Introduction
Epstein-Barr virus (EBV) is an established cause of
various types of lymphoma including Burkitt lymphoma and Hodgkins
lymphoma, and nasopharyngeal carcinoma (1). Similar to Helicobacter pylori,
EBV is accepted as an infective agent that plays an important
pathogenic role in gastric cancer (2,3). Gastric
carcinoma with lymphoid stroma (GCLS) is a rare histological
subgroup of gastric cancers, constituting approximately 1–4% of all
gastric carcinomas. Approximately 80% of the reported GCLS cases
are associated with an EBV infection (1,4). To
diagnose GCLS accurately, the immunohistochemical approach of the
tumor was important in addition to sophisticated observation of the
endoscopic findings, while standardized diagnostic criteria are
lacking (2,4). Early gastric cancer has favorable
outcomes with curative resection including gastrectomy and
endoscopic submucosal resection (4,5). The
current prognosis for GCLS is favorable due to its lower lymph node
metastatic rate and a higher survival rate despite deep submucosal
invasion of tumor cells.
In the present study, we report a case of
EBV-associated early GCLS accompanying lymph node metastasis
treated by laparoscopic distal gastrectomy.
Case report
A 61-year-old woman was referred to Kochi Medical
School Hospital as a result of an evaluation of a gastric mass
lesion that was initially diagnosed by a local medical doctor.
Laboratory investigations, including serum carcinoembryonic antigen
and cancer antigen 19-9 screening showed no significant
abnormalities. Esophagogastroduodenoscopy (EGD) revealed an
elevated lesion with a central irregularly depressed area in the
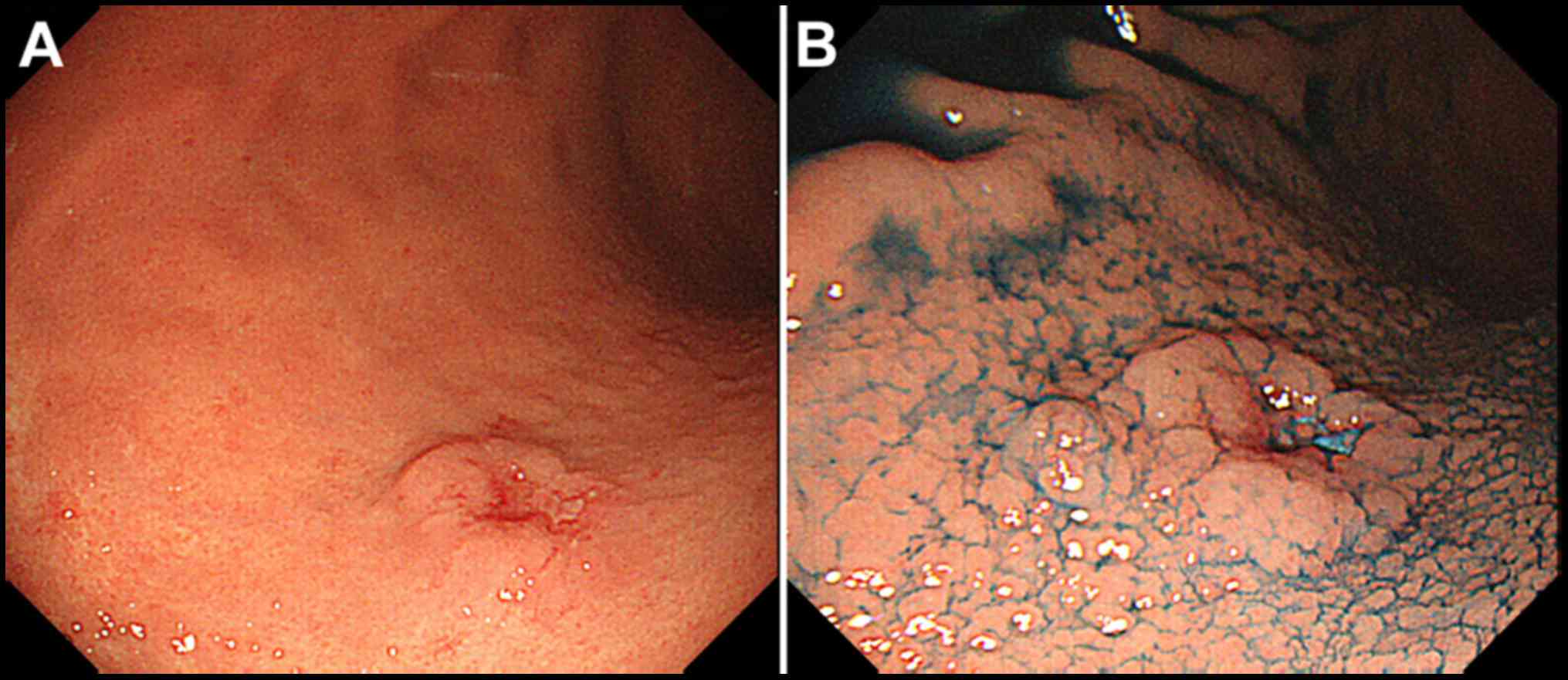
posterior wall of the middle third of the stomach (Fig. 1A). The elevated lesion descended in a
gradual slope to the surrounding mucosa when indigo carmine dye was
used in chromoendoscopy (Fig. 1B).
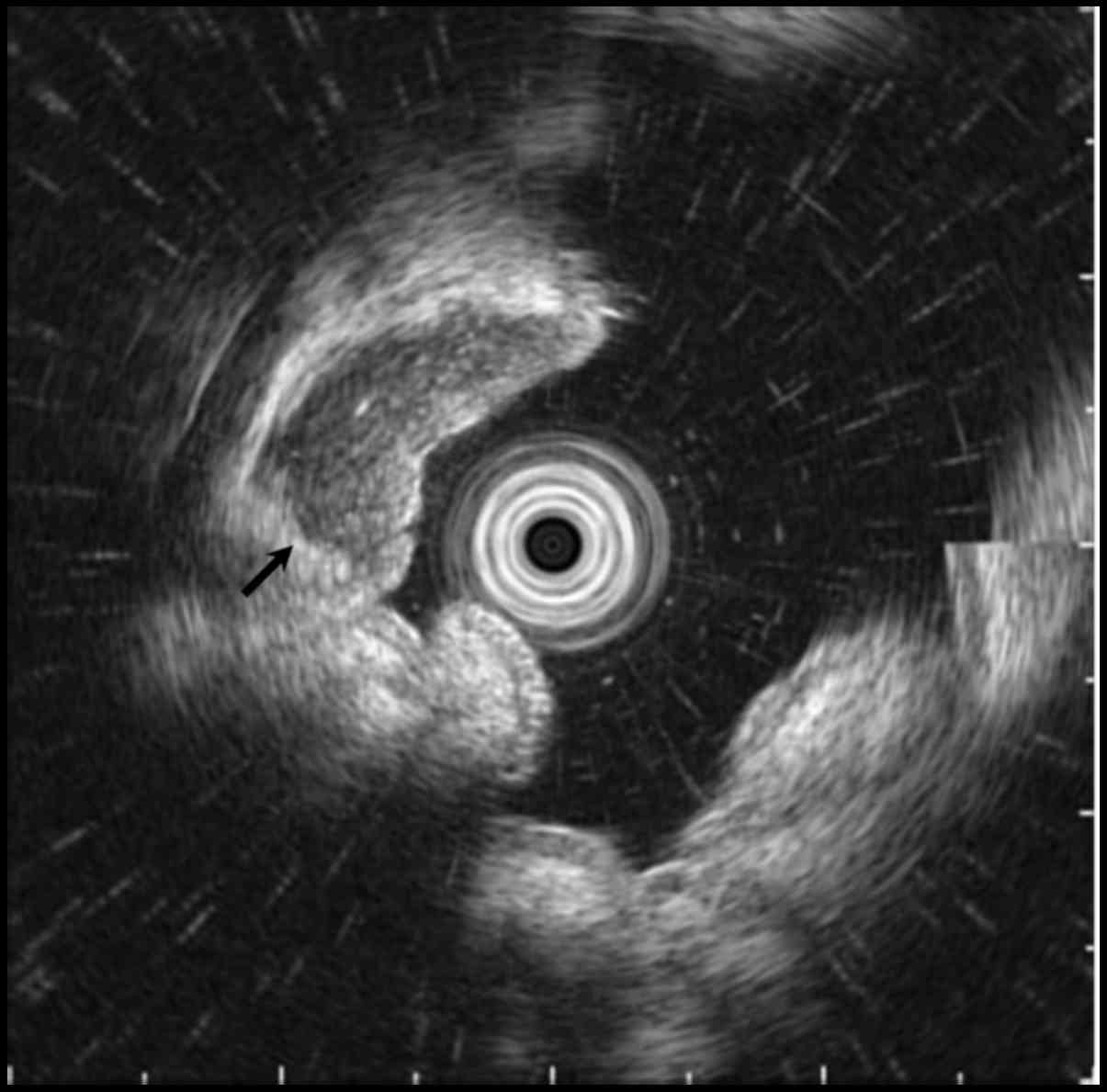
Endoscopic ultrasonography (EUS) revealed a well-circumscribed
hypoechoic mass located predominantly within the submucosa and the
mucosa (Fig. 2, arrow). Biopsy
specimens of the lesion showed prominent lymphocyte infiltration
with a lymphoepithelial lesion, suspected of being carcinoma with
lymphoid stroma.
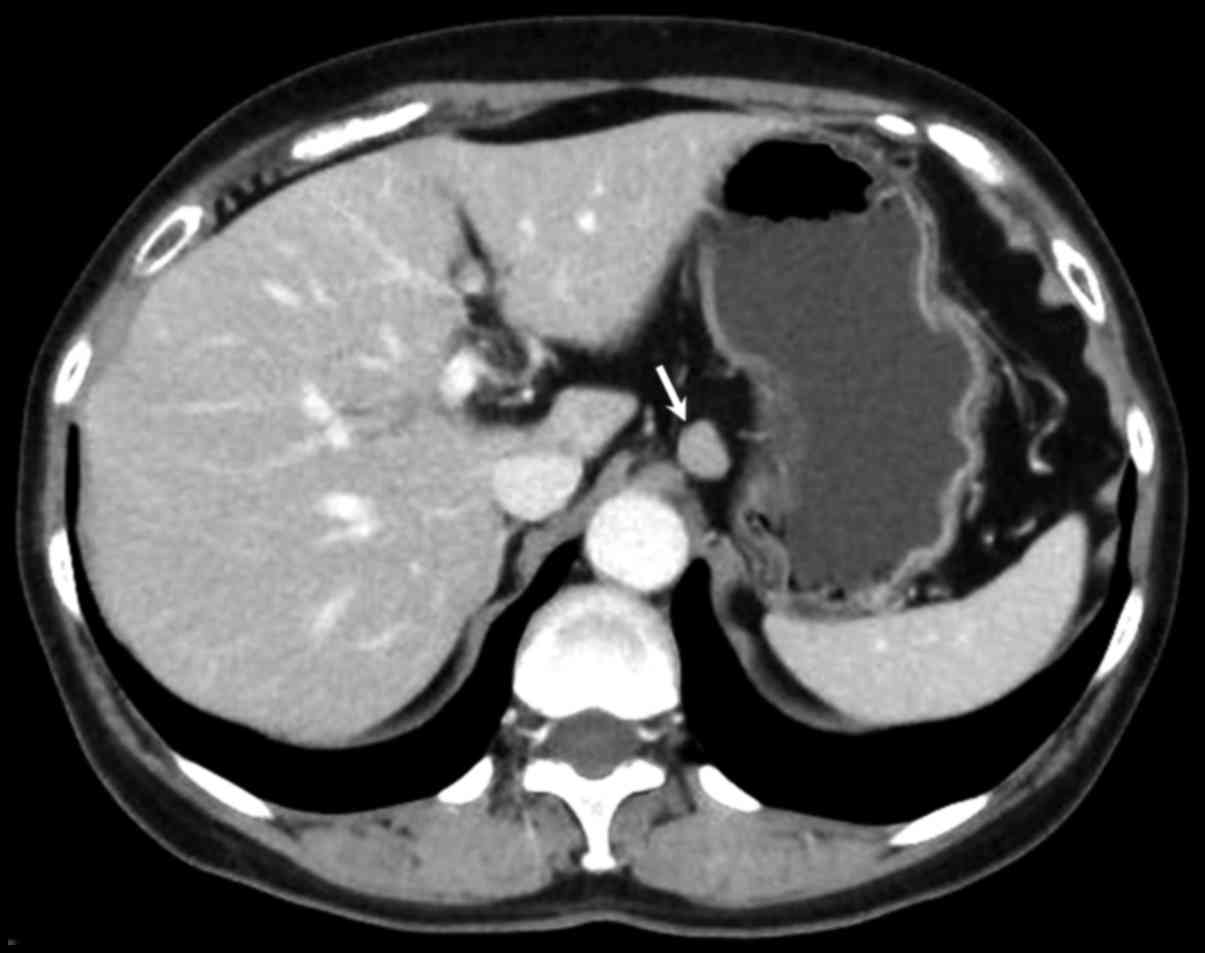
Abdominal contrast-enhanced computed tomography (CT)
revealed a well-defined mass with homogeneous enhancement
approximately 1.2 cm in diameter in the middle part of the stomach.
The CT analysis also revealed lymphadenopathy in the perigastric
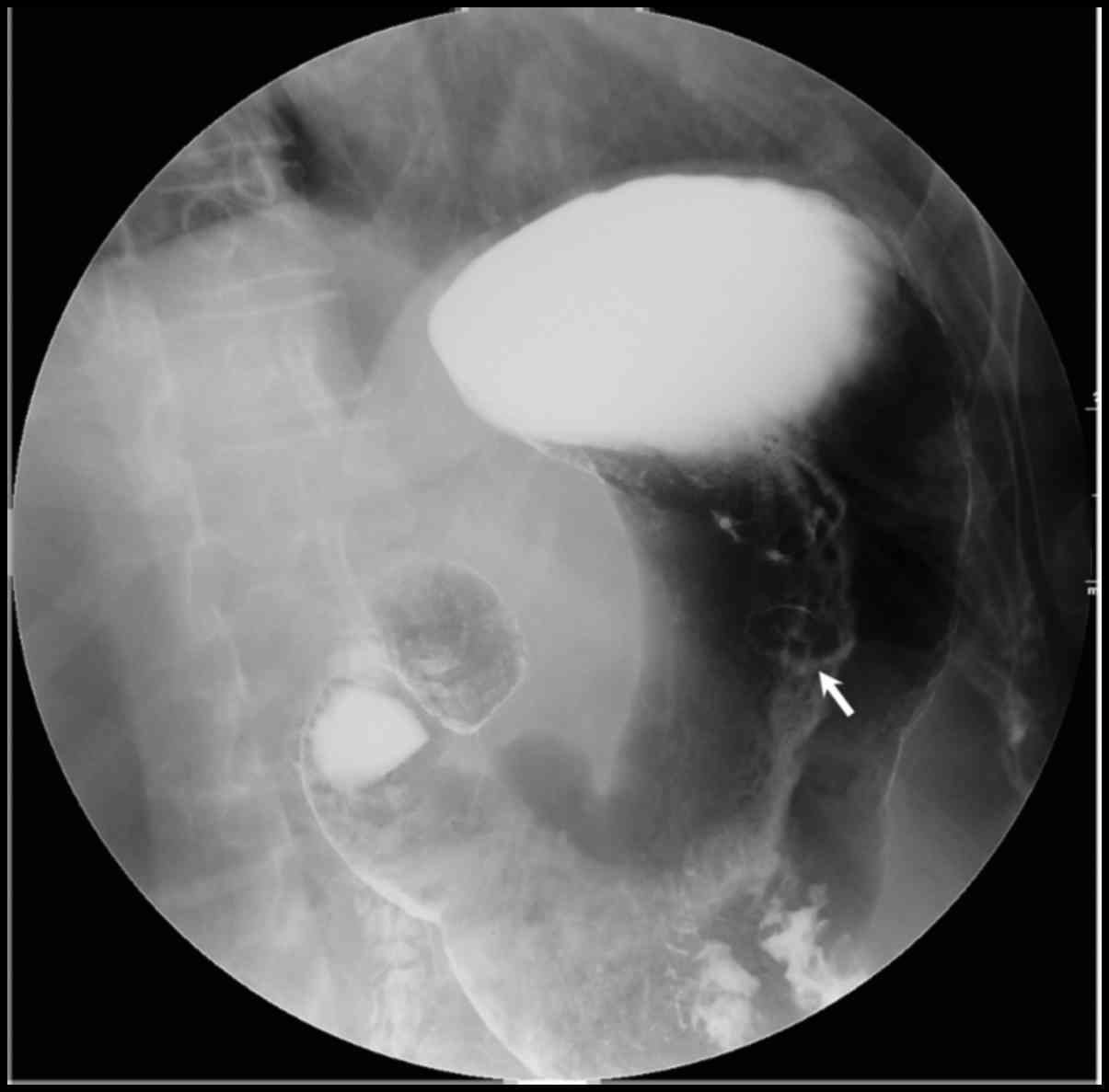
area with a maximum size of 1.4 cm in diameter (Fig. 3, arrow). Double-contrast upper
gastrointestinal imaging shows a round filling defect with central
collection of Barium measuring 1.2 cm with a clear margin in the
middle part of the stomach (Fig. 4,
arrow).
As a result of these investigations we suspected
that the patient had a gastric carcinoma with lymphoid stroma
accompanying lymph node metastases. The patient underwent
laparoscopic distal gastrectomy with reginal lymphadenectomy
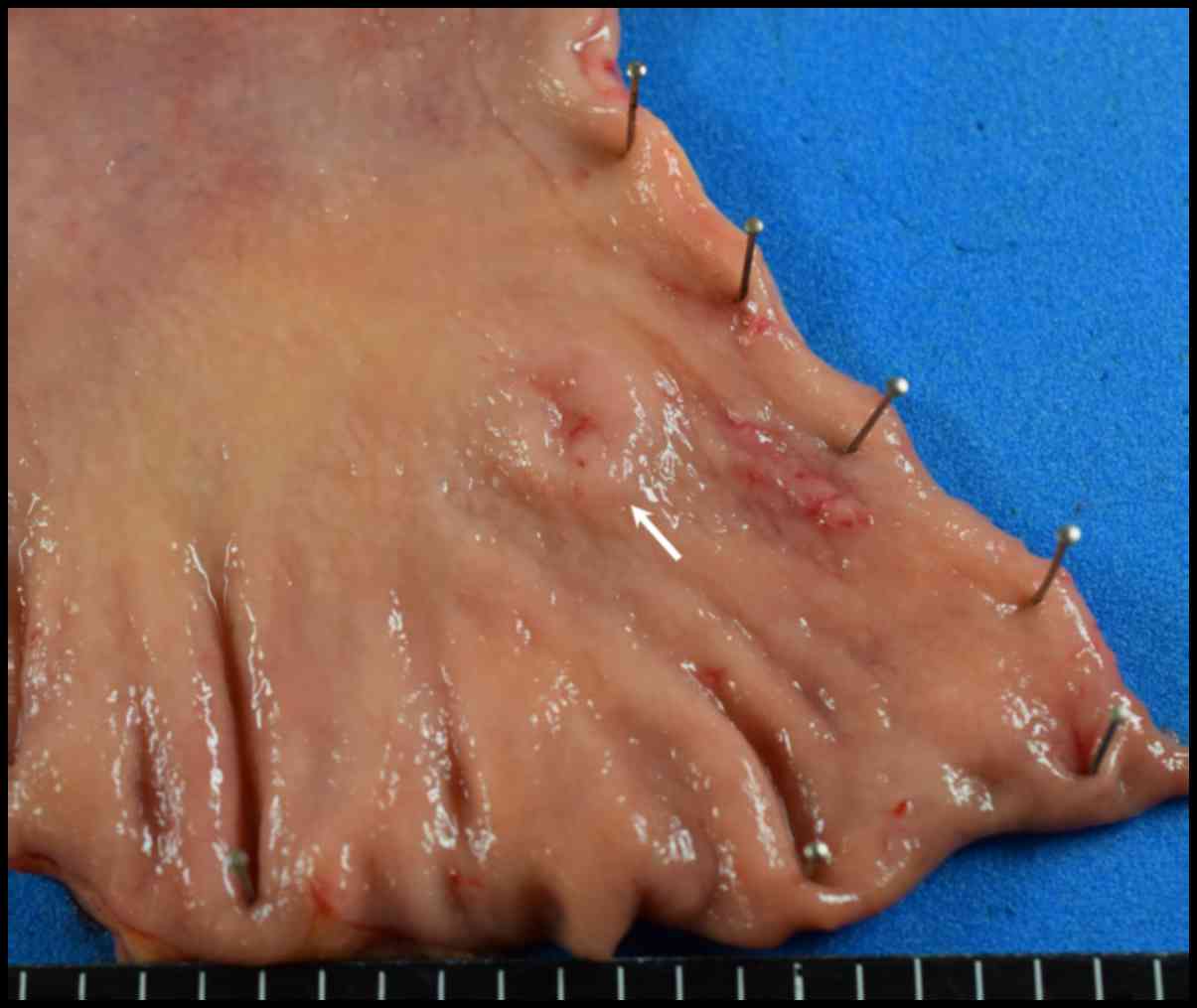
followed by Billroth I reconstruction. Macroscopic examination of
the resected specimen showed a slightly elevated lesion-like
submucosal tumor with a central depression measuring 1.2×1.2 cm
(Fig. 5, arrow).
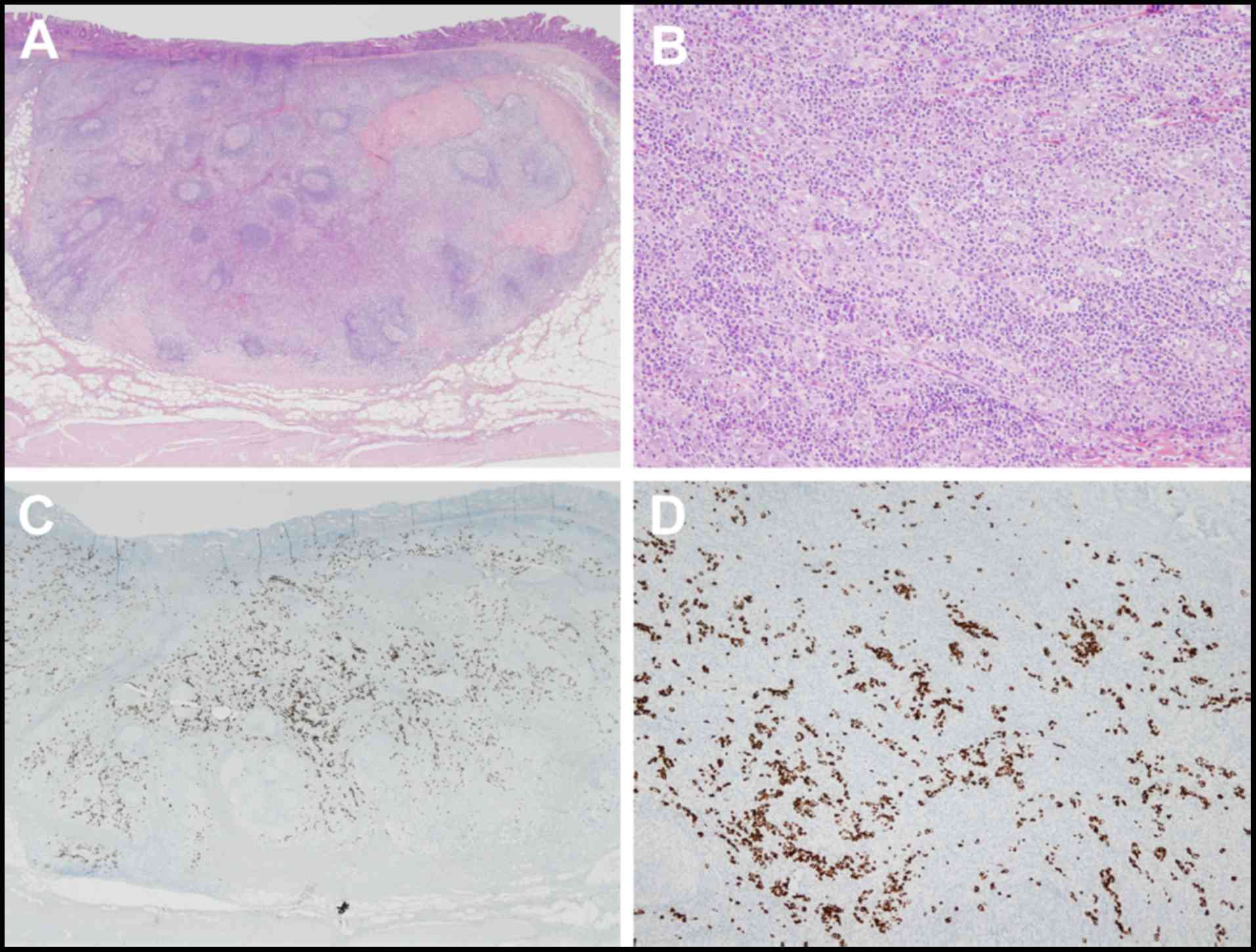
Microscopic examination of the patient specimens
showed a bulging mass consists of poorly differentiated
adenocarcinoma. There was expansive growth into the submucosa that
formed tubular structures and cancer nests with a submucosal depth
of invasion of 4,000 µm (Fig. 6A and
B). The tumor cells were positive for EBV-encoded RNA by in
situ hybridization (EBER-ISH; Fig.
6C and D), and the expression of Ki-67 in cancer cells was 25%.
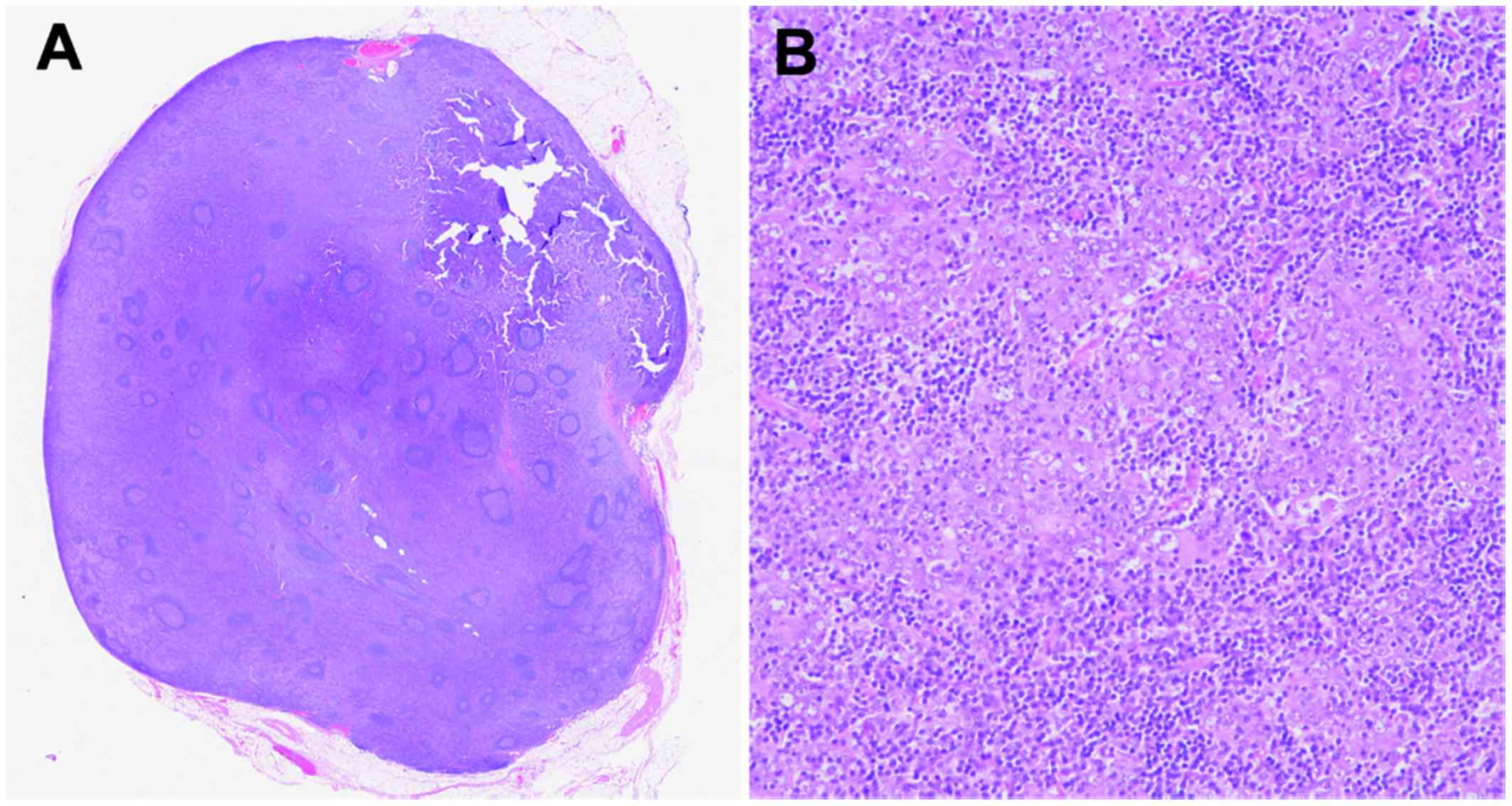
In addition, there was one lymph node metastasis in 13 dissected
lymph nodes, which was detected as lymphadenopathy in the CT
imaging but there was no lymphovascular infiltration. The lymph
node station number with metastasis was 3 according to anatomical
definition of Japanese classification of gastric carcinoma
(6), which was 2.0 cm in diameter,
consisting of diffuse spreading of adenocarcinoma in the lymph node
(Fig. 7A and B). The results of the
other immunohistochemical investigations of the primary gastric
cancer and metastatic lymph node showed positive immunostaining for
cytokeratin (AE1/AE3) and negative for CDX-2. Marked infiltration
of lymphoid cells was observed in the tumor stroma and these were
negative for EBER-ISH. The postoperative course was uneventful and
the patient has been well without evidence of recurrence for two
months following the operation.
Discussion
GCLS has distinct clinical characteristics that
occur in old age and predominantly in males. These tumors arise in
the cardia or middle portion of the stomach and have prominent
lymphocyte infiltration, particularly in the submucosa (7,8).
EBER-ISH is the gold standard for determining EBV infection in
histological sections. Carcinoma cells and dysplastic epithelial
cells are positive for EBV, but not the normal epithelium or
lymphoid stroma in EBV-associated GCLS. The estimate of EBV
positivity in gastric cancer is 8.7% overall according to a
meta-analysis, and the frequencies are not statistically different
among different anatomical locations (1). Although EBV is considered to be the
main cause of the lymphocytic response, the mechanism by which EBV
contributes to the carcinogenesis of gastric mucosa has not been
elucidated (9).
The rate of metastasis to the lymph node in
submucosal cancers with GCLS is significantly lower than in
conventional early gastric cancer, especially those involving an
EBV infection (2,8). The spread of tumors through the gastric
wall may be prevented by abundant lymphocytic reactions, which
regulate the anti-tumor effect of immunity, resulting in a more
favorable prognosis as a result of the immune response to the tumor
(8,9). An investigation of 41 GCLS in early
gastric cancer reported that there was no lymph node metastasis if
the submucosal depth of invasion was ≤2,000 µm from the muscularis
mucosa, while the incidence rate of lymph node metastasis was 28.6%
if the SM depth of invasion was >2,000 µm (8).
Regarding the association between the sizes of
gastric carcinoma and lymph node metastasis, Kim et al
reported that there was no lymph node metastasis in the tumors with
sizes <1.0 cm, and 2-dimensional tumor size was the only
significant risk factor for lymph node metastasis in the analysis
for 574 patients with differentiated minute submucosal cancer
(10). Shin et al reported
that tumor size was smaller in GCLS, 2.1 cm, than in no-GCLS, 3.1
cm, and only two patients with GCSL (3.4%) showed lymph node
metastasis in the analysis of 1696 patients with early gastric
cancer (11). Therefore, the lymph
node metastasis of small size GCLS as the present case seems to be
extremely rare.
In the present case, it was difficult to detect the
submucosal invasion during EGD without EUS, because the majority of
the lesion was a bulging mass with abundant lymphoid stroma that
was located in the submucosa by pathological investigation.
Standard radical gastrectomy with regional lymphadenectomy is
important, recommending in cases with submucosal invasion of tumor
cells to a depth of more than 2,000 µm, regardless of tumor size.
EUS seems to be an essential modality to estimate the depth of
tumor invasion in the diagnostic and therapeutic management of
GCLS.
Since GCLS is a rare disease, clinicians should
recognize the features of this entity to make an accurate diagnosis
and select the appropriate treatment. Further studies and
assessments of additional cases are required to establish
standardized recommendation criteria and management for this
entity.
References
|
1
|
Murphy G, Pfeiffer R, Camargo MC and
Rabkin CS: Meta-analysis shows that prevalence of Epstein-Barr
virus-positive gastric cancer differs based on sex and anatomic
location. Gastroenterology. 137:824–833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liang Q, Yao X, Tang S, Zhang J, Yau TO,
Li X, Tang CM, Kang W, Lung RW, Li JW, et al: Integrative
identification of Epstein-Barr virus-associated mutations and
epigenetic alterations in gastric cancer. Gastroenterology.
147:1350–1362.e4. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lim H, Park YS, Lee JH, Son DH, Ahn JY,
Choi KS, Kim DH, Choi KD, Song HJ, Lee GH, et al: Features of
gastric carcinoma with lymphoid stroma associated with Epstein-Barr
virus. Clin Gastroenterol Hepatol. 13:1738–1744.e2. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watanabe H, Enjoji M and Imai T: Gastric
carcinoma with lymphoid stroma. Its morphologic characteristics and
prognostic correlations. Cancer. 38:232–243. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Namikawa T, Kitagawa H, Iwabu J,
Okabayashi T, Sugimoto T, Kobayashi M and Hanazaki K:
Clinicopathological properties of the superficial spreading type
early gastric cancer. J Gastrointest Surg. 14:52–57. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Japanese Gastric Cancer Association, .
Japanese classification of gastric carcinoma: III English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi MG, Jeong JY, Kim KM, Bae JM, Noh JH,
Sohn TS and Kim S: Clinical significance of gastritis cystica
profunda and its association with Epstein-Barr virus in gastric
cancer. Cancer. 118:5227–5233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huh CW, Jung DH, Kim H, Kim H, Youn YH,
Park H, Kim JW, Choi SH, Noh SH and Kim JH: Clinicopathologic
features of gastric carcinoma with lymphoid stroma in early gastric
cancer. J Surg Oncol. 114:769–772. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang KH, Wang RF, Yang MH, Wu CW, Fang
WL, Li AF, Chi CW and Kao HL: Advanced gastric cancer patients with
lymphoid stroma have better survival than those without. J Surg
Oncol. 107:523–528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim TJ, Lee H, Min YW, Min BH, Lee JH, Kim
KM, Kim MJ, Kim K, Rhee PL and Kim JJ: One-dimensional and
2-dimensional tumor size measurement for prediction of lymph node
metastasis in differentiated early gastric cancer with minute
submucosal invasion. Gastrointest Endosc. 85:730–736. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shin DH, Kim GH, Lee BE, Lee JW, Ha DW,
Jeon HK, Baek DH, Song GA, Ahn SJ and Park DY: Clinicopathologic
features of early gastric carcinoma with lymphoid stroma and
feasibility of endoscopic submucosal dissection. Surg Endosc. April
13–2017. View Article : Google Scholar : (Epub ahead of
print). View Article : Google Scholar
|