Introduction
Multidetector computed tomography (MDCT) (1–4) and
fluorodeoxyglucose-positron emission tomography (FDG-PET) (5,6) are used
to detect and diagnose pulmonary lesions. Early detection improves
prognosis, but the pulmonary lesion detection rates are
approximately 20% (1–4) and <3% (5,6) for
these two methods, respectively. CT-guided lung biopsy is a
well-established method for pulmonary lesion diagnosis (7–9). The
diagnostic accuracy of this method ranges from 64 to 97% (10–14). The
most common complication associated with CT-guided lung biopsy is
pneumothorax development, and the rate of this complication varies
widely (i.e., 8–69%) (10–16). Various investigators have analyzed
factors thought to affect the diagnostic accuracies and
complication rates for CT-guided lung biopsy (10–20).
Risk factors for the development of pulmonary lung
biopsy-associated pneumothorax include age, smoking history, lesion
volume, lesion position, depth from the pleura to the tumor, biopsy
needle angle, and needle thickness (10–20).
Operator experience is also a risk factor (17). If the patient develops CT-guided lung
biopsy-associated pneumothorax, it is difficult to correct the
condition using deaeration (21) or
drainage (13). It is, therefore,
necessary to decrease the rate of biopsy-associated
pneumothorax.
The aim of the present study was to evaluate risk
factors, including biopsy needle angle, for CT-guided lung
biopsy-associated pneumothorax development. We used a retrospective
analysis of a large series of patients with pulmonary lesions who
underwent this procedure.
Patients and methods
Patients
We retrospectively analyzed 325 patients who
underwent CT-guided lung biopsy between April 1, 2010 and September
30, 2015 at the Institute of Biomedical Research and Innovation
(Kobe, Japan). All the patients were scanned using CT (Bright
Speed; GE Healthcare UK Ltd, Little Chalfont, Buckinghamshire, UK).
CT image thickness was 2.5 mm. Examinations were performed based
strictly on clinical indications alone. Informed consent was
obtained from each patient before the procedure was performed. The
biopsies were performed by experienced physicians. This
retrospective study was approved by the ethics committee of the
Institute of Biomedical Research and Innovation (Kobe, Japan).
Procedures
Each patient underwent a percutaneous lung biopsy
using conventional CT scan guidance with a co-axial image. Each
patient was placed in the prone or spine position during the
procedure. Before the scan was initiated, the patient practiced
breath holding at deep expiration and deep inspiration.
The CT scan was used to accurately locate the lesion
and determine the shortest route for the biopsy. The CT scan range
included the typical pulmonary lesion size (i.e., 30–40 mm). The
protocol specified the use of effective 110–150 mA at 120 kV, a
0.5-sec rotation time, a collimation (beam width) of 12 mm, a
14.4-mm x-ray beam width, and a 2.5-mm slice thickness. The lung
function reconfiguration function was used and the field of view
(FOV) was 22 cm.
The biopsies were performed using a needle system
with a 19-gauge introducer needle (Co-Axial Introducer Needle,
Angiotech, Gainesville, FL, USA) and a 22-gauge needle for fine
needle aspiration (HAKKO Sonoguide PTC, needle type-B,
Hakko-Medical, Chikuma, Japan). A 20-gauge automated cutting biopsy
needle with 15- or 25-mm notches was used for the core needle
biopsy (Bard Magnum, Medicon, Osaka, Japan).
The CT-guided lung biopsy examination was performed
using a standard procedure. First, CT images of the lesion position
in the right or left lung were obtained. The CT landmark was the
center of the lesion. A radiopaque grid was placed on the patient's
skin in position over the lesion, and the skin puncture point and
the lesion center were determined using images that included this
grid. The puncture point was determined after the operator measured
the skin surface to pleura distance, the needle path distance, and
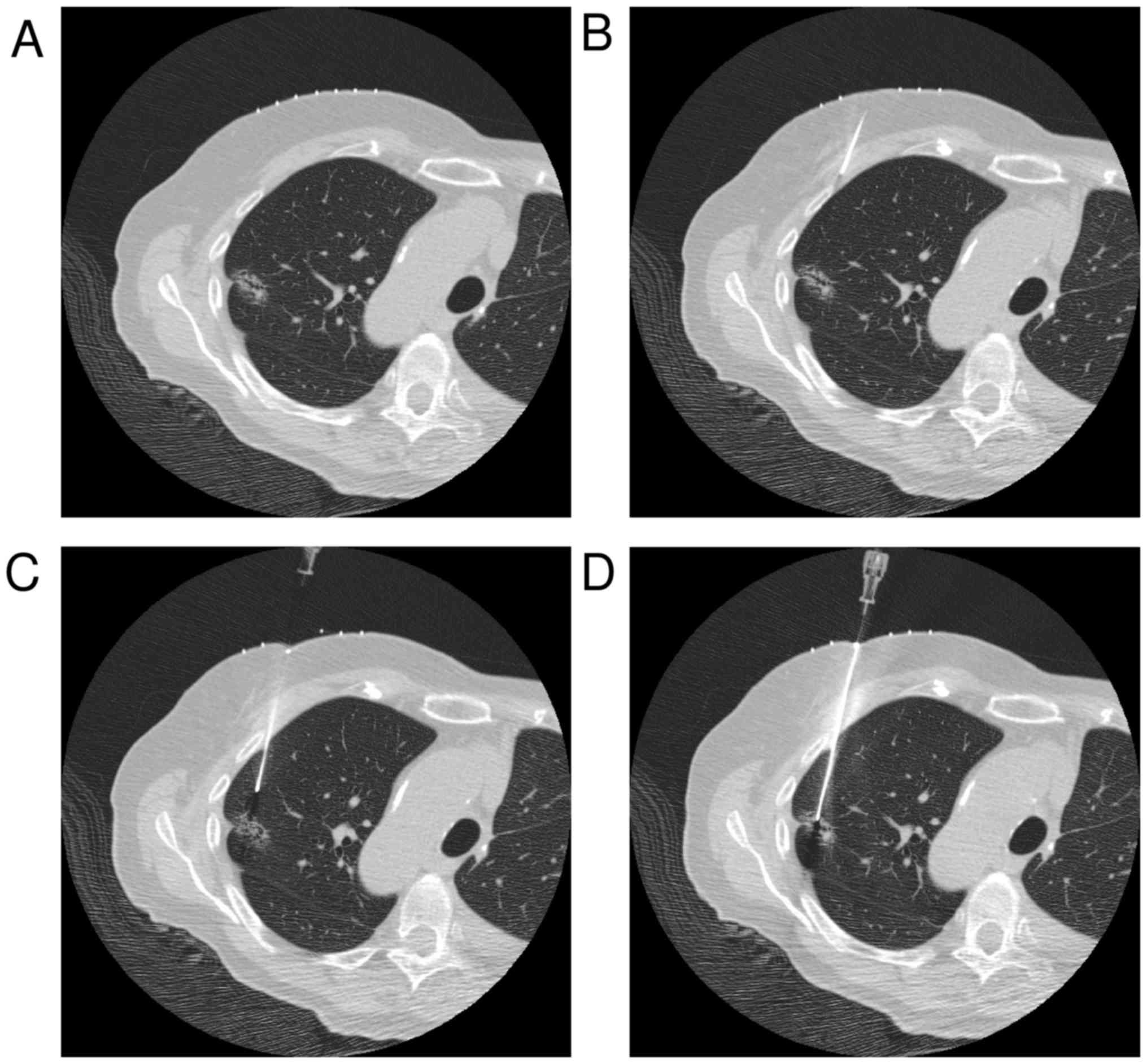
the needle angle (Fig. 2A). The skin
incision was made after 10 ml 1% xylocaine was administered
subcutaneously as a local anesthetic. The initial puncture was
stopped at the pleura surface and did not puncture the pleura
(Fig. 2B). CT images were obtained,
and the puncture point was determined after the needle tip to
lesion distance, the needle path distance, and the needle angle
were measured (with punctured pleura, Fig. 2C). When skin to lesion distance was
long, the operator made the puncture along the access route until
the lesion was encountered. If the needle angle was incorrect, the
operator changed it to an appropriate angle (with punctured lesion,
Fig. 2D). Specimens obtained were
immediately immersed in 10% buffered formalin solution. A chest CT
scan of the lung apices to the diaphragm was routinely performed at
the end of the procedure to detect the presence of pneumothorax or
intrapulmonary hemorrhage.
Measurement yield
The angle between the pleura and needle, and the
maximum diameters between the pleura and needle tip on the lung
window were measured (window wide, 1700; window level, −600).
Fig. 3 shows the results for the
needle angle, which was measured from the operator's position
(performed during sessions 5 or 6). Lesions abutting the pleural
surface were excluded from the analysis. Patients with and without
pneumothorax were separated into two groups. Level 1 pneumothorax
occurred at the cranial lung apex at the clavicle level. Level 2
was between levels 1 and 3. Level 3 pneumothorax was a complete or
near complete collapse of the lung.
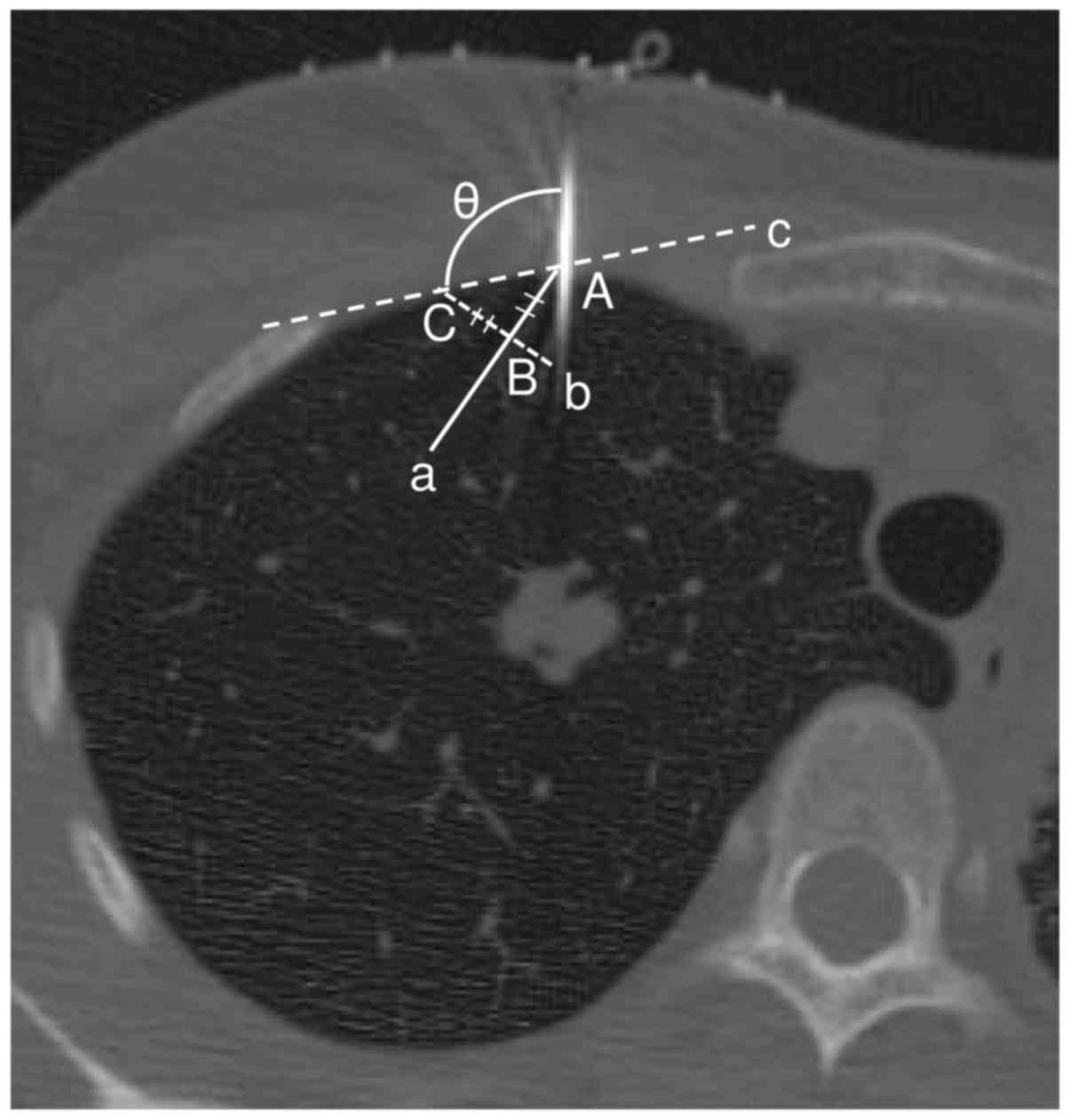 | Figure 3.The angle of the needle. The angle
measured a pulmonary surface using the simple tangent line method
on a CT image. Point A was the point where the needle crossed with
the pulmonary surface. Point B was 10 mm into the pulmonary surface
from point A. Line, a, was between point A and point B. Line, b,
was straight through line, a, through point B. Line, b, was at a
right angle to line, a. Point C was 10 mm from point B on the
pulmonary surface. Line, c, was the line that linked point C to
point A. The needle angle (θ) was measured (°) between the needle
and the pulmonary surface. |
Statistical analysis
The independent risk factors for pneumothorax were
determined using multivariate logistic regression analysis. Data on
multiple variables related to the patient, lesion, and procedure
were collected for statistical analysis. Linear two-sided t-tests
or χ2-tests were performed for the between-variable
analyses. P<0.05 was considered to indicate a statistically
significant result.
Results
Patient characteristics, and multivariate and
univariate analyses. We retrospectively analyzed the data from 325
consecutive patients who underwent CT-guided lung biopsies from
April 1, 2010 to September 30, 2015. The CT dose index (CTDIw)
(22) was 0.49±0.14 mGy (range,
0.28–1.19 mGy). Table I presents the
results for patient demographic characteristics and the results of
further multivariate analyses. Table
II presents the results of univariate analyses used to
investigate associations between risk factors and pneumothorax
development. Biopsy-related pneumothorax occurred in 49.2%
(160/325) of the procedures. A total of 5.5% (18/325) of the
procedures were discontinued as a result of the development of
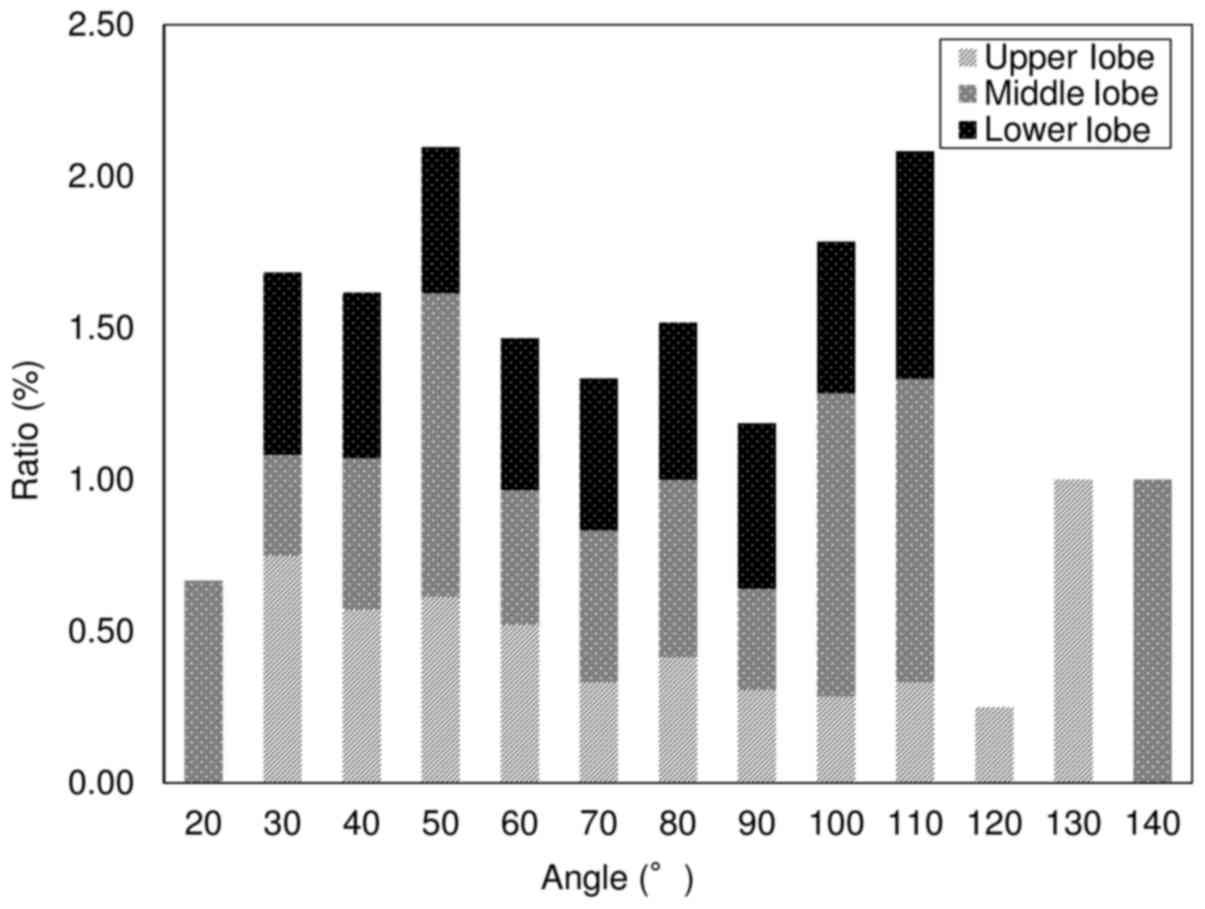
pneumothorax. Fig. 4 presents the
results of univariate analyses used to investigate the
relationships between lung lobe and pneumothorax development; 40.8%
occurred in the upper (58/142), 17.6% in the middle (25/142), and
41.5% in the lower (59/142) lung lobes.
 | Table I.Patient and biopsy
characteristics. |
Table I.
Patient and biopsy
characteristics.
| Patient | Values |
|---|
| Age (years) | 69.3±11.1 |
| Sex, no. |
|
| Male | 180 (55) |
|
Female | 145 (45) |
| Biopsy needle angle;
degree |
|
| Session
5 | 70.9±24.0 |
| Session
6 | 71.8±22.8 |
| From
session 5 to session 6 | 5.1±8.1 |
| Lobe, no. |
|
|
Upper | 133 (40.9) |
|
Middle | 48 (14.7) |
|
Lower | 144 (44.3) |
 | Table II.Results of univariate analyses to
determine risk factors for pneumothorax. |
Table II.
Results of univariate analyses to
determine risk factors for pneumothorax.
| Factors | Without pneumothorax
(n=165) | With pneumothorax
(n=142) | Discontinuance
(n=18) | P-valuea | P-valueb |
|---|
| Patient |
|
|
|
|
|
| Age,
year | 69.3±9.3 | 69.7±11.3 | 71.0±7.8 | 0.6831 | 0.4404 |
| Sex |
|
|
|
|
|
|
Female | 73 (22.4) | 80 (28.0) | 11 (3.4) |
|
|
|
Male | 92 (28.3) | 62 (21.2) | 7 (2.1) |
|
|
| Lobe |
|
|
|
|
|
|
Upper | 72 (22.5) | 58 (17.8) | 4 (1.2) |
|
|
| Middle or
Lingular | 21 (6.5) | 25 (7.7) | 1 (0.3) |
|
|
|
Lower | 72 (22.2) | 59 (18.2) | 13 (4.0) |
|
|
|
Upper-Middle |
|
|
| 0.2855 | 0.8928 |
|
Upper-Lower |
|
|
| 0.5339 | 0.0387 |
|
Middle-Lower |
|
|
| 0.5264 | 0.1827 |
| The angle of
needle |
|
|
|
|
|
| Session
5 | 73.2±22.1 | 70.5±23.9 | 70.2±21.4 | 0.5195 | 0.6841 |
| Session
6 | 72.6±22.7 | 71.0±22.9 | 72.4±18.9 | 0.5411 | 0.9839 |
| From session 6 to
5 | 4.6±4.49 | 6.7±10.3 | 3.55±2.80 | 0.1817 | 0.4584 |
The angle of biopsy needle and
pleura
Table II presents
the results for the relationship between needle angle and the
pleura, and pneumothorax development. Of those procedures in
session 5, 73.2±22.1 had no pneumothorax and 70.5±23.9% experienced
pneumothorax (P=0.5195); 70.2±21.4 were discontinued because
pneumothorax developed (P=0.6841). Of those procedures in session
6, 72.6±22.7% had no pneumothorax and 71.0±22.9% had pneumothorax
(P=0.5411), while 72.4±18.9% were discontinued because pneumothorax
developed (P=0.9839).
When the needle angle was <90°, 40.3% (131/325)
of the procedures were performed without the development of
pneumothorax and 40.0% (130/325) with the development of
pneumothorax. A total of 4.3% (14/325) of the procedures were
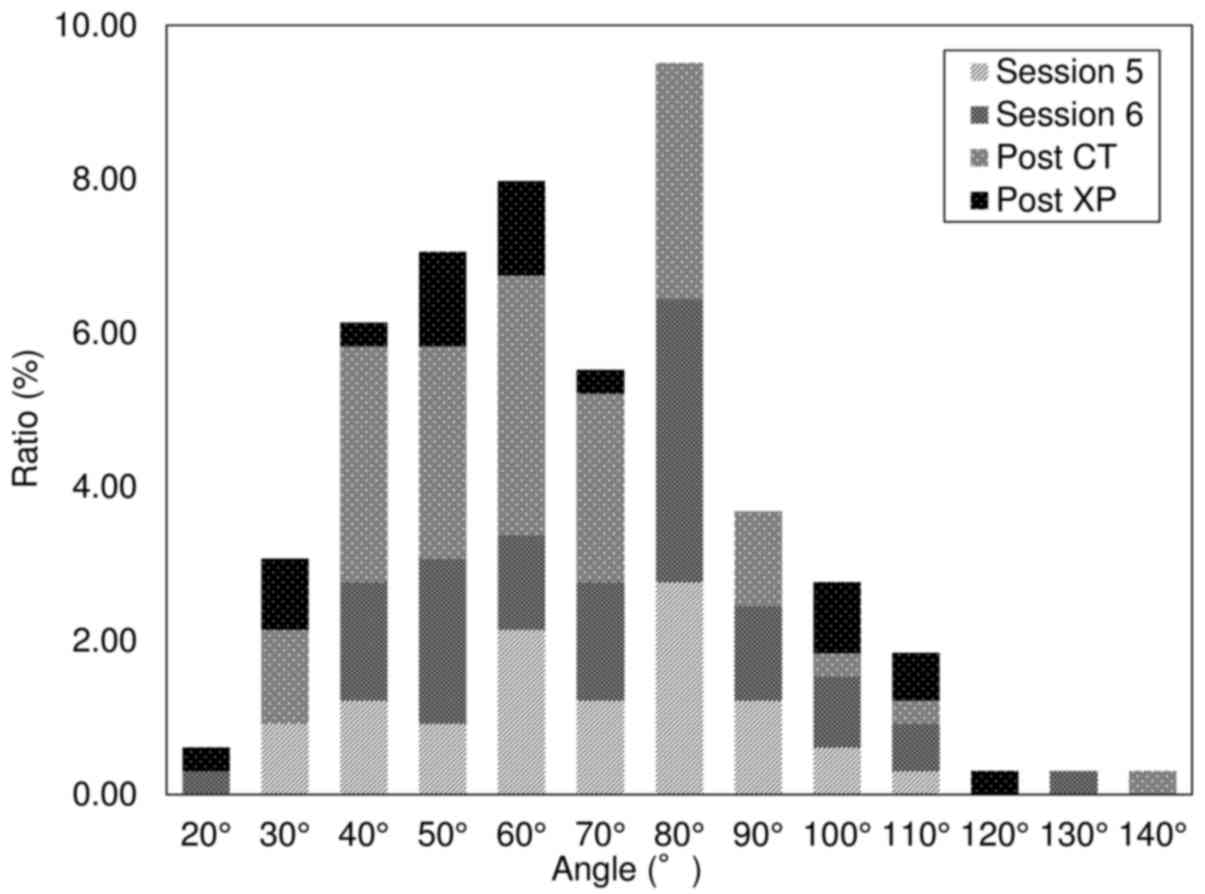
discontinued as a result of pneumothorax development. Fig. 5 shows the results of the univariate
analyses to determine the relationship between needle angle and
pneumothorax development. Of those, 23.1% of the procedures were in
session 5 (37/160), 27.5% were in session 6 (44/160), 36.9% were
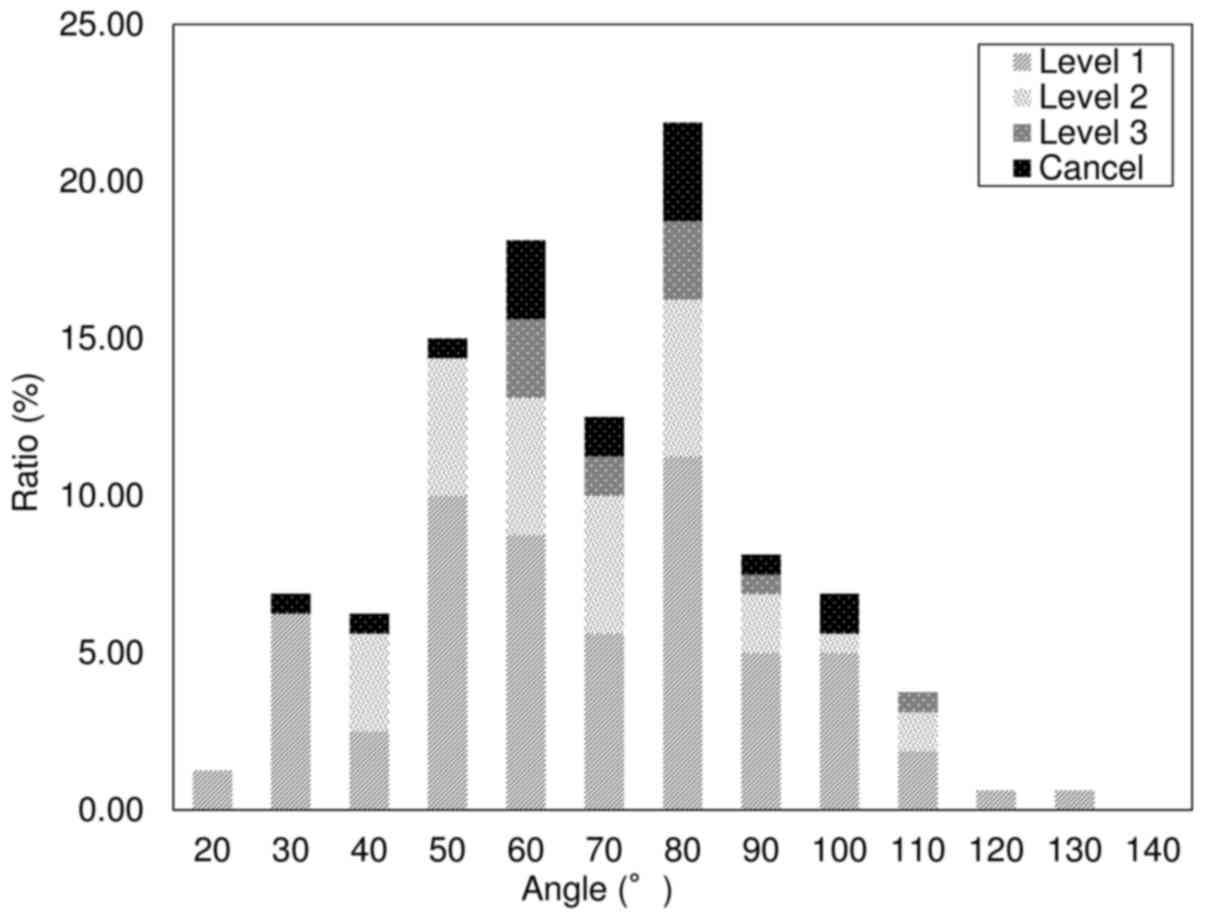
post CT (59/160), and 12.5% were X-P (20/160). Fig. 6 presents the results of the
univariate analyses to summarize pneumothorax severity and
discontinuation of the procedure; 59.4% of the patients had mild
(95/160), 25.0% had moderate (40/160), and 7.5% had severe (12/160)
pneumothorax. A total of 11.2% (18/160) of the procedures were
discontinued.
Discontinuation of CT lung biopsy
The lung biopsy was discontinued in 18 cases; 2.5%
(4/160) of these had upper, 0.6% (1/160) had middle, and 8.1%
(13/160) had lower lung lobe pneumothorax. Of those procedures that
were discontinued because pneumothorax developed 33.3% (6/18) were
a level 1, 50.0% (9/18) were a level 2, and 16.7% (3/18) were a
level 3 pneumothorax. A total of 61.1% (11/18) of the procedures
were in session 5 and 38.9% (7/18) in session 6.
Discussion
The incidence of pneumothorax was 49.2% (160/325) in
this study population. CT-guided lung biopsy becomes more difficult
or may even be discontinued if pneumothorax develops. Two important
risk factors that affect pneumothorax rate are the distance from
the pleura to the lesion and the distance from the pleura to the
needle tip.
Of the biopsies that were discontinued because of
pneumothorax development, 2.5% was upper lung lobe, 0.6% was middle
lobe, and 8.1% was lower lobe biopsies. The lower lung lobe is, in
particular, affected by diaphragmatic movement. During the
procedure, the patient was instructed to hold their breath to
suppress diaphragmatic movement. However, it is impossible to
completely stop diaphragmatic movement; breath holding will only
reduce it. Increased motion of the lower lobe during respiration
may cause inaccurate placement of the biopsy needle into the
lesion. Janssens et al reported that females aged >65
years and males aged >75 years have age-related reduced
respiratory muscle strength and experience difficulty maintaining a
held breath for extended periods of time (21). We hypothesize that the patients
affected by pneumothorax in this study had a reduced ability to
maintain breath holding for the required length of time. The
patients were in a moving gantry during sessions 3, 5, and 6, and
may not have heard the operator's instructions to hold their breath
during inspiration or expiration. Hanley et al (23) reported that when diaphragmatic
movement decreases, it suppresses the abdomen, and the patient may
have difficulty stopping their breath. Wong et al (24) reported that diaphragmatic movement
decreases are greater if mouth breathing, not nose breathing, is
used. The incidence of pneumothorax was likely lower in our patient
population compared with other patient populations because we
instructed patients to use this technique during the procedure.
As the patient was in an unnatural position during
CT, the patient was instructed to practice breath holding at the
end of deep inspiration and expiration. We also used CT images to
confirm that the access route bypassed the ribs and arteries. The
angle between the biopsy needle and pleura was <90° in 84.6%
(275/325) of the procedures. The positioning (supine, prone, or
oblique) was maintained to increase the ease with which the
operator could insert the needle. The angle between the needle and
pleura was <90° in the anterior-posterior and right-left
positions. During the biopsy, the operator stood to the left side
of the CT couch. We tried to acquire the target angle by setting a
pillow or a towel between the patient and the couch (Fig. 1). The angle was typically between 40°
and 90° (76.6%, 249/325) because the operator used this method to
secure a puncture access route (e.g., towels were placed under the
clavicle when the lesion was in the lung apex).
More accurate examination of the access route from
the skin surface to the lesion may result from careful examination
of the 3-D image of the relationships between the lung, lesion, and
bone before the day of the procedure. To reduce the incidence of
pneumothorax, the positioning of the patient, the acquiring route,
and the level of experience of the operator should be reviewed
(17). A hold system (Vac-Lok Bean
Bag: MEDTEC, Orange City, IA, USA) is an ideal system for patient
positioning (25). It has high
repeatability and is relatively comfortable for the patient. The
patient is also likely to be more cooperative during the biopsy if
breath holding is practiced starting from the day before the
procedure.
Our study was limited by the use of retrospective
design, because unexamined risk factors may have been present that
were associated with discontinuing the procedure as a result of
pneumothorax development. Our data also did not include information
on tumor-positive rates. However, these results contribute to
increased understanding regarding the conditions that should be
present to increase the probability of successful CT-guided lung
biopsy.
In conclusion, this study revealed the factors that
affect the risk of developing pneumothorax and that affect the risk
that pneumothorax may result in discontinuation of CT-guided lung
biopsy (e.g., needle angle, lobe where the lesion is present, and
patient positioning). The success rate of CT-guided lung biopsy can
be improved if the factors that affect pneumothorax development are
considered. The access route is simple to locate, easy to puncture
by the operator, and diaphragmatic movement can be successfully
managed.
References
|
1
|
Diederich S, Wormanns D and Heindel W:
Low-dose CT: New tool for screening lung cancer? Eur Radiol.
11:1916–1924. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Henschke CI, Yankelevits DF, Libby DM,
McCauley D, Pasmantier M, Altorki NK, Smith JP and Miettinen OS:
Early lung cancer action project: Annual screening using single
helical CT. Ann N Y Acad Sci. 952:124–134. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Henschke CI, Yankelevits DF, Mirtcheva R,
McGuinness G and McCauley D; Miettinen OS; ELCAP Group, : CT
screening for lung cancer: Frequency and significance of part-solid
and nonsolid nodules. AJR Am J Roentgenol. 178:1053–1057. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Henschke CI, Yankelevits DF, Libby DM and
Kimmel M: CT screening for lung cancer: The first ten years. Cancer
J. 8 Suppl 1:S47–S54. 2002.PubMed/NCBI
|
|
5
|
Pastorino U, Bellomi M, Landoni C, De
Fiori E, Arnaldi P, Picchio M, Pelosi G, Boyle P and Fazio F: Early
lung cancer detection with spiral CT and positron emission
tomography in heavy smokers: 2-year results. Lancet. 362:593–597.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bastarrika G, García-Velloso MJ, Lozano
MD, Montes U, Torre W, Spiteri N, Campo A, Seijo L, Alcaide AB,
Pueyo J, et al: Early lung cancer detection using spiral computed
tomography and positron emission tomography. Am J Respir Crit Care
Med. 171:1378–1383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harter LP, Moss AA, Goldberg HI and Gross
BH: CT-guided fine-needle aspirations for diagnosis of benign and
malignant disease. AJR Am J Roentgenol. 140:363–367. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li H, Boiselle PM, Shepard JO,
Trotman-Dickenson B and McLoud TC: Diagnostic accuracy and safety
of CT-guided percutaneous needle aspiration biopsy of the lung:
Comparison of small and large pulmonary nodules. AJR Am J
Roentgenol. 167:105–109. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klein JS and Zarka MA: Transthoracic
needle biopsy: An overview. J Thorac Imaging. 12:232–249. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takeshita J, Masago K, Kato R, Hata A,
Kaji R, Fujita S and Katakami N: CT-guided fine needle aspiration
and core needle biopsy of pulmonary lesions: A single-center
experience on 750 biopsies in Japan. AJR Am J Roentgenol.
204:29–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tomiyama N, Yasuhara Y, Nakajima Y, Adachi
S, Arai Y, Kusumoto M, Eguchi K, Kuriyama K, Sakai F, Noguchi M, et
al: CT-guided needle biopsy of lung lesions: A survey of severe
complication based on 9783 biopsies in Japan. Eur J Radiol.
59:60–64. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Poulou LS, Tsagouli P, Ziakas PD, Politi
D, Trigidou R and Thanos L: Computed tomography-guided needle
aspiration and biopsy of pulmonary lesions: A single-center
experience in 1000 patients. Acta Radiol. 54:640–645. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hiraki T, Mimura H, Gobara H, Iguchi T,
Fujiwara H, Sakurai J, Matsui Y, Inoue D, Toyooka S, Sano Y and
Kanazawa S: CT fluoroscopy-guided biopsy of 1,000 pulmonary lesions
performed with 20-gauge coaxial cutting needles: Diagnostic yield
and risk factors for diagnostic failure. Chest. 136:1612–1617.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsukada H, Satou T, Iwashima A and Souma
T: Diagnostic accuracy of CT-guided automated needle biopsy of lung
nodules. AJR Am J Roentgenol. 175:239–243. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laurent F, Latrabe V, Vergier B, Montaudon
M, Vernejoux JM and Dubrez J: CT-guided transthoracic needle biopsy
of pulmonary nodules smaller than 20 mm: Results with an automated
20-gauge coaxial cutting needle. Clin Radiol. 55:281–287. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saji H, Nakamura H, Tsuchida T, Tsuboi M,
Kawate N, Konaka C and Kato H: The incidence and the risk of
pneumothorax and chest tube placement after percutaneous CT-guided
lung biopsy: The angle of the needle trajectory is a novel
predictor. Chest. 121:1521–1566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yeow KM, Su IH, Pan KT, Tsay PK, Lui KW,
Cheung YC and Chou AS: Risk factors of pneumothorax and bleeding:
Multivariate analysis of 660 CT-guided coaxial cutting needle lung
biopsies. Chest. 126:748–754. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kazerooni EA, Lim FT, Mikhail A and
Martinez FJ: Risk of pneumothorax in CT-guided transthoracic needle
aspiration biopsy of the lung. Radiology. 198:371–375. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Topal U and Ediz B: Transthoracic needle
biopsy: Factors effecting risk of pneumothorax. Eur J Radiol.
48:263–267. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yeow KM, Tsay PK, Cheung YC, Lui KW, Pan
KT and Chou AS: Factors affecting diagnostic accuracy of CT-guided
coaxial cutting needle lung biopsy: Retrospective analysis of 631
procedures. J Vasc Interv Radiol. 14:581–588. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Janssens JP, Pache JC and Nicod LP:
Physiological changes in respiratory function associated with
ageing. Eur Respir J. 13:197–205. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zompatori M, Fasano L, Mazzoli M, Sciascia
N, Cavina M, Pacilli AM and Paioli D: Spiral CT evaluation of
pulmonary emphysema using a low-dose technique. Radiol Med.
104:13–24. 2002.(In English, Italian). PubMed/NCBI
|
|
23
|
Hanley J, Debois MM, Mah D, Mageras GS,
Raben A, Rosenzweig K, Mychalczak B, Schwartz LH, Gloeggler PJ,
Lutz W, et al: Deep inspiration breath-hold technique for lung
tumors: The potential value of target immobilization and reduced
lung density in dose escalation. Int J Radiat Oncol Biol Phys.
45:603–611. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong JW, Sharpe MB, Jaffray DA, Kini VR,
Robertson JM, Stromberg JS and Martinez AA: The use of active
breathing control (ABC) to reduce margin for breathing motion. Int
J Radiat Oncol Biol Phys. 44:911–919. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takayama K, Mizowaki T, Kokubo M, Kawada
N, Nakayama H, Narita Y, Nagano K, Kamino Y and Hiraoka M: Initial
validations for pursuing irradiation using a gimbals tracking
system. Radiother Oncol. 93:45–49. 2009. View Article : Google Scholar : PubMed/NCBI
|