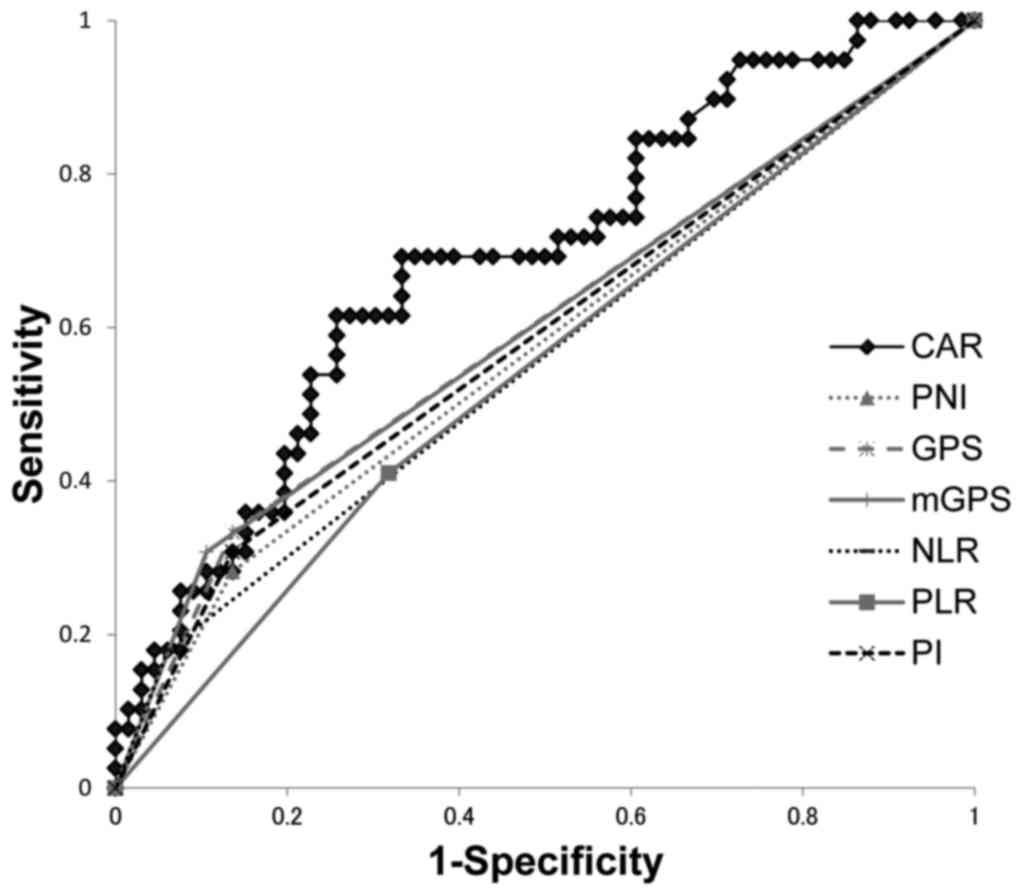
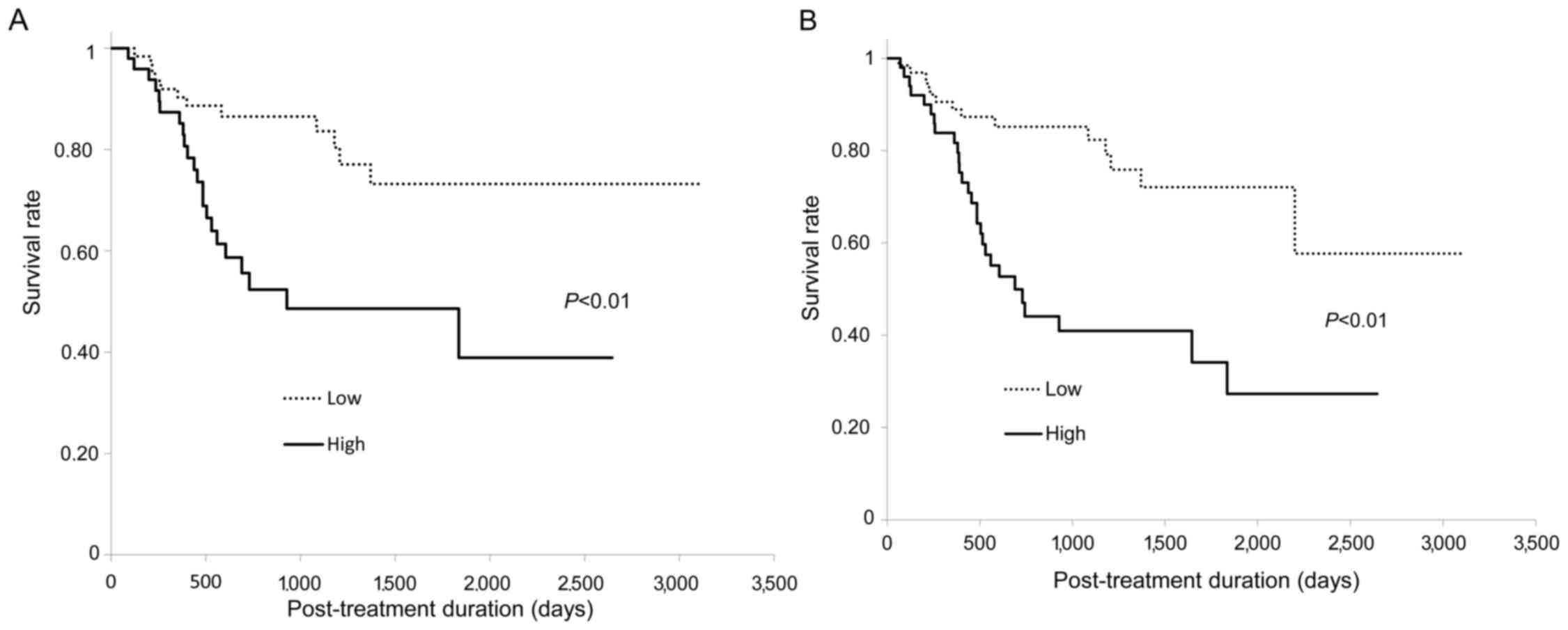
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakamura M, Iwahashi M, Nakamori M, Ojima
T, Katsuda M, Iida T, Hayata K, Kato T and Yamaue H: A new
prognostic score for the survial of patients with esophageal
squamous cell carcinoma. Surg Today. 44:875–883. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou X, Du Y, Huang Z, Xu J, Qiu T, Wang
J, Wang T, Zhu W and Liu P: Prognostic value of PLR in various
cancers: A meta-analysis. PLoS One. 9:e1011192014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kawashima M, Murakawa T, Shinozaki T,
Ichinose J, Hino H, Konoeda C, Tsuchiya T, Murayama T, Nagayama K,
Nitadori J, et al: Significance of the glasgow prognostic score as
a prognostic indicator for lung cancer surgery. Interact Cardiovasc
Thorac Surg. 21:637–643. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Templeton AJ, McNamara MG, Šeruga B,
Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: A systematic review and meta-analysis. J
Natl Cancer Inst. 106:dju1242014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu XL, Yu HQ, Hu W, Song Q and Mao WM: A
novel inflammation-based prognostic score, the C-reactive
protein/albumin ratio predicts the prognosis of patients with
operable esophageal squamous cell carcinoma. PLoS One.
10:e01386572015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He S, Wang Y, Chen H, Yang L, Liang S, Lu
L and Chen Y: C-reactive protein/albumin ratio (CAR) as a
prognostic factor in patients with non-metastatic nasopharyngeal
carcinoma. J Cancer. 7:2360–2366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishizuka M, Nagata H, Takagi K, Iwasaki Y,
Shibuya N and Kubota K: Clinical significance of the C-reactive
protein to albumin ratio for survival after surgery for colorectal
cancer. Ann Surg Oncol. 23:900–907. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishizuka M, Nagata H, Takagi K, Horie T
and Kubota K: Inflammation-based prognostic score is a novel
predictor of postoperative outcome in patients with colorectal
cancer. Ann Surg. 246:1047–1051. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kato A, Tsuji T, Sakao Y, Ohashi N, Yasuda
H, Fujimoto T, Takita T, Furuhashi M and Kumagai H: A comparison of
systemic inflammation-based prognostic scores in patients on
regular hemodialysis. Nephron Extra. 3:91–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyazaki T, Yamasaki N, Tsuchiya T,
Matsumoto K, Kunizaki M, Kamohara R, Hatachi G, Doi R, Obata T and
Nagayasu T: Ratio of C-reactive protein to albumin is a prognostic
factor for operable non-small-cell lung cancer in elderly patients.
Surg Today. 47:836–843. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martin HL, Ohara K, Kiberu A, Van Hagen T,
Davidson A and Khattak MA: Prognostic value of systemic
inflammation-based markers in advanced pancreatic cancer. Intern
Med J. 44:676–682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suzuki Y, Okabayashi K, Hasegawa H,
Tsuruta M, Shigeta K, Kondo T and Kitagawa Y: Comparison of
preoperative inflammation-based prognostic scores in patients with
colorectal cancer. Ann Surg. Dec 15–2016.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu XL, Yu HQ, Hu W, Song Q and Mao WM: A
novel inflammation-based prognostic score, the C-reactive
protein/albumin ratio predicts the prognosis of patients with
operable esophageal squamous cell carcinoma. PLoS One.
10:e01386572015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Forrest LM, McMillan DC, McArdle CS,
Angerson WJ and Dunlop DJ: Evaluation of cumulative prognostic
scores based on the systemic inflammatory response in patients with
inoperable non-small-cell lung cancer. Br J Cancer. 89:1028–1030.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McMillan DC, Crozier JE, Canna K, Angerson
WJ and McArdle CS: Evaluation of an inflammation-based prognostic
score (GPS) in patients undergoing resection for colon and rectal
cancer. INt J Colorectal Dis. 22:881–886. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Borda F, Borda A, Jiménez J, Zozaya JM,
Prieto C, Gómez M, Urman J and Ibáñez B: Predictive value of
pre-treatment hypoalbuminemia in prognosis of resected colorectal
cancer. Gastroenterol Hepatol. 37:289–295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Crumley AB, Stuart RC, McKernan M and
McMillan DC: Is hypoalbuminemia an independent prognostic factor in
patients with gastric cancer? World J Surg. 34:2393–2398. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wong VK, Malik HZ, Hamady ZZ, Al-Mukhtar
A, Gomez D, Prasad KR, Toogood GJ and Lodge JP: C-reactive protein
as a predictor of prognosis following curative resection for
colorectal liver metastases. Br J Cancer. 96:222–225. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kinoshita A, Onoda H, Takano K, Imai N,
Saeki C, Fushiya N, Miyakawa Y, Nishino H and Tajiri H:
Pretreatment serum C-reactive protein level predicts poor prognosis
in patients with hepatocellular carcinoma. Med Oncol. 29:2800–2808.
2012. View Article : Google Scholar : PubMed/NCBI
|