Introduction
In Indian women, cervical cancer is among the most
common malignancies, and is a major cause of mortality (1–3). India
has a higher incidence of cervical cancer compared with the
developed countries (1). The
age-adjusted incidence rate of cervical cancer varies widely among
Indian registries, from 4.91 to 23.07/100,000 populations; much
higher than the world (7.9/100,000 population). Poor to moderate
living standards, lack of proper screening, and a high (≥10% in
women aged >30 years) prevalence of human papillomavirus
infection could be the potential reasons responsible for the higher
incidence in India (4).
The treatment of choice for early-stage cervical
cancer is radical surgery, whereas, concurrent chemoradiation is
preferred for locally advanced stages. However, the treatment
options are very limited for patients with recurrent or metastatic
cancers. In women with recurrent or limited metastatic disease,
surgery or radiotherapy may be effective in only in few cases
(5,6). After the clinical alert from the
National Cancer Institute (NCI) in 1999 (7), concurrent chemoradiotherapy has become
the standard of treatment for locally advanced cervical cancer
(8); it enhances the
radiosensitivity and local tumor efficacy of cytotoxic agents, with
eradication of micrometastasis and ultimately improves the pelvic
control and survival (9).
Chemotherapy with a platinum agent
(carboplatin/cisplatin) and a taxane based regimen is considered
the standard of care for cervical cancer in terms of longer overall
survival (OS) and a better quality of life for the patients
(5,7). Furthermore, carboplatin has advantages
over cisplatin in terms of a better safety profile (low incidence
of neuropathy, nephrotoxicity and emetogenesis) (10).
Docetaxel has dose limiting toxicities of acute
hypersensitivity reactions, and fluid retention due to the
excipients (polysorbate-80 and ethanol) used in the conventional
formulations, which require corticosteroid premedication (11). DoceAqualip, a nanosomal docetaxel
lipid suspension (NDLS), was developed with lipids generally
recognized as safe (GRAS) by the US Food and Drug Administration
(USFDA) and is devoid of polysorbate-80 and ethanol. DoceAqualip
has been shown to be more effective than the conventional docetaxel
in the treatment of breast cancer (12) and has demonstrated a promising
overall response and safety without the need of corticosteroid in
the treatment of breast cancer, advanced gastric adenocarcinoma,
ovarian cancer, hormone refractory prostate cancer, and non-small
cell lung cancer (11–13).
Here, we report a case of a Stage IIIB cervical
cancer patient who was treated with concurrent carboplatin and
DoceAqualip chemoradiotherapy.
Case report
A 52-year-old woman presented with complaints of
abnormal vaginal bleeding along with white discharge since five
months. Her vaginal examination revealed a hard indurated friable
growth replacing the cervix. Per rectal examination revealed
involvement of bilateral parametrium.
Her past medical history included type 2 diabetes
and she was taking combination tablet of glimepiride 1 mg and
metformin 500 mg twice daily for 3 years. Histopathological
examination of the cervical biopsy specimen revealed moderately
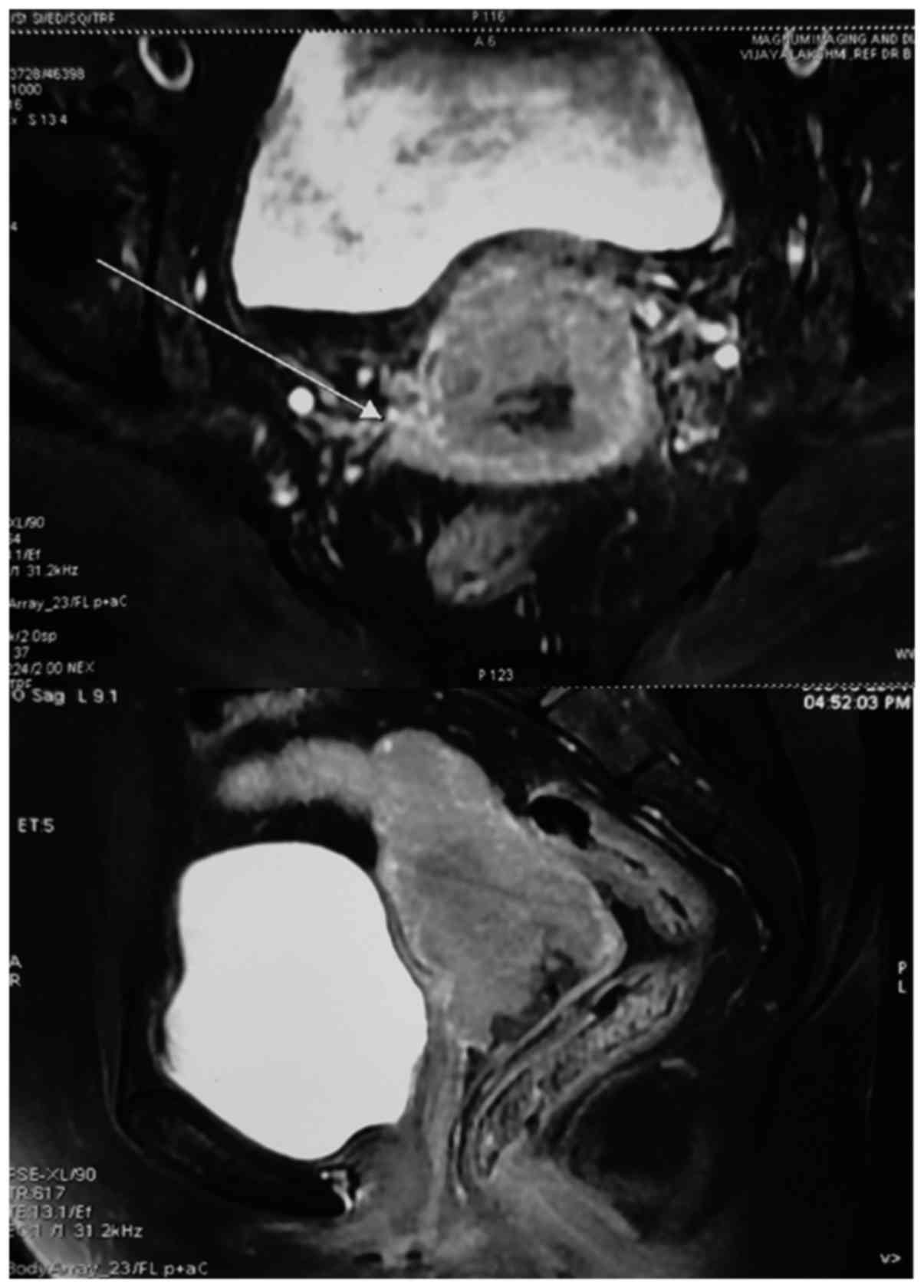
differentiated squamous cell carcinoma. The magnetic resonance
imaging (MRI) of pelvis revealed a large mass of 55×45 mm in size
in the uterine cervix infiltrating the lower third of the
myometrium with enlarged bilateral internal iliac nodes, measuring
1.6 cm on the left side and 1.4 cm on the right side (Fig. 1). Based on these findings, the
patient was diagnosed with squamous cell carcinoma cervix Stage
IIIB, with Eastern Cooperation Oncology Group (ECOG) score of 2 and
was planned for concurrent chemoradiotherapy.
The patient was started on chemotherapy with
docetaxel-carboplatin regimen on December 26, 2014. Carboplatin and
nanosomal docetaxel lipid suspension (NDLS; DoceAqualip) were
administered intravenously (IV) at doses corresponding to AUC 5–6
(area under the curve; total dose 450 mg) and 75 mg/m2
(body surface area of 1.67 kg/m2), respectively for 4
cycles (every 3 weekly cycle). DoceAqualip was given without
steroid premedication, at a total dose of 120 mg during the first 2
cycles and the dose was reduced to 100 mg for cycles 3 and 4 as the
patient developed Grade II neutropenia after 2 cycles of
radiotherapy. Post completion of 2 cycles of DoceAqualip, pegylated
filgrastim (6 mg) was administered as secondary prophylaxis 24 h
after DoceAqualip administration to avoid the risk of neutropenia.
The patient did not develop any hypersensitivity reactions (HSR) or
any other serious adverse event despite not using any
corticosteroids as premedication. No abnormal laboratory
investigations were reported throughout the course of the
treatment.
The patient also received concomitant radiotherapy
from December 31, 2014 to March 10, 2015 at a dose of 50 Gy to the
pelvis and 6120 cGy to bilateral parametrium plus 2 high dose rate
intracavitary (HDR ICA)- 15 Gy to point A (paracervical reference
point) (6).
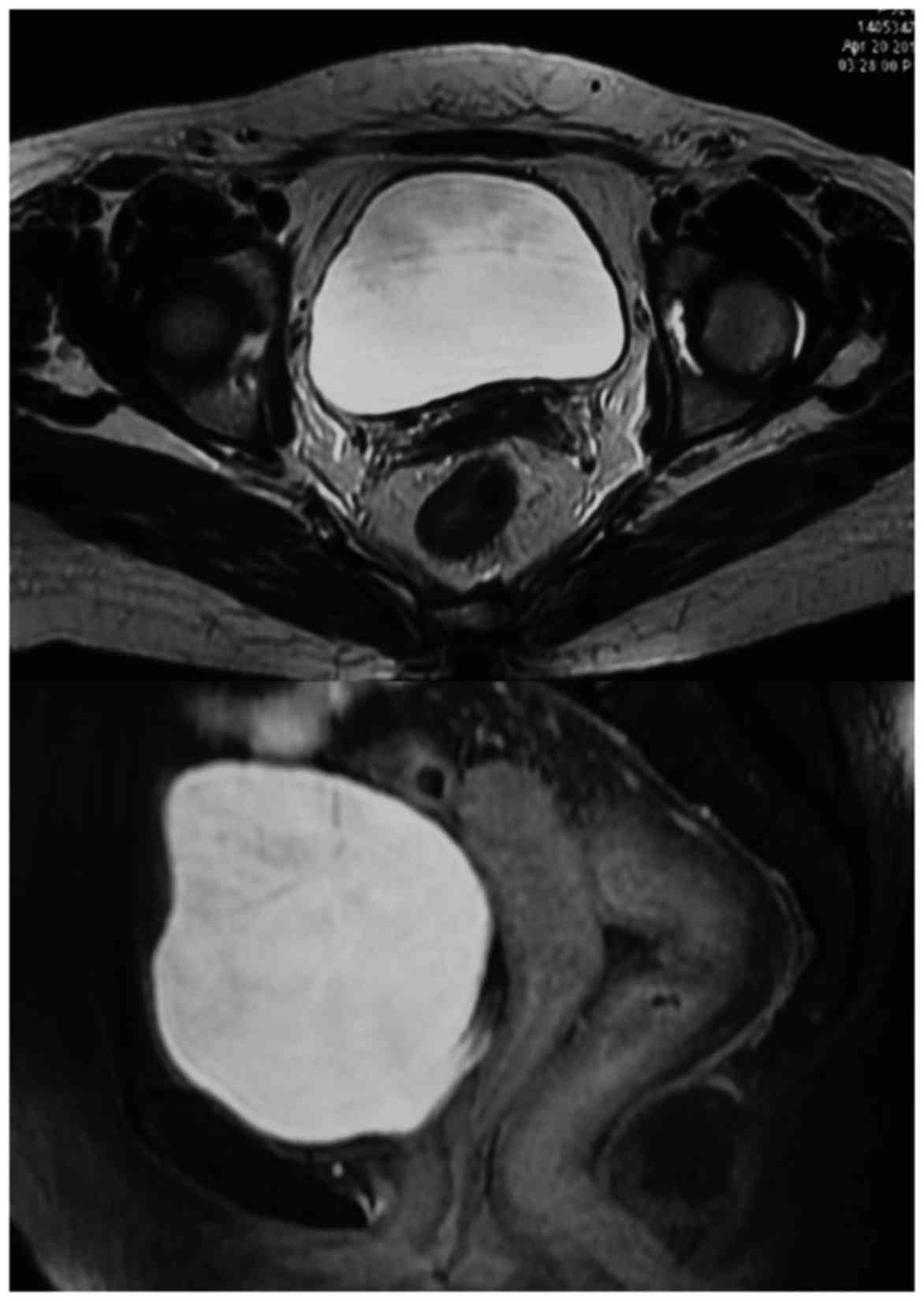
Post chemoradiotherapy, her MRI done on April 20,
2015 did not reveal any evidence of residual mass in the cervix and
no evidence of enlarged internal iliac or para aortic nodes
(Fig. 2). The response could best be
graded as a complete response (CR) as per RECIST 1.1 criteria. Her
fluorodeoxyglucose (FDG) positron emission tomography-computed
tomography (PET-CT) scan done on October 12, 2015 revealed no
active disease. The follow-up FDG PET CT whole body scan done on
June 5, 2017 did not show any evidence of metabolically active
lesion in the uterine cervix or anywhere else in the body.
Consent was obtained from the patient for the
publication of this case report and informed consent document was
signed by the patient.
Discussion
Cervical carcinoma is one of the leading
malignancies in Indian women with a higher burden as compared to
western countries. In India, it accounts for 16% of all cancers in
urban women and 37% of the cancers in rural women (3). Concurrent chemoradiation using
cisplatin has shown 30–50% reduction in the risk of death, and is
regarded the standard treatment for patients with cervical cancer
(7,14,15).
Carboplatin, an analogue of cisplatin, with a similar mechanism of
action, has been used for the treatment of locally advanced
cervical carcinoma. It has advantages over cisplatin in terms of
decreased nephrotoxicity, neurotoxicity and emetogenesis (16). This favourable toxicity profile when
compared to cisplatin may result in better patient adherence to the
treatment plan (15). A phase II
clinical study in patients with recurrent or metastatic squamous
carcinoma of the uterine cervix revealed substantially better
toxicity profile of carboplatin than cisplatin with similar
efficacy (17).
Taxanes are commonly used in the treatment of
locally advanced cervical cancer in combination with platinum
compounds (18). Docetaxel and
carboplatin concurrent chemotherapy has shown to be effective in
the treatment of cervical cancer with low incidence of
nephrointestinal, neurointestinal, and gastrointestinal toxicities
(8). In a phase I study in 20
patients with locally advanced or recurrent cervical cancer, weekly
docetaxel and carboplatin (145 cycles) showed a high efficacy in a
dose-dense setting. The study demonstrated an overall response rate
of 65%, and the treatment was well-tolerated (19).
Similarly, in a pilot study that assessed the
efficacy and safety of docetaxel and carboplatin combination
therapy in advanced or recurrent uterine cervix cancer in 17
patients yielded 76% overall response rate (CR in 2 patients,
partial response in 11, and stable disease in 4) with no disease
progression reported. Overall, it was concluded that combination
chemotherapy with docetaxel (60 mg/m2) and carboplatin
(AUC 6) was effective and well-tolerated in the treatment of
uterine cervix cancer. However, the incidence of Grade 3/4
neutropenia was very high (76%), and patients with Grade 4
neutropenia or febrile neutropenia required prophylactic
granulocyte colony stimulating factor (G-CSF, 5 µg/kg)
administration (20).
Takekida and colleagues further confirmed the
efficacy and safety of docetaxel and carboplatin regimen in the
treatment of cervical cancer. In a study of 66 patients (62
treatment naïve and 4 recurrent cancer), docetaxel (60
mg/m2) and carboplatin (AUC 6) combination therapy
yielded an overall clinical response rate of 63.7% (44/66, 95%
confidence interval, 52.1–75.3). Neutropenia was the major
haematological toxicity, which rapidly reversed with G-CSF use
(8).
Docetaxel is formulated in polysorbate-80 and
ethanol, which are known to cause infusion-related toxicities and
hypersensitivity reactions, requiring corticosteroid premedication.
Thus, a new formulation nanosomal docetaxel lipid suspension (NDLS)
was developed using GRAS lipids that is free of polysorbate-80 and
ethanol, and is found to be safe in earlier studies (11–13).
This is the first case report on the use of
DoceAqualip in the treatment of cervical cancer. In the current
report, the patient who had Stage IIIB cervical cancer, was treated
with DoceAqualip and carboplatin combination at generally
recommended doses (120 mg followed by 100 mg, and AUC 6,
respectively) along with radiation therapy, and achieved CR.
Overall, the treatment was well-tolerated, even when there was no
corticosteroid premedication, hypersensitivity reaction or any
other serious adverse event was not reported. Two-year follow-up
investigations did not reveal any recurrence of the carcinoma.
Ashraf et al, reported that patients receiving DoceAqualip
monotherapy did not require G-CSF support whereas dual combination
therapy with carboplatin required G-CSF support at each treatment
cycle (13). Furthermore, there is a
>20% risk of febrile neutropenia in patients receiving
combination chemotherapy (21). In
this case also, the patient developed Grade 2 neutropenia after 2nd
dose of radiotherapy; hence, DoceAqualip dose was reduced from 120
to 100 mg after cycle 2 to avoid radiotherapy interruption and
further dose delays. Also, after 2 cycles, the patient was given
prophylactic G-CSF (pegylated filgrastim) support after each cycle
of dual combination therapy with DoceAqualip and carboplatin.
Furthermore, it has been established that external-beam and
intracavitary radiation along with the concurrent chemotherapy
significantly improves survival in locally advanced cervical cancer
patients, which was also followed for this patient (6).
The current report highlights the efficacy and
safety of DoceAqualip in the treatment of advanced cervical cancer.
These results need to be further confirmed in a larger clinical
trial.
In conclusion, NDLS (DoceAqualip), a new formulation
developed to overcome toxicity and hypersensitivity reactions
caused by polysorbate-80 and ethanol content of the conventional
docetaxel formulations, has shown to be effective and
well-tolerated in the treatment of cervical cancer without the need
for corticosteroid premedication.
Acknowledgements
We would like to thank Mr. Shreekant Sharma (Lambda
Therapeutic Research) for his support in developing the
concept/medical writing, additional editorial support and follow-up
with the journal/publisher, and Dr Venugopal Madhusudhana (Lambda
Therapeutic Research) for additional editorial assistance. Dr
Deepak Bunger and Dr Mujtaba Khan are employees of Intas
Pharmaceuticals Ltd.
References
|
1
|
Asthana S, Chauhan S and Labani S: Breast
and cervical cancer risk in India: An update. Indian J Public
Health. 58:5–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harjani RR, Janaki MG, Somashekhar M,
Ponni A, Alva RC, Koushik K, Kannan RA and Sathyamurthy A:
Feasibility of Concurrent Chemoradiation in Cervical Cancer
Patients From Rural Background. Clin Ovarian Other Gynecol Cancer.
7:29–32. 2014. View Article : Google Scholar
|
|
3
|
Nandakumar A, Ramnath T and Chaturvedi M:
The magnitude of cancer cervix in India. Indian J Med Res.
130:219–221. 2009.PubMed/NCBI
|
|
4
|
Bobdey S, Sathwara J, Jain A and
Balasubramaniam G: Burden of cervical cancer and role of screening
in India. Indian J Med Paediatr Oncol. 37:278–285. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boussios S, Seraj E, Zarkavelis G,
Petrakis D, Kollas A, Kafantari A, Assi A, Tatsi K, Pavlidis N and
Pentheroudakis G: Management of patients with recurrent/advanced
cervical cancer beyond first line platinum regimens: Where do we
stand? A literature review. Crit Rev Oncol Hematol. 108:164–174.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morris M, Eifel PJ, Lu J, Grigsby PW,
Levenback C, Stevens RE, Rotman M, Gershenson DM and Mutch DG:
Pelvic radiation with concurrent chemotherapy compared with pelvic
and para-aortic radiation for high-risk cervical cancer. N Engl J
Med. 340:1137–1143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
National Cancer Institute, . NCI Issues
Clinical Announcement on Cervical Cancer: Chemotherapy Plus
Radiation Improves Survival. February 22;
|
|
8
|
Takekida S, Fujiwara K, Nagao S, Yamaguchi
S, Yoshida N, Kitada F, Kigawa J, Terakawa N, Nishimura R and Phase
II: Phase II study of combination chemotherapy with docetaxel and
carboplatin for locally advanced or recurrent cervical cancer. Int
J Gynecol Cancer. 20:1563–1568. 2010.PubMed/NCBI
|
|
9
|
Chen CC, Wang L, Lin JC and Jan JS: The
prognostic factors for locally advanced cervical cancer patients
treated by intensity-modulated radiation therapy with concurrent
chemotherapy. J Formos Med Assoc. 114:231–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Monk BJ, Alberts DS, Burger RA, Fanta PT,
Hallum AV III, Hatch KD and Salmon SE: In vitro phase II comparison
of the cytotoxicity of a novel platinum analog, nedaplatin (254-S),
with that of cisplatin and carboplatin against fresh, human
cervical cancers. Gynecol Oncol. 71:308–312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Naik R and Khan MA: Doceaqualip in a
patient with prostate cancer who had an allergic reaction to
conventional docetaxel: A case report. Mol Clin Oncol. 6:341–343.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahmad A, Sheikh S, Taran R, Srivastav SP,
Prasad K, Rajappa SJ, Kumar V, Gopichand M, Paithankar M, Sharma M,
et al: Therapeutic efficacy of a novel nanosomal docetaxel lipid
suspension compared with taxotere in locally advanced or metastatic
breast cancer patients. Clin Breast Cancer. 14:177–181. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ashraf M SR, Khan MA, Shah M, Bhat Y and
Wani ZA: Efficacy and safety of a novel nanosomal docetaxel lipid
suspension (NDLS) as an anti cancer agent a retrospective study.
Ann Oncol. 27(suppl_9): mdw579.008. 2016. View Article : Google Scholar
|
|
14
|
Kong TW, Chang S-J, Paek J, Yoo S-C, Yoon
J-H, Chang K-H, Chun M and Ryu H-S: Comparison of concurrent
chemoradiation therapy with weekly cisplatin versus monthly
fluorouracil plus cisplatin in FIGO stage IIB-IVA cervical cancer.
J Gynecol Oncol. 23:235–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nam EJ, Lee M, Yim GW, Kim JH, Kim S, Kim
SW, Kim JW and Kim YT: Comparison of carboplatin- and
cisplatin-based concurrent chemoradiotherapy in locally advanced
cervical cancer patients with morbidity risks. Oncologist.
18:843–849. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Adams M, Kerby IJ, Rocker I, Evans A,
Johansen K and Franks CR; The Swons Gynaecological Cancer Group, :
A comparison of the toxicity and efficacy of cisplatin and
carboplatin in advanced ovarian cancer. Acta Oncol. 28:57–60. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weiss GR, Green S, Hannigan EV, Boutselis
JG, Surwit EA, Wallace DL and Alberts DS: A phase II trial of
carboplatin for recurrent or metastatic squamous carcinoma of the
uterine cervix: A Southwest Oncology Group study. Gynecol Oncol.
39:332–336. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwab CL, English DP, Roque DM and Santin
AD: Taxanes: Their impact on gynecologic malignancy. Anticancer
Drugs. 25:522–535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rein DT, Kurbacher CM, Breidenbach M,
Schöndorf T, Schmidt T, König E, Göhring UJ, Blohmer JU and
Mallmann P: Weekly carboplatin and docetaxel for locally advanced
primary and recurrent cervical cancer: A phase I study. Gynecol
Oncol. 87:98–103. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagao S, Fujiwara K, Oda T, Ishikawa H,
Koike H, Tanaka H and Kohno I: Combination chemotherapy of
docetaxel and carboplatin in advanced or recurrent cervix cancer. A
pilot study. Gynecol Oncol. 96:805–809. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lyman GH, Dale DC, Legg JC, Abella E,
Morrow PK, Whittaker S and Crawford J: Assessing patients' risk of
febrile neutropenia: Is there a correlation between
physician-assessed risk and model-predicted risk? Cancer Med.
4:1153–1160. 2015. View
Article : Google Scholar : PubMed/NCBI
|