
followed by a cytoplasmic region comprising one nucleotide-binding
domain (NBD) and one small regulatory domain. The NBDs and
regulatory domains are the most highly conserved elements among the
ABCA subfamily. The structure of ABCA1 reveals a polar cluster on
one side of TMD1 close to the intracellular boundary (11). The structure of the ABCA1 presented
herein represents a major step towards the mechanistic
understanding of ABCA1-mediated lipid export and nascent HDL
biogenesis (12). ABCA1 has been
shown to be necessary for the synthesis of HDL by exporting
cholesterol out of the cells (13).
Functional ABCA1 mediates the expansion of the N-terminus of apoA-I
on the cell surface, followed by the release of nascent HDL, which
is the only path for elimination of cholesterol from the body
(14). Two missense mutations of
human ABCA1 proteins linked to Tangier disease and familial HDL
deficiency were previously found to be associated with diminished
cholesterol efflux activity, and the functionality of these ABCA1
mutants in terms of tumor inhibition and cholesterol efflux was
tested in prostate cancer cells lacking endogenous ABCA1 expression
(15). Over 50 mutations in the
ABCA1 gene were recently identified. The human ABCA1 has been shown
to be closely associated with the development of various human
cancers, including prostate, colon and breast cancer (16–18).
Prostate cancer patients with higher serum low-density lipoprotein
(LDL) levels exhibited significantly shorter overall survival. Low
ABCA1 expression has also been associated with shorter survival in
patients with prostate cancer. This may be explained by the use of
lipid as an energy source during growth and metastasis of prostate
cancer cells. ABCA1 has been shown to play a critical role in the
synthesis of HDL particles by exporting cellular cholesterol
(19,20).
 | Table I.Structure and function of ABCA
subfamily proteins. |
Table I.
Structure and function of ABCA
subfamily proteins.
| Proteins | Structure | Function | (Refs.) |
|---|
| ABCA1 | Length, 2,261 amino
acids; contains two TMDs, and a polar cluster on one side of | Synthesis of HDL,
exporting cholesterol out of the cells, lipid efflux activity.
Mutation of the |
|
|
| TMD1 close to the
intracellular boundary. | ABCA1 gene may
induce Tangier disease and familial hypoalphalipoproteinemia. Tumor
suppressor function in cancer. |
|
| ABCA2 | Coding region 7.3
kb in size, codes for a 2,436-amino acid polypeptide, comprises 48
exons. | Highly expressed in
brain tissue; may play a role in macrophage lipid metabolism and
neural development. | (34) |
| ABCA3 | Consists of 33
exons encoding a 1,704-amino acid (~150 kDa) protein. | Mutation of the
ABCA3 gene is the most common cause of surfactant deficiency and is
associated with cataract-microcornea syndrome. | (35) |
| ABCA4 | Transcribes a large
retina-specific protein with two TMDs, two glycosylated ECDs and
two NBDs. | An inward-directed
retinoid flippase; mutation of ABCA4 induces Stargardt's
disease. |
|
| ABCA5 | Consists of 1,642
amino acid residues, with two sets of six transmembrane segments
and an NBD. | Mutations in ABCA5
cause hair overgrowth and may be associated with a lysosomal
disease, particularly in cardiomyocytes and follicular cells. | (36) |
| ABCA7 | Spans a region of
~32 kb and comprises 46 exons. | Predisposes to
Alzheimer's disease. | (37) |
| ABAC9 | Consists of 39
exons, genomic region of ~85 kb chromosome 17q24.2. | Involved in
monocyte differentiation and macrophage lipid homeostasis. | (38) |
| ABCA10 | The coding sequence
of ABCA10 is 4.6 kb in size and codes for a 1,543-amino acid
protein. | Involved in
macrophage lipid homeostasis. |
|
| ABCA12 | Located on the long
(q) arm of chromosome 2 between positions 34 and 35. | Active in some
types of skin cells, linked to harlequin-type ichthyosis. |
|
| ABCA13 | Coding region is
6.7 kb in size and encodes a protein consisting of 2,143 amino
acids with a predicted molecular weight of 240 kDa. | Linked to
psychiatric disorders such as schizophrenia, bipolar disorder and
depression. | (39) |
Considering these findings, further investigation
into ABCA1 as a tumor suppressor in prostate cancer is required. We
herein review the role of ABCA1 in prostate cancer and the ongoing
research on its association with cancer proliferation, invasion and
migration, apoptosis and multidrug resistance. The aim of the
present review was to demonstrate that ABCA1 represents a promising
new target in the treatment of prostate cancer.
Effect of ABCA1 on the progression of
prostate cancer
The ABCA1 protein mediates cellular cholesterol
transfer to apoA-I through the plasma membrane. ABCA1 has been
found to play a crucial role in the etiology of various
neurological and cardiovascular diseases, including inflammation
and metabolic syndrome (21), and
may also be involved in the initiation and development of prostate
cancer. A causal association between ABCA1-mediated tumor
inhibition and mitochondrial cholesterol depletion has been
identified through testing the dependence of ABCA1 anticancer
activity on its efflux capacity (22). In addition, transforming growth
factor (TGF)-β signaling plays a key role in prostate cancer
occurrence and maintenance of cancer stem cell characteristics. The
growth inhibitory effect of TGF-β may be due to overexpression of
ABCA1 in prostate cancer cells (23). The expression of ABCA1 is associated
with nuclear receptor of liver X receptor (LXR) and LXR forms a
heterodimer with retinoid X receptor and regulates transcription of
ABCA1 by binding to the DR-4 promoter element (24). Furthermore, TGF-β may increase ABCA1
expression through activation of LXR signaling. It has been
demonstrated that LXR activation, with accompanying upregulation of
ABCA1 expression, may decrease cholesterol levels and reduce the
growth of prostate cancer cell xenograft tumors in mice (25). Furthermore, LXR agonists have been
shown to induce expression of ABCA1, inhibit tumor growth and
reduce progression to androgen independence in a xenograft model of
prostate cancer (26). The antitumor
effects of these compounds may involve a variety of mechanisms:
Statins inhibit GTPases such as Ras and Rho family proteins via
blocking protein prenylation and/or farnesylation, and LXR agonists
induce cell cycle arrest through upregulation of p27. Conversely,
intracellular cholesterol promotes prostate cancer progression as a
substrate for de novo androgen synthesis and through
regulation of Akt signaling (22).
Hypermethylation of the ABCA1 promoter has been
shown to silence ABCA1 expression and is associated with high-grade
prostate cancer (27). The
interaction of apoA-I with ABCA1 activates the signaling molecule
Janus kinase 2 (JAK2), which optimizes the cholesterol efflux
activity of ABCA1 by phosphorylation (28). ABCA1-mediated JAK2 activation also
activates signal transducer and activator of transcription 3
(STAT3), which significantly attenuates the expression of
proinflammatory cytokines in macrophages. The anti-inflammatory
effects of the apoA-I/ABCA1/STAT3 pathway, however, is dependent on
the activity of suppressor of cytokine signaling 3. Taken together,
these findings suggest that the interaction of apoA-I/ABCA1
activates cholesterol efflux and STAT3 branching pathways to
synergistically inhibit inflammation in macrophages (29). It was also demonstrated that the
apoA-I/ABCA1 interaction with the JAK2/STAT3 pathway to inhibit
macrophage inflammatory responses is independent of ABCA1 lipid
transport function. ABCA1 mutation carriers also exhibit an
increased incidence of systemic and plaque inflammation (30). It has been observed that aberrant
regulation of tristetraprolin and human antigen R plays an
important role in the progression of prostate cancer and other
inflammation-related cancers, as well as in cancer cell
proliferation, apoptosis, angiogenesis, invasion and
chemoresistance (31).
Epidemiological data have associated a reduced risk
of prostate cancer with low serum cholesterol, as well as with the
use of statins, demonstrating the role of cholesterol metabolism in
the development of aggressive prostate cancer (32). The efflux of intracellular
cholesterol is mediated by reverse cholesterol transporters, such
as scavenger receptor class B type I, a membrane receptor with
extensive tissue distribution and a number of physiological
functions (33). A reduction in
Akt-dependent survival signaling may also contribute to the
anticancer activity of ABCA1. In particular, ~70% of advanced PCa
exhibits loss of phosphatase and tensin homolog (PTEN) or
consequent activation of the phosphoinositide 3-kinase (PI3K)/Akt
pathway, which leads to enhanced cell survival, migration and
castration-resistant growth (40).
Intracellular cholesterol promotes prostate cancer
progression, as it is a substrate for de novo androgen
synthesis, and by regulation of Akt signaling. Akt increased the
expression of ABCA1 by delaying its degradation, but does not alter
the mRNA levels (41). The
protective effect of the ABCA1 protein is mediated by Akt2,
possibly by stabilizing ABCA1 resistance to calpain-mediated
proteolysis. ABCA1 reduces LDL receptor expression, decreases
intracellular cholesterol and relies on LXRα. Of note, the
epidermal growth factor receptor (EGFR) signal opposes the effects
of LXRα on cholesterol homeostasis, whereas EGFR inhibitors
interact with LXRα agonists to destroy cancer cells. Inhibition of
activation of LXRα by sterol metabolites may be an effective
strategy for targeting EGFR-KRAS signaling against cancer (42).
The promoter region of the ABCA1 gene has been shown
to be epigenetically silenced by hypermethylation in the LNCaP
human prostate cancer cell line. This suggests that loss of ABCA1
expression may lead to a significant alteration in cholesterol
homeostasis and may indicate a fundamental association between high
circulating cholesterol levels and prostate cancer growth (43). Epidemiological studies have
identified a correlation between high serum cholesterol levels and
prostate cancer, as well as a protective effect of statin use
(44).
In conclusion, hypermethylation and gene
inactivation of ABCA1 promoters leads to accumulation of
cholesterol in prostate cancer cells. Thus, this cellular
cholesterol efflux pathway may be an important determinant of
prostate cancer aggressiveness, as well as a potential therapeutic
target.
Association of ABCA1 with apoptosis and
autophagy in prostate cancer
Overexpression of ABCA1 leads to decreased cellular
cholesterol and tumor growth inhibition in prostate cancer cells.
ABCA1 antitumor activity requires an efflux function and appears to
be mediated by reduced mitochondrial cholesterol combined with an
increased potential for release of cell death-promoting molecules,
such as cytochrome C, from mitochondria. The loss-of-function
phenotype for somatic mutations identified in human colon cancer
suggests the role of ABCA1 as a tumor suppressor, albeit in the
context of increased cholesterol synthesis sufficient to raise the
levels of mitochondrial cholesterol. This antitumor effect may be
due to apoptosis, as overexpression of ABCA1 has been shown to
increase mitochondrial release of cytochrome C in vitro and
in vivo (45).
Furthermore, downregulation of ABCA1 is present in
most prostate cancer specimens, and ABCA1 expression levels are
inversely associated with Gleason score. DNA hypermethylation on
the ABCA1 promoter in LNCaP cells effectively inhibits basal
expression and prevents the complete induction of trans-activators.
The loss of ABCA1 protein expression directly results in high
intracellular cholesterol levels, thereby contributing to an
environment conducive to tumor progression. In addition, ABCA1
hypermethylation was exclusively detected in intermediate- and
high-grade prostate cancer. Thus, epigenetic inactivation of ABCA1
may be intimately associated with prostate cancer progression,
suggesting that dysregulation of cellular cholesterol levels in
prostate epithelial cells creates an environment conducive to tumor
progression.
Autophagy is an intracellular protein-degradation
process that is conserved across eukaryotes, including yeast and
humans, which responds to cellular stress conditions to maintain a
healthy cellular status by degrading and recycling cytoplasmic
contents via the lysosomal route (46). Under nutrient starvation conditions,
intracellular proteins are transported to lysosomes and vacuoles
via membranous structures known as autophagosomes, and are
degraded. It has been demonstrated that autophagy participates in
the regulation of lipid metabolism and cholesterol homeostasis,
with a special emphasis on macrophage-derived foam cells. Autophagy
has emerged as an alternative lipid metabolic pathway through the
lysosomal degradative pathway, making this process a potential
therapeutic target for diseases associated with disorders of lipid
metabolism (47). Of note, all these
lipoproteins increase autophagic flux in macrophages, and autophagy
depletion in these cells results in diminished cholesterol efflux
to apoA-I. The autophagy inhibitor 3-methyladenine and an ATG5
siRNA were found to significantly attenuate autophagy, subsequently
suppressing the ABCA1-mediated cholesterol efflux (48). Biochemical studies have demonstrated
that the accumulation of cholesterol esters in prostate cancer
cells is the result of loss of the tumor suppressor PTEN and
activation of the PI3K/Akt pathway. AMP-activated protein kinase
(AMPK)-dependent ABCA1 expression also involves the mammalian
target of rapamycin (mTOR) pathway and inhibits the
extracellular-signal regulated kinase (ERK). AMPK-induced ABCA1
expression leads to cholesterol efflux from human macrophages
(49). Through the ERK1/2 pathway,
intracellular ABCA1 exerts a protective effect against oxidative
stress caused by ischemia. The PI3K/Akt/mTOR signaling pathway is a
classic autophagy pathway. PI3K and Akt suppression may inhibit
mTOR phosphorylation at the Ser2448 site, thereby
enhancing the expression of autophagy-related proteins and inducing
autophagy (50).
It has been demonstrated that elevated mitochondrial
cholesterol promotes resistance to cell death in prostate cancer
and may lead to malignant cell transformation (51). Inhibitory RNA (RNAi) knockdown of
squalene synthase, the enzyme which catalyzes the first committed
step of cholesterol synthesis, leads to decreased cell
proliferation and survival of prostate cancer cells in vitro
(52). ABCA1 promotes a reduction in
mitochondrial cholesterol levels, mitochondrial permeability
transition-associated cytochrome C release, and
non-apoptotic/necrotic cell death in response to cell stress.
Ultimately, ABCA1-mediated cell death depends on cholesterol for
modulation of mitochondrial function, while ABCA1 deficiency allows
for elevated mitochondrial cholesterol and ultimately promotes
cancer cell survival.
ABCA1 and multidrug resistance (MDR)
MDR is a major cause of failure of prostate cancer
chemotherapy and is associated with an increased mortality risk
(53). The PI3K/Akt signaling
cascade may be correlated with MDR1/P-glycoprotein (P-gp) and plays
a critical role in the MDR phenotype. MDR-associated protein (MRP)
1 is expressed in a variety of human malignancies (54). ABCA1 may export anticancer drugs and
decrease intracellular drug concentration, which is associated with
limited success of anticancer chemotherapy, attributed to the the
cross-resistance of tumor cells and referred to as MDR. The role of
ABCA1 in MDR has been well-characterized, and measuring the
expression of ABCA1 may be used for predicting the response to
anticancer drugs.
Cell viability in prostate cancer may be regulated
by modifying critical pathways mediating novel androgen signaling
by AR-targeting microRNAs. In this study, androgens stimulating
miR-19a, and miR-19a directly repressing ABCA1 mRNA expression, may
represent a possible mechanism underlying androgen-mediated
repression of ABCA1, promoting PCa cell proliferation (55). The activation of ABCA1 was also shown
to inhibit the proliferation of androgen-dependent prostate cancer
cells, and androgen treatment in LNCaP cells significantly
inhibited the expression of ABCA1 mRNA (56). In vitro studies demonstrated
that LXR agonists activate transcription of the MRP2 gene to
promote excretion of endogenous and xenobiotic compounds from
hepatocytes into the bile (57).
ABCA1 also transfers phospholipids, preferentially
phosphatidylcholine and cholesterol, to lipid-free apoA-I to
produce pre-β-HDL, which also binds directly to MDR1 and regulates
substrate recognition via MDR1 (58). Eukaryotic ABC proteins may retain
similar substrate binding pockets and move substrates in an
ATP-dependent manner. The prototype of eukaryote ABC proteins may
be those involved in membrane lipid transport.
If overexpression of ABCA1 promotes MDR in tumors,
ABCA1 gene mutations may lead to drug resistance and alter
substrate specificity. Thus, the study of ABCA1 gene mutations and
their role in MDR is crucial for individualized patient
treatment.
In conclusion, ABCA1 plays an important role in the
prevention and treatment of prostate cancer through regulation of
cellular cholesterol efflux (Fig.
1). The association of lower ABCA1 expression with shorter
survival in prostate cancer prompts ongoing research to further
elucidate the mechanism underlying ABCA1 regulation of tumor
growth. Various cross-regulatory mechanisms may be the focus of
future research. Studies on ABCA1 will contribute to the
understanding of drug transport and the role of cholesterol in
cancer growth, and may lead to optimization of treatment for
prostate and other cancers. Additionally, the study of ABCA1 gene
mutations and associated differences in expression and
functionality may lay the foundation for future pharmacogenetics in
prostate cancer treatment. Ultimately, studies in ABCA1 may provide
a novel therapeutic target in cancer.
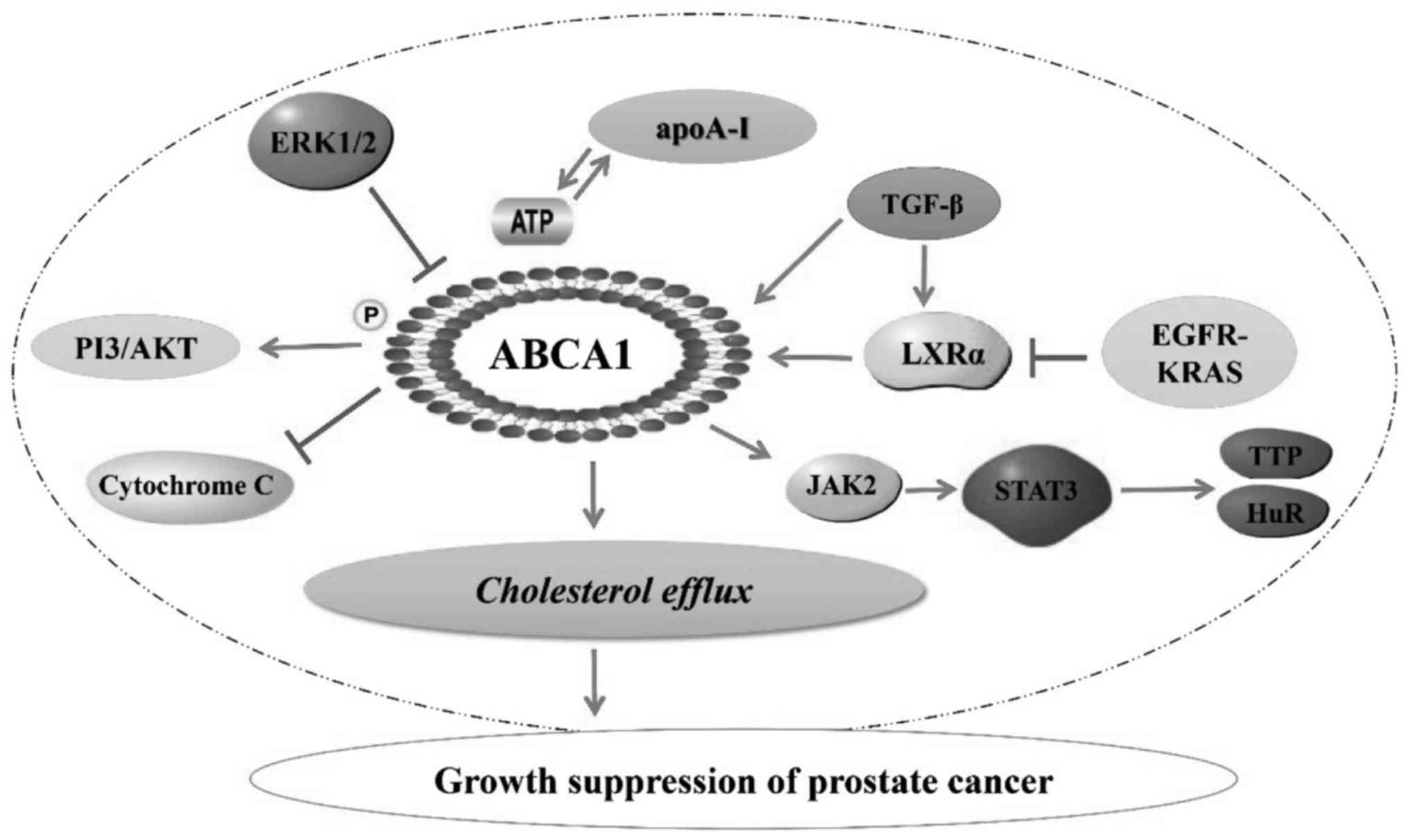 | Figure 1.Association of ABCA1 with prostate
cancer. ABCA1, ATP-binding cassette transporter A1; PI3,
phosphoinositide 3 kinase; ERK, extracellular signal-regulated
kinase; apoA-I, apolipoprotein A-I; TGF, transforming growth
factor; LXR, liver X receptor; EGFR, epidermal growth factor
receptor; JAK2, Janus kinase 2; STAT3, signal transducer and
activator of transcription 3; TTP, tristetraprolin; HuR, human
antigen R. |
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant no. 81541163); the Natural
Science Foundation of Hunan Province (grant no. 2015JJ3101); the
Open Fund Based on Innovation Platform of Hunan Provincial
Education Department (grant no. 15K111); the Hunan Provincial
Cooperative Innovation Center for Molecular Target New Drug Study
(grant no. 2014-405); and the Innovation Program of Graduate
Education in Cooperative Innovation Center for Molecular Target New
Drug of University of South China (grant no. 0223-0002-00028).
Glossary
Abbreviations
Abbreviations:
|
ABC
|
ATP-binding cassette
|
|
LDL
|
low-density lipoprotein
|
|
apoA-I
|
apolipoprotein A-I
|
|
TGF-β
|
transforming growth factor-β
|
|
JAK2
|
Janus kinase 2
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
HDL
|
high-density lipoprotein
|
|
LXR
|
liver X receptor
|
|
MDR
|
multidrug resistance
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
P-gp
|
P-glycoprotein
|
|
MRP
|
multidrug resistance-associated
protein
|
|
EGFR
|
epidermal growth factor receptor
|
References
|
1
|
Zong Y, Goldstein AS and Huang J: The
molecular basis for ethnic variation and histological subtype
differences in prostate cancer. Sci China Life Sci. 56:780–787.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adlakha YK, Khanna S, Singh R, Singh VP,
Agrawal A and Saini N: Pro-apoptotic miRNA-128-2 modulates ABCA1,
ABCG1 and RXRα expression and cholesterol homeostasis. Cell Death
Dis. 4:e7802013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yue S, Li J, Lee SY, Lee HJ, Shao T, Song
B, Cheng L, Masterson TA, Liu X, Ratliff TL and Cheng JX:
Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT
activation underlies human prostate cancer aggressiveness. Cell
Metab. 19:393–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stopsack KH, Gerke TA, Andrén O, Andersson
SO, Giovannucci EL, Mucci LA and Rider JR: Cholesterol uptake and
regulation in high-grade and lethal prostate cancers.
Carcinogenesis. 38:806–811. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cruz PM, Mo H, McConathy WJ, Sabnis N and
Lacko AG: The role of cholesterol metabolism and cholesterol
transport in carcinogenesis: A review of scientific findings,
relevant to future cancer therapeutics. Front Pharmacol. 4:1192013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flores K, Manautou JE and Renfro JL:
Gender-specific expression of ATP-binding cassette (Abc)
transporters and cytoprotective genes in mouse choroid plexus.
Toxicology. 386:84–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Durmus S, Hendrikx JJ and Schinkel AH:
Apical ABC transporters and cancer chemotherapeutic drug
disposition. Adv Cancer Res. 125:1–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaminski WE, Piehler A and Wenzel JJ: ABC
A-subfamily transporters: Structure, function and disease. Biochim
Biophys Acta. 1762:510–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qian H, Zhao X, Cao P, Lei J, Yan N and
Gong X: Structure of the human lipid exporter ABCA1. Cell.
169:1228–1239.e10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fitzgerald ML, Mendez AJ, Moore KJ,
Andersson LP, Panjeton HA and Freeman MW: ATP-binding cassette
transporter A1 contains an NH2-terminal signal anchor sequence that
translocates the protein's first hydrophilic domain to the
exoplasmic space. J Biol Chem. 276:15137–15145. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lamping E, Baret PV, Holmes AR, Monk BC,
Goffeau A and Cannon RD: Fungal PDR transporters: Phylogeny,
topology, motifs and function. Fungal Genet Biol. 47:127–142. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lund-Katz S and Phillips MC: High density
lipoprotein structure-function and role in reverse cholesterol
transport. Subcell Biochem. 51:183–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sene A, Khan AA, Cox D, Nakamura RE,
Santeford A, Kim BM, Sidhu R, Onken MD, Harbour JW, Hagbi-Levi S,
et al: Impaired cholesterol efflux in senescent macrophages
promotes age-related macular degeneration. Cell Metab. 17:549–561.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang S and Smith JD: ABCA1 and nascent HDL
biogenesis. Biofactors. 40:547–554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murano T, Yamaguchi T, Tatsuno I, Suzuki
M, Noike H, Takanami T, Yoshida T, Suzuki M, Hashimoto R, Maeno T,
et al: Subfraction analysis of circulating lipoproteins in a
patient with Tangier disease due to a novel ABCA1 mutation. Clin
Chim Acta. 452:167–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bi DP, Yin CH, Zhang XY, Yang NN and Xu
JY: MiR-183 functions as an oncogene by targeting ABCA1 in colon
cancer. Oncol Rep. 35:2873–2879. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sharma M, Tuaine J, McLaren B, Waters DL,
Black K, Jones LM and McCormick SP: Chemotherapy agents alter
plasma lipids in breast cancer patients and show differential
effects on lipid metabolism genes in liver cells. PLoS One.
11:e01480492016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaneko T, Kanno C, Ichikawa-Tomikawa N,
Kashiwagi K, Yaginuma N, Ohkoshi C, Tanaka M, Sugino T, Imura T,
Hasegawa H and Chiba H: Liver X receptor reduces proliferation of
human oral cancer cells by promoting cholesterol efflux via
up-regulation of ABCA1 expression. Oncotarget. 6:33345–33357. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sviridov DO, Drake SK, Freeman LA and
Remaley AT: Amphipathic polyproline peptides stimulate cholesterol
efflux by the ABCA1 transporter. Biochem Biophys Res Commun.
471:560–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang M, Li L, Xie W, Wu JF, Yao F, Tan
YL, Xia XD, Liu XY, Liu D, Lan G, et al: Apolipoprotein A-1 binding
protein promotes macrophage cholesterol efflux by facilitating
apolipoprotein A-1 binding to ABCA1 and preventing ABCA1
degradation. Atherosclerosis. 248:149–159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sultana A, Cochran BJ, Tabet F, Patel M,
Torres LC, Barter PJ and Rye KA: Inhibition of inflammatory
signaling pathways in 3T3-L1 adipocytes by apolipoprotein A-I.
FASEB J. 30:2324–2335. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smith B and Land H: Anticancer activity of
the cholesterol exporter ABCA1 gene. Cell Rep. 2:580–590. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Her NG, Jeong SI, Cho K, Ha TK, Han J, Ko
KP, Park SK, Lee JH, Lee MG, Ryu BK and Chi SG: PPARδ promotes
oncogenic redirection of TGF-β1 signaling through the activation of
the ABCA1-Cav1 pathway. Cell Cycle. 12:1521–1535. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tazoe F, Yagyu H, Okazaki H, Igarashi M,
Eto K, Nagashima S, Inaba T, Shimano H, Osuga J and Ishibashi S:
Induction of ABCA1 by overexpression of hormone-sensitive lipase in
macrophages. Biochem Biophys Res Commun. 376:111–115. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dufour J, Viennois E, De Boussac H, Baron
S and Lobaccaro JM: Oxysterol receptors, AKT and prostate cancer.
Curr Opin Pharmacol. 12:724–728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chuu CP, Hiipakka RA, Kokontis JM, Fukuchi
J, Chen RY and Liao S: Inhibition of tumor growth and progression
of LNCaP prostate cancer cells in athymic mice by androgen and
liver X receptor agonist. Cancer Res. 66:6482–6486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee BH, Taylor MG, Robinet P, Smith JD,
Schweitzer J, Sehayek E, Falzarano SM, Magi-Galluzzi C, Klein EA
and Ting AH: Dysregulation of cholesterol homeostasis in human
prostate cancer through loss of ABCA1. Cancer Res. 73:1211–1218.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao GJ, Yin K, Fu YC and Tang CK: The
interaction of ApoA-I and ABCA1 triggers signal transduction
pathways to mediate efflux of cellular lipids. Mol Med. 18:149–158.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang C, Houston BA, Storey C and LeBoeuf
RC: Both STAT3 activation and cholesterol efflux contribute to the
anti-inflammatory effect of apoA-I/ABCA1 interaction in
macrophages. J Lipid Res. 57:848–857. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bochem AE, van der Valk FM, Tolani S,
Stroes ES, Westerterp M and Tall AR: Increased systemic and plaque
inflammation in ABCA1 mutation carriers with attenuation by
statins. Arterioscler Thromb Vasc Biol. 35:1663–1669. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ramírez CM, Lin CS, Abdelmohsen K, Goedeke
L, Yoon JH, Madrigal-Matute J, Martin-Ventura JL, Vo DT, Uren PJ,
Penalva LO, et al: RNA binding protein HuR regulates the expression
of ABCA1. J Lipid Res. 55:1066–1076. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ohno Y, Ohori M, Nakashima J, Okubo H,
Satake N, Hashimoto T and Tachibana M: Association between
preoperative serum total cholesterol level and biochemical
recurrence in prostate cancer patients who underwent radical
prostatectomy. Mol Clin Oncol. 4:1073–1077. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cardenas E and Ghosh R: Vitamin E: A dark
horse at the crossroad of cancer management. Biochem Pharmacol.
86:845–852. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaminski WE, Piehler A, Püllmann K,
Porsch-Ozcürümez M, Duong C, Bared GM, Büchler C and Schmitz G:
Complete coding sequence, promoter region, and genomic structure of
the human ABCA2 gene and evidence for sterol-dependent regulation
in macrophages. Biochem Biophys Res Commun. 281:249–258. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Paolini A, Baldassarre A, Del Gaudio I and
Masotti A: Structural features of the ATP-Binding Cassette (ABC)
transporter ABCA3. Int J Mol Sci. 16:19631–19644. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kubo Y, Sekiya S, Ohigashi M, Takenaka C,
Tamura K, Nada S, Nishi T, Yamamoto A and Yamaguchi A: ABCA5
resides in lysosomes, and ABCA5 knockout mice develop lysosomal
disease-like symptoms. Mol Cell Biol. 25:4138–4149. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Broccardo C, Osorio J, Luciani MF, Schriml
LM, Prades C, Shulenin S, Arnould I, Naudin L, Lafargue C, Rosier
M, et al: Comparative analysis of the promoter structure and
genomic organization of the human and mouse ABCA7 gene encoding a
novel ABCA transporter. Cytogenet Cell Genet. 92:264–270. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Piehler A, Kaminski WE, Wenzel JJ,
Langmann T and Schmitz G: Molecular structure of a novel
cholesterol-responsive A subclass ABC transporter, ABCA9. Biochem
Biophys Res Commun. 295:408–416. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Maeß MB, Stolle K, Cullen P and Lorkowski
S: Evidence for an alternative genomic structure, mRNA and protein
sequence of human ABCA13. Gene. 515:298–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Marques RB, Aghai A, de Ridder CMA,
Stuurman D, Hoeben S, Boer A, Ellston RP, Barry ST, Davies BR,
Trapman J and van Weerden WM: High efficacy of combination therapy
Using PI3K/AKT inhibitors with androgen deprivation in prostate
cancer preclinical models. Eur Urol. 67:1177–1185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Okoro EU, Guo Z and Yang H: Akt
isoform-dependent regulation of ATP-Binding cassette A1 expression
by apolipoprotein E. Biochem Biophys Res Commun. 477:123–128. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gabitova L, Restifo D, Gorin A, Manocha K,
Handorf E, Yang DH, Cai KQ, Klein-Szanto AJ, Cunningham D, Kratz
LE, et al: Endogenous sterol metabolites regulate growth of
EGFR/KRAS-dependent tumors via LXR. Cell Rep. 12:1927–1938. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Solomon KR, Allott EH, Freeman MR and
Freedland SJ: Words of wisdom. Re: Dysregulation of cholesterol
homeostasis in human prostate cancer through loss of ABCA1. Eur
Urol. 63:1128–1129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hayashi N, Matsushima M, Yamamoto T,
Sasaki H, Takahashi H and Egawa S: The impact of
hypertriglyceridemia on prostate cancer development in patients
aged ≥60 years. BJU Int. 109:515–519. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chou JL, Huang RL, Shay J, Chen LY, Lin
SJ, Yan PS, Chao WT, Lai YH, Lai YL, Chao TK, et al:
Hypermethylation of the TGF-β target, ABCA1 is associated with poor
prognosis in ovarian cancer patients. Clin Epigenetics. 7:12015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Annu Rev Genet.
43:67–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Puri P and Chandra A: Autophagy modulation
as a potential therapeutic target for liver diseases. J Clin Exp
Hepatol. 4:51–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Han XB, Li HX, Jiang YQ, Wang H, Li XS,
Kou JY, Zheng YH, Liu ZN, Li H, Li J, et al: Upconversion
nanoparticle-mediated photodynamic therapy induces autophagy and
cholesterol efflux of macrophage-derived foam cells via ROS
generation. Cell Death Dis. 8:e28642017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lin XL, Liu MH, Hu HJ, Feng HR, Fan XJ,
Zou WW, Pan YQ, Hu XM and Wang Z: Curcumin enhanced cholesterol
efflux by upregulating ABCA1 expression through AMPK-SIRT1-LXRα
signaling in THP-1 macrophage-derived foam cells. DNA Cell Biol.
34:561–572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jiang Y, Kou J, Han X, Li X, Zhong Z, Liu
Z, Zheng Y, Tian Y and Yang L: ROS-dependent activation of
autophagy through the PI3K/Akt/mTOR pathway is induced by
hydroxysafflor yellow A-sonodynamic therapy in THP-1 macrophages.
Oxid Med Cell Longev. 2017:85191692017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wu X, Daniels G, Lee P and Monaco ME:
Lipid metabolism in prostate cancer. Am J Clin Exp Urol. 2:111–120.
2014.PubMed/NCBI
|
|
52
|
Brusselmans K, Timmermans L, Van de Sande
T, Van Veldhoven PP, Guan G, Shechter I, Claessens F, Verhoeven G
and Swinnen JV: Squalene synthase, a determinant of Raft-associated
cholesterol and modulator of cancer cell proliferation. J Biol
Chem. 282:18777–18785. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang H, Jia XH, Chen JR, Yi YJ, Wang JY,
Li YJ and Xie SY: HOXB4 knockdown reverses multidrug resistance of
human myelogenous leukemia K562/ADM cells by downregulating P-gp,
MRP1 and BCRP expression via PI3K/Akt signaling pathway. Int J
Oncol. 49:2529–2537. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen JR, Jia XH, Wang H, Yi YJ, Wang JY
and Li YJ: Timosaponin A-III reverses multi-drug resistance in
human chronic myelogenous leukemia K562/ADM cells via
downregulation of MDR1 and MRP1 expression by inhibiting PI3K/Akt
signaling pathway. Int J Oncol. 48:2063–2070. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mo W, Zhang J, Li X, Meng D, Gao Y, Yang
S, Wan X, Zhou C, Guo F, Huang Y, et al: Identification of novel
AR-targeted microRNAs mediating androgen signalling through
critical pathways to regulate cell viability in prostate cancer.
PLoS One. 8:e565922013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sekine Y, Demosky SJ, Stonik JA, Furuya Y,
Koike H, Suzuki K and Remaley AT: High-density lipoprotein induces
proliferation and migration of human prostate androgen-independent
cancer cells by an ABCA1-dependent mechanism. Mol Cancer Res.
8:1284–1294. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chisaki I, Kobayashi M, Itagaki S, Hirano
T and Iseki K: Liver X receptor regulates expression of MRP2 but
not that of MDR1 and BCRP in the liver. Biochim Biophys Acta.
1788:2396–2403. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kimura Y, Morita SY, Matsuo M and Ueda K:
Mechanism of multidrug recognition by MDR1/ABCB1. Cancer Sci.
98:1303–1310. 2007. View Article : Google Scholar : PubMed/NCBI
|















