Introduction
Primary intracranial leptomeningeal gliomas (PLG),
is a rare entity that arises from the heterotopic glial tissue in
the subarachnoid space as first described by Wolbach (1). PLGs have been divided into two
different forms, solitary and diffuse. Diffuse PLG is often
difficult to exclude the exophytic growth from the underlying brain
or seeding from a distant primary lesion in the central nervous
system (2,3). On the other hand, solitary PLG is
extremely rare that only 16 cases including the present case have
been reported in the literature, on basis of currently available
data (2–14). We herein report a case of
intracranial focal solitary PLG, which mimicked an extra-axial
tumor, to better characterize this rare tumor.
Case report
History and examination
A 55-year-old woman who had suffered from headache
and nausea for a few weeks admitted to our hospital. She had no
obvious abnormality on neurological examinations. Her past medical
history was not appreciable except for thymoma removal surgery in
her forties, and her family history was unremarkable. Informed
consent was obtained from the patient and her family. Magnetic
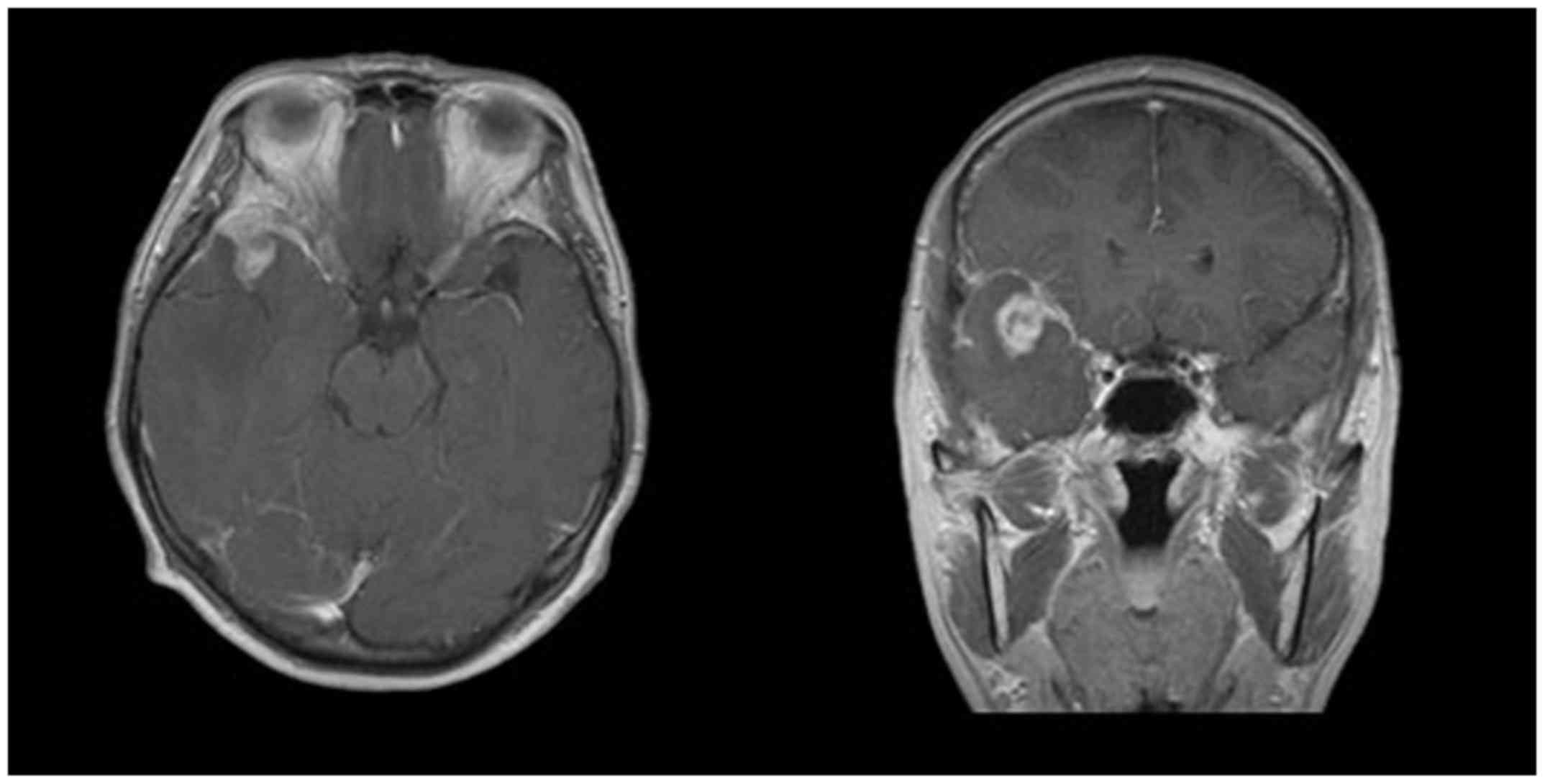
resonance imaging (MRI) demonstrated a brain tumor occupying the
tip of middle fossa to the frontal base with a maximal diameter of
25 mm. The greater part of the lesion showed low signal intensity
on T1 weighted images and iso signal intensity on T2 weighted
images. Middle cerebral artery was involved by the tumor. A massive
perifocal edema was found in the adjacent brain. The lesion was
strongly and heterogeneously enhanced after contrast medium
injection (Fig. 1). Internal carotid
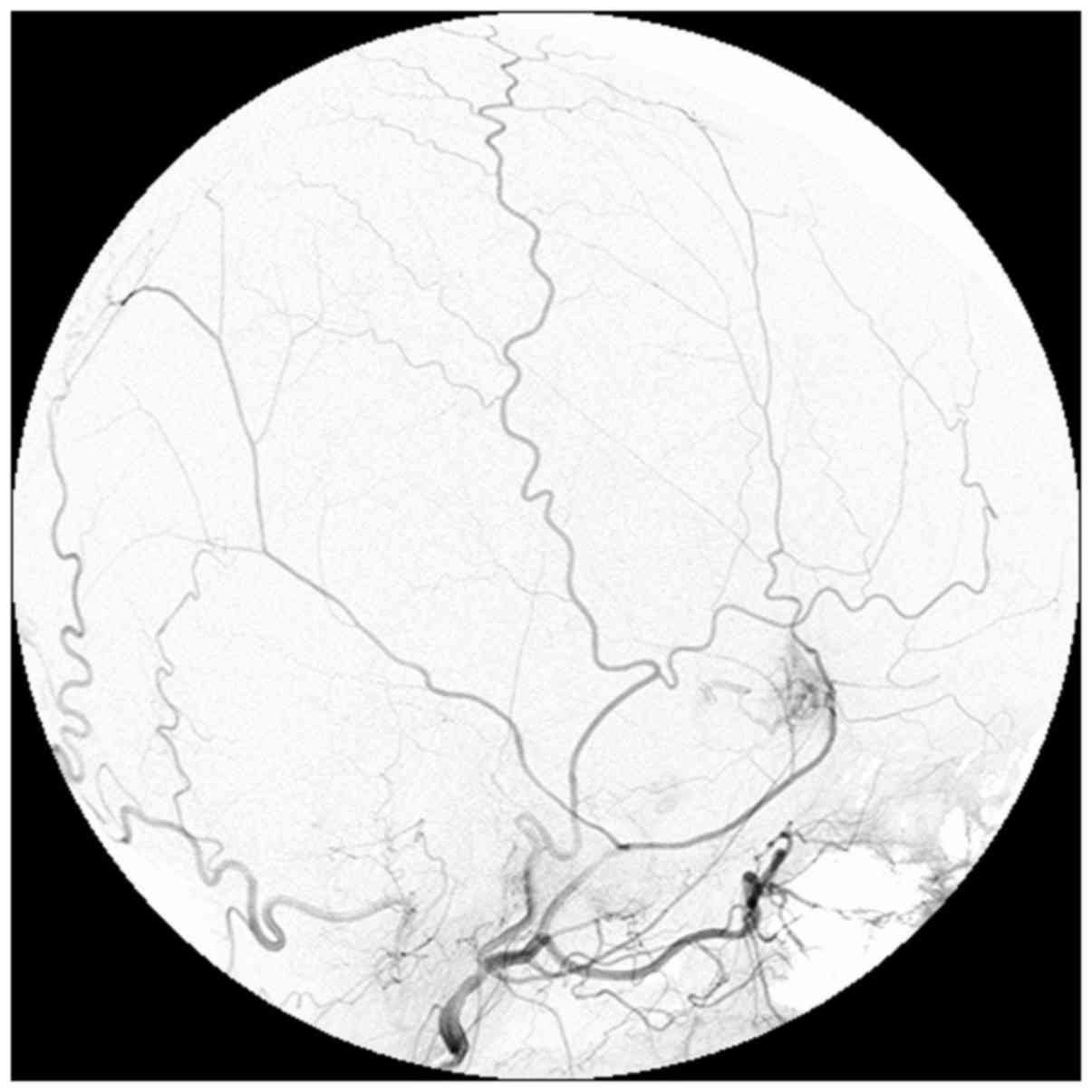
artery angiography did not show any abnormality. However, external
carotid artery angiography showed a tumor stain from the middle
meningeal artery in where the mass was located on MRI (Fig. 2). Such radiological findings were
interpreted as diagnostic hemangiopericytoma or meningioma.
Surgery
A frontotemporal craniotomy was made. Dura matter
was adhered to the surface of the temporal cortex and was not easy
to separate. This connecting tissue was coagulated and cut one by
one to flip the dura matter under microscopy. Numerous small
vessels were found within this connective tissue that easily bled.
The tumor was found immediately after opening the Sylvian fissure,
and was distinguished from the surrounding brain parenchyma in this
region. The tumor was soft and easily bled. As opening the Sylvian
fissure further, the M1 segment of the middle cerebral artery was
found to be involved within the tumor mass. Tumor was removed as
much as possible but avoided removing the tumor involving the
perforators of the middle cerebral artery. When reaching deeply
into the Sylvian fissure, the tumor infiltrated into the temporal
lobe and there was no clear border between the tumor and the
adjacent normal parenchyma. Therefore, right temporal lobectomy was
made at 50 mm from the temporal tip to remove the entire mass.
Histopathological examination
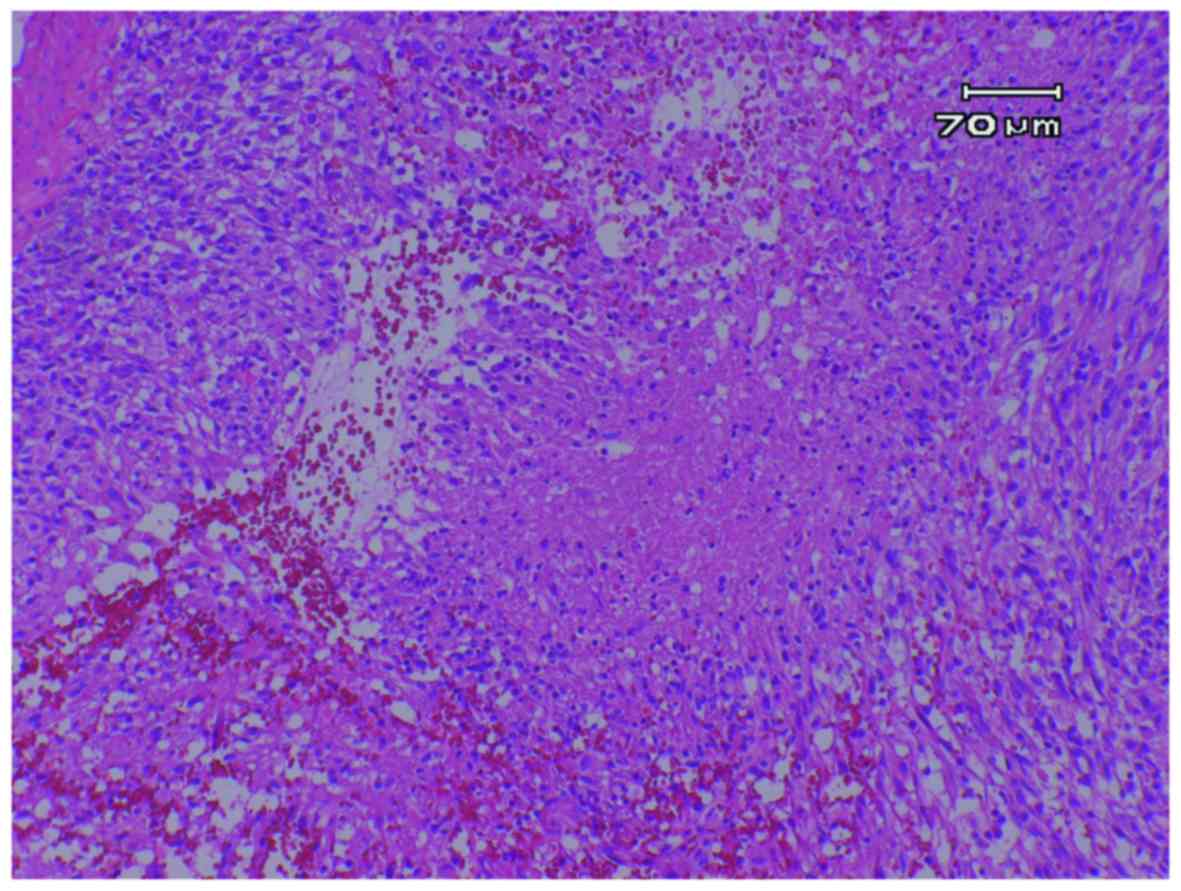
The specimen exhibited nuclear and cytoplasmic
pleomorphism and nuclear atypia. Highly atypical astrocytes with
numerous mitotic nuclei were found overall. Necrosis with
pseudopalisading and microvascular proliferation were seen in
several areas (Fig. 3). The tumor
cells showed intense staining for glial fibrillary acidic protein
indicating their astrocytic nature and high Ki-67 proliferative
index of ≥40%. An obvious invasion of the tumor into the temporal
lobe was observed. The tumor cells were immunohistochemically
negative for isocitrate dehydrogenase (IDH)1-R132H and p53, and
positive for epidermal growth factor receptor. Feature of
hemangiopericytoma was not seen all over the section with negative
silver staining. Therefore, the pathological diagnosis was
determined to be a glioblastoma, IDH-wild type, a glioma of World
Health Organization grade IV, originating from a heterotopic glial
cluster in the vicinity of the right Sylvian cistern.
Post-operative course
Under diagnosis as glioblastoma, the tumor bed was
irradiated with 60 Gy in 30 times of fraction. She also received
administration of temozolomide chemotherapy according to the Stupp
regimen. The patient was discharged from the hospital without any
neurological deficits.
Six months after the initial surgery, the patient
complained motor weakness in both lower limbs. Spinal MRI revealed
multiple enhanced masses throughout the spinal cord, consistent
with dissemination. Brain MRI also indicated a local re-growth of
the original tumor in where the tumor was left. A second
angiography was performed, but no tumor stain was seen from both
internal and external carotid arteries. Additional radiation
against the spinal lesions and advanced chemotherapy were done but
the tumor became uncontrollable. The patient died 10 months after
the initial surgery. Autopsy was not performed.
Discussion
Solitary PLG is extremely rare and there is no
consensus on the diagnostic criteria or treatment. The pathogenesis
of solitary PLG remains unclear, and it is speculated that it
originates from the leptomeningeal heterotopic neuroglial nests
that separated from the bulk of the central nervous system during
embryogenesis and subsequently underwent neoplastic transformation
in rare instances (15,16). Heterotopic neuroglial nests in the
subarachnoid space are seen in ~1% of normal individuals; the
incidence is higher (25%) in patients with various congenital
malformations of the brain and spinal cord (16). However, it remains unclear,
particularly with respect to diffuse PLG, whether such lesions
should be considered genuine primitive tumor or as seeding of
unknown intra-parenchymal glioma (2). More rarely they were reported as
solitary and focal tumor form with or without leptomeningeal
spreading, mimicking an extra-axial central nervous system tumor
such as meningioma, acoustic neurinoma, or metastasis (6,11). From
the basis of currently available data, descriptive information
summarizing the previously reported and described cases of solitary
and focal form of PLGs are listed in Table I.
 | Table I.Summary of reported cases of solitary
primary intracranial leptomeningeal gliomas. |
Table I.
Summary of reported cases of solitary
primary intracranial leptomeningeal gliomas.
|
|
|
|
|
| CT | MRI |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| No. | Authors | Year | Age, years | Sex | Plane-CT | Enhanced-CT | T1 | T2 | T1-Gadolinium | Angiography | Location | Pathology | Outcome |
|---|
| 1 | Horoupian et
al (4) | 1979 | 49 | F | Mass | Homogeneous
enhancement | NA | NA | NA | No staining | Frontal to
parietal | Astrocytoma | Recurrence at 18
months |
| 2 | Shuangshoti et
al (5) | 1984 | 49 | F | Mass | Homogeneous
enhancement | NA | NA | NA | NA | Para-seller | Mixed glioma | No recurrence for 24
months |
| 3 | Sceats et al
(6) | 1986 | 53 | F | NA | Homogeneous
enhancement | NA | NA | NA | No staining | Cerebellopontine
angle | Grade II
astrocytoma | Well
post-surgery |
| 4 | Kakita et al
(7) | 1992 | 74 | F | NA | Homogeneous
enhancement | NA | NA | NA | NA | Parietal | Glioblastoma | Died in 2 months |
| 5 | Oka et al
(8) | 1994 | 75 | M | Mass | Homogeneous
enhancement | NA | NA | NA | MCA | Frontal to parietal
convexity | Anaplastic
oligo-astrocytoma | Well
post-surgery |
| 6 | Opeskin et al
(9) | 1994 | 59 | M | Mass | NA | Mass |
|
| No staining | Cerebellum | Desmoplastic
astrocytoma | Died in 7 months |
| 7 | Krief et al
(2) | 1994 | 26 | F | Normal | NA | Normal | Normal | Clear pathologic
leptomeningeal enhancement | NA | Parietal | High grade
glioma | NA |
| 8 | Ng and Poon (10) | 1998 | 79 | M | Normal | NA | Suggesting meningeal
disease or brain edema |
| Homogeneous
enhancement | NA | Temporal to
parietal | Fibrillary
astrocytoma | Died in 1 month |
| 9 | Sell et al
(11) | 2000 | 62 | M | Mass | NA | NA | NA | Heterogeneous
enhancement | PICA | Cerebellum | High grade astrocytic
tumor | Died in 4 month |
| 10 | Cirak et al
(12) | 2000 | 2 | F | Mass | NA | NA | NA | Homogenous
enhancement | NA | Brain stem | Astrocytoma | NA |
| 11 | Wakabayashi et
al (3) | 2002 | 72 | F | Mass,
calcification | Heterogeneous
enhancement | Hypo/iso | Hyper | Heterogeneous
enhancement | MMA | Bilateral
frontal | Glioblastoma | Died in 18
months |
| 12 | Wakabayashi et
al (3) | 2002 | 33 | M | Mass, cyst,
calcification | NA | Hypo/iso | NA | Heterogeneous
enhancement | MMA | Temporal | Glioblastoma | Femur metastasis at
39 months, Good at 51 months |
| 13 | Wakabayashi et
al (3) | 2002 | 74 | M | Mass, cyst | Heterogeneous
enhancement | Iso | Hyper | Ring like
enhancement | MMA | Frontal |
Oligodendroglioma | Local recurrence at
10 months, good at 55 months |
| 14 | De Tommasi et
al (13) | 2007 | 78 | F | Mass, cyst | NA | Polycystic | NA | NA | NA | Frontal to
parietal | Pilocytic
astrocytoma | No recurrence for
24 months |
| 15 | Kim et al
(14) | 2013 | 45 | M | Mass | NA | Hypo/low | Iso/hyper | Heterogeneous
enhancement | NA | Frontal | Anaplastic
oligoastrocytoma | No recurrence for
12 months |
| 16 | Present case | 2014 | 55 | F | Mass, edema | NA | Low | Iso | Heterogeneous
enhancement | MMA | Temporal | Glioblastoma | Died in 10
months |
Radiological features of intracranial solitary PLGs
are not well documented, but frequently observed as extra-axial
tumors mimicking meningioma. Computerized tomography (CT) findings
were reported in 14 cases. From the CT scan findings, most of the
authors indicated an iso density mass only available to identify
the tumor. But some tumors included intra-tumoral high density or
cystic component, indicating calcification and cyst (3). Enhanced CT was taken in 8 cases and
most of the tumors showed homogeneous enhancement. MRI was taken in
10 cases. MRI after gadolinium injection showed that most tumors
were enhanced but in several patterns. One case reported by
Wakabayashi et al (3)
presented a ring-like enhancement effect, which was a
characteristic enhancing pattern of an intra-axial glioma. The same
authors indicated that careful analysis of MRI patterns suggested
glioma rather than meningioma in their cases. Taken together, such
CT and MRI patterns seems to depend on the pathological
characteristics and malignancy of the tumor.
From the previous description of angiography of 8
cases out of 15 previous cases, which vessel gives blood supply to
solitary PLG seems controversial. Three tumors were fed by the
branches of the external carotid artery and one from the internal
carotid artery (but not from the external carotid artery). One
solitary PLG in the posterior fossa was fed by the posterior
inferior cerebellar artery. The remaining 3 cases did not show
tumor staining from any arteries. In the present case, tumor stain
was clearly seen from the middle meningeal artery but not from the
internal carotid artery. This vascular supply was consistent with
the intra-operative finding that numerous capillary vessels
connecting the dura matter and the tumor. The presence of tumor
staining again seems to rely on the pathological malignancy of the
tumor. Indeed, 3 cases that did not show any tumor stain were all
diagnosed pathologically as lower grade glioma. In conclusion,
because the pathology of solitary PLGs can vary from astrocytic to
oligodendrocytic glioma, both from lower grade to higher grade, it
makes it different to define the characteristics by any
radiological diagnostic tools.
The PLG was first described by Wolbach in 1907
(1). Cooper and Kernohan in 1951
suggested that meningeal gliomas have no apparent attachment or
neoplastic process into the brain or spinal cord in any cases
(16). Therefore, it might be
emphasized as a tumor that grows in the subarachnoid space without
any invasion into the brain parenchyma, although there is no
consensus on the diagnostic criteria for such rare neoplastic
condition (14). However, it was
mentioned that lesions were sometimes linked to the parenchyma by
glial bridges extending via the perivascular spaces into the
superficical layers of the cortex. Krief et al (2) reported a case of solitary PLG that
showed small pathological cortical involvement that was not seen by
pre-surgical MRI. Further, some reports indicated a tendency of
intra-operative parenchymal invasion (3,8). In the
present case, the diagnosis of intracranial solitary PLG was made
based on the evidence that the pre-surgical angiogram showing tumor
stain from the middle meningeal artery and because the majority of
the tumor existed in the subarachnoid space although the tumor
obviously invaded the temporal parenchyma confirmed by both
intra-operative and pathological finding. In the present case,
tumor probably originated from a heterotopic glial cluster in the
vicinity of the right Sylvian cistern. The present case shows that
solitary PLG might invade the parenchyma, disseminate to the spinal
cord, and make multiple lesions depending on the tumor character.
The present tumor was IDH-wild type glioblastoma but genomic
details are not analyzed in the previous reports. IDH status might
vary among solitary PLG cases considering the wide variety of
pathological feature.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CT
|
computerized tomography
|
|
MRI
|
magnetic resonance imaging
|
|
PLG
|
primary intracranial leptomeningeal
glioma
|
|
IDH
|
isocitrate dehydrogenase
|
References
|
1
|
Wolbach SB: Congenital Rhabdomyoma of the
Heart. J Med Res. 16:495–520.7. 1907.PubMed/NCBI
|
|
2
|
Krief O, Monnier L, Cornu P, Foncin JF,
Dormont D and Marsault C: MR of isolated leptomeningeal glioma.
AJNR Am J Neuroradiol. 15:1782–1784. 1994.PubMed/NCBI
|
|
3
|
Wakabayashi K, Shimura T, Mizutani N,
Koide A, Yamagiwa O, Mori F, Nishiyama K, Tanaka R and Takahashi H:
Primary intracranial solitary leptomeningeal glioma: A report of 3
cases. Clin Neuropathol. 21:206–213. 2002.PubMed/NCBI
|
|
4
|
Horoupian DS, Lax F and Suzuki K:
Extracerebral leptomeningeal astrocytoma mimicking a meningioma.
Arch Pathol Lab Med. 103:676–679. 1979.PubMed/NCBI
|
|
5
|
Shuangshoti S, Kasantikul V, Suwanwela N
and Suwanwela C: Solitary primary intracranial extracerebral
glioma. Case report. J Neurosurg. 61:777–781. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sceats DJ Jr, Quisling R, Rhoton AL Jr,
Ballinger WE and Ryan P: Primary leptomeningeal glioma mimicking an
acoustic neuroma: Case report with review of the literature.
Neurosurgery. 19:649–654. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kakita A, Wakabayashi K, Takahashi H,
Ohama E, Ikuta F and Tokiguchi S: Primary leptomeningeal glioma:
Ultrastructural and laminin immunohistochemical studies. Acta
Neuropathol. 83:538–542. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oka H, Kawano N, Morii S, Suwa T, Irikura
K and Saitoh T: Intracranial extracerebral glioma]. Noshuyo Byori
11: 193–200, 1994. Noshuyo Byori. 11:193–200. 1994.(In
Japanese).
|
|
9
|
Opeskin K, Anderson RM and Nye DH: Primary
meningeal glioma. Pathology. 26:72–74. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ng HK and Poon WS: Primary leptomeningeal
astrocytoma. Case report. J Neurosurg. 88:586–589. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sell M, Mitrovics T and Sander BC: Primary
nodular meningeal glioma mimicking metastatic tumor of the
cerebellum with diffuse infra- and supratentorial leptomeningeal
spread. Clin Neuropathol. 19:126–130. 2000.PubMed/NCBI
|
|
12
|
Cirak B, Caksen H, Ugras S and Unal O:
Primary leptomeningeal astrocytoma in a child. Pediatr Int.
42:389–391. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De Tommasi A, Occhiogrosso G, De Tommasi
C, Luzzi S, Cimmino A and Ciappetta P: A polycystic variant of a
primary intracranial leptomeningeal astrocytoma: Case report and
literature review. World J Surg Oncol. 5:722007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim YG, Kim EH, Kim SH and Chang JH:
Solitary primary leptomeningeal glioma: Case report. Brain Tumor
Res Treat. 1:36–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bailey P and Robitaille Y: Primary diffuse
leptomeningeal gliomatosis. Can J Neurol Sci. 12:278–281. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cooper IS and Kernohan JW: Heterotopic
glial nests in the subarachnoid space; histopathologic
characteristics, mode of origin and relation to meningeal gliomas.
J Neuropathol Exp Neurol. 10:16–29. 1951. View Article : Google Scholar : PubMed/NCBI
|