Introduction
Chronic obstructive pulmonary disease (COPD) is the
fourth leading cause of death and results in >2.5 million deaths
per year worldwide (1). Lung cancer
is frequently seen in patients with COPD and has been found to be
the most frequent cause of death in patients with mild COPD
(2). In COPD cohort studies, the
incidence ratios for lung cancer ranged from 4.2 (3) to 16.7 per 1,000 person-years (4). It is crucial to improve the management
of both COPD and lung cancer.
Although lung cancer should be treated appropriately
according to the guidelines, lung cancer patients with COPD are at
high risk when undergoing pulmonary resection because of their
reduced pulmonary function and increased postoperative morbidity
(5,6). The perioperative management of patients
with COPD and lung cancer, especially with preoperative
rehabilitation and bronchodilators, is important for the
improvement of surgical outcomes. Bronchodilators are one of the
therapeutic options for stable COPD (7). Three types of bronchodilators are
commercially available: inhaled β2-agonists, anticholinergics, and
corticosteroids. Combining bronchodilators with different
mechanisms and durations of action may increase the degree of
bronchodilation while producing equivalent or lesser side effects
(8). Short-term combination therapy
using formoterol and tiotropium has been shown to have a bigger
impact on the forced expiratory volume in one second
(FEV1) than single components for COPD without lung
cancer surgery (9,10). Moreover, a few studies have shown
that combinations of a long-acting β2-agonist (LABA) and a
long-acting muscarinic antagonist (LAMA) produced a significant
increase in lung function (11,12).
Previous studies reported the effectiveness of
perioperative LAMA therapy in patients with COPD requiring lung
cancer surgery (13,14), while the effects of LAMA/LABA
combination therapy in patients with COPD requiring lung cancer
surgery are currently unclear. We hypothesized that the addition of
LABA to preoperative treatment with LAMA would optimize
preoperative lung function and reduce the risk for postoperative
pulmonary complications. The objectives of this retrospective study
were the following: i) to compare the effectiveness of
perioperative LAMA/LABA therapy and LAMA therapy on lung function
and the postoperative complications of patients with
moderate-to-very severe COPD requiring lung cancer surgery, and ii)
to examine the impact of the severity of COPD on overall survival
after lung cancer surgery.
Materials and methods
Study design and population
From January 2005 to December 2015, 920 consecutive
patients with primary lung cancer underwent surgical resection at
our institution. Of these patients, 82 patients with
moderate-to-severe COPD who required lobectomy were enrolled in a
retrospective study. The severity of COPD was classified according
to the Global Initiative for Chronic Obstructive Lung Disease
(GOLD) criteria (7). We started
preoperative LAMA/LABA therapy for all patients with COPD from 2013
and have performed this treatment for thirteen patients. Patients
received LAMA therapy, as historical control according to the same
inclusion and exclusion criteria, are 19 patients from 2005 to
2012. Patients meeting the following exclusion criteria were
eliminated from the present analysis: No preoperative inhaled
therapy (n=25); a medical history of previous treatment for COPD
(n=10); a medical history of inhaled steroids (n=6); no lung
function test after inhaled therapy (n=5); preoperative LAMA
therapy from 2013 (n=3) and no perioperative rehabilitation (n=1).
The improvement of preoperative pulmonary function and the
postoperative morbidity were compared between the patients with
preoperative LAMA and LAMA/LABA therapy. All patients underwent
chest and abdominal computed tomography (CT) scans, brain magnetic
resonance imaging and fluoro-2-deoxyglucose positron emission
tomography/CT for clinical staging for lung cancer. The following
parameters were assessed from the medical records: Patient age,
gender, pathological stage, histology, surgical procedure,
pulmonary function, GOLD COPD stages, and prognosis. This study was
reviewed and approved by the Institutional Review Board
(M16021).
Smoking cessation
All patients in this study were confirmed to have
ceased smoking more than 2 weeks before surgery.
Preoperative bronchodilators
In the LAMA group, the patients received inhaled
tiotropium bromide (n=19) from >2 weeks before surgery to at
least 1 month after surgery without interruption.
In the LAMA/LABA group, the patients received
inhaled tiotropium bromide and LABA [formoterol (n=8), indacaterol
(n=4)] or combined LABA/LAMA [indacaterol/glycopyrrolate (n=1)]
from >2 weeks before surgery to at least 1 month after surgery
without interruption.
Postoperative complications
Postoperative pulmonary complications were defined
as: i) pneumonia, defined by the presence of at least three of the
following criteria: persistent lung infiltrate on chest X-ray,
fever >38.3°C, white blood cell count >10,000 mm3
or <3,000 mm3; ii) acute respiratory failure, defined
as postoperative ventilator dependence >12 h or reintubation for
mechanical ventilation; iii) chronic respiratory failure, defined
as the need for continuous oxygen therapy for more than 1 month
after discharge. Postoperative cardiovascular complications were
defined as arrhythmias (atrial fibrillation, paroxysmal
supraventricular tachycardia, ventricular tachycardia), angina
pectoris, myocardial infarction, congestive heart failure and
thromboembolic events. Finally, surgical mortality was defined as
death within 30 days following surgery.
Statistical analysis
The data are presented as the mean ± standard
deviation (SD) or as medians with interquartile ranges. Categorical
variables are shown as the percentage of the sample. Comparisons
between the two groups were assessed by Student's t-test for
normally distributed variables or by the Mann-Whitney U test for
non-normally distributed variables. Differences were considered to
be statistically significant when the P-value<0.05. Overall
survival (OS) was defined as the time from the date of surgery
until the date of the last follow-up for living patients or until
death. Survival curves were prepared using the Kaplan-Meier method
and were compared univariately using the log-rank test. To
determine which factors were significantly associated with
survival, a multivariate analysis using a Cox proportional hazards
model was performed. All statistical analyses were performed using
JMP version 11.0 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
The clinical characteristics of patients are shown
in Table I. There was no significant
difference between patients in the LAMA group and the LAMA/LABA
group with regard to age, sex, pathological stage (AJCC 8th),
Charlson index, histology, SpO2, PaO2,
pulmonary function, and GOLD COPD stages.
 | Table I.Patients characteristics. |
Table I.
Patients characteristics.
| Variables | LABA + LAMA group
(n=13) | LAMA group
(n=19) | P-value |
|---|
| Age, years | 70.9±1.8 | 70.8±1.5 | 0.97 |
| Sex |
|
|
|
| Male | 12 (92%) | 17 (89%) | 0.79 |
|
Female | 1 (8%) | 2 (11%) |
|
| Pathological stage
(AJCC 8th), (I/II/III) | 7/3/3 | 11/3/5 | 0.87 |
| Charlson index | 3.30±0.2 | 3.47±0.1 | 0.42 |
| Histology,
(Sq/Ad/LCNEC) | 7/5/1 | 11/6/2 | 0.91 |
| SpO2,
% | 96.2±0.3 | 96.3±0.3 | 0.85 |
| PaO2,
Torr | 82.8±2.4 | 81.3±2.0 | 0.64 |
| Pulmonary
function |
|
|
|
|
FEV1/FVC, % | 53.8±3.5 | 52.3±2.5 | 0.71 |
|
FEV1, l | 1.47±0.11 | 1.33±0.09 | 0.34 |
|
FEV1, %
predicted | 66.1±3.26 | 59.5±2.63 | 0.12 |
| %
DLCO | 81.2±7.20 | 79.3±5.71 | 0.84 |
| COPD-GOLD stages,
(II/III) | 12/1 | 15/4 | 0.31 |
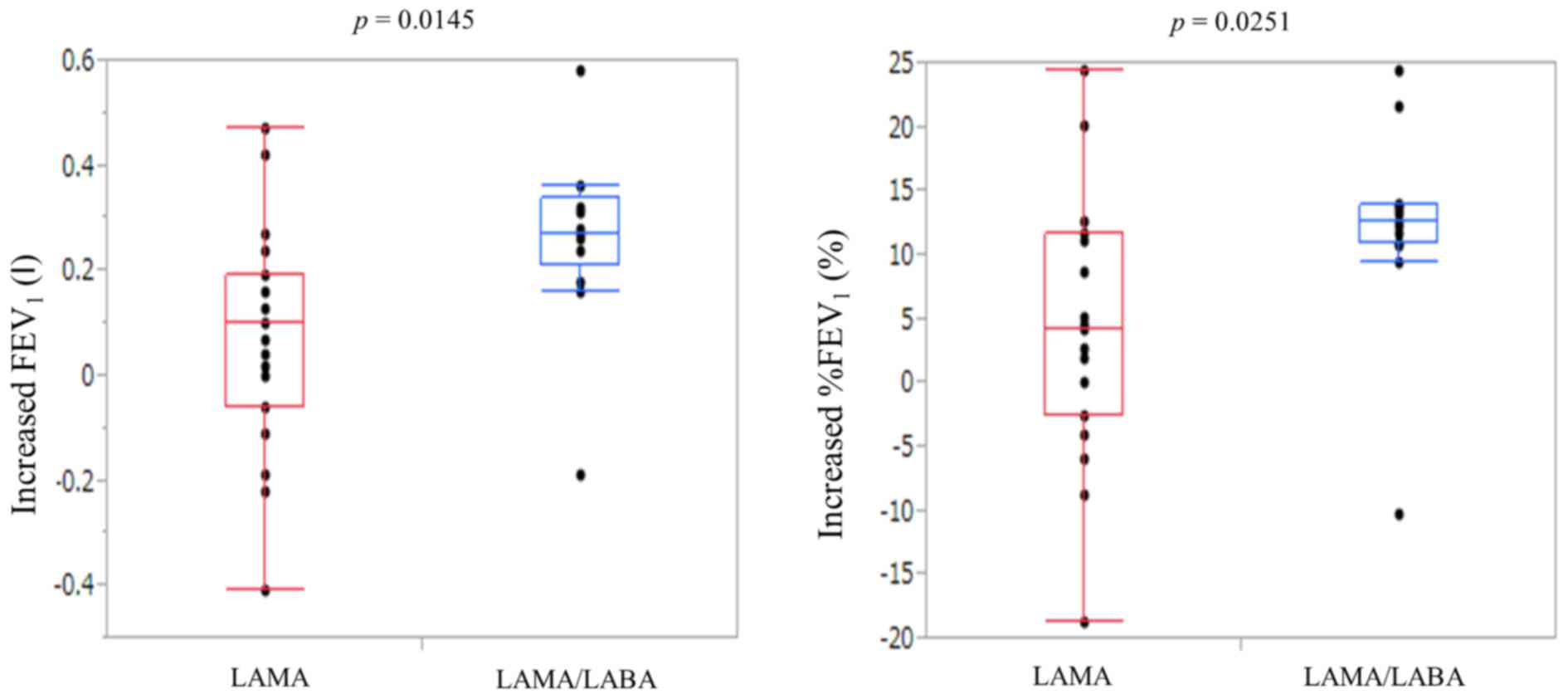
Efficacy after bronchodilators
The treatment effects after LAMA therapy or
LAMA/LABA therapy are shown in Table
II. LAMA therapies resulted in the improvement of
FEV1 (post-therapy FEV1 1.41±0.09 l) and
%FEV1 (post-therapy %FEV1 63.7±2.95%).
LAMA/LABA therapies resulted in the improvement of FEV1
(post-therapy FEV1 1.73±0.12 l) and %FEV1
(post-therapy %FEV1 78.3±3.74%). The increases in
FEV1 and %FEV1 were significantly higher for
LAMA/LABA therapy than LAMA (post-therapy-pre-therapy
FEV1 0.26±0.05 vs. 0.07±0.05 l, P=0.0145;
post-therapy-pre-therapy %FEV1 12.2±2.53 vs. 4.21±2.16%,
P=0.0251, respectively) (Fig. 1).
More patients in the LAMA/LABA group had a marked improvement of
>10% in %FEV1 after bronchodilators than patients in
the LAMA group (85 vs. 32%, P=0.0046).
 | Table II.Measurements after
bronchodilators. |
Table II.
Measurements after
bronchodilators.
| Measurement | LAMA + LABA group
(n=13) | LAMA group
(n=19) | P-value |
|---|
| FEV1,
l | 1.73±0.12 | 1.41±0.09 | 0.0310 |
| FEV1, %
predicted | 78.3±3.74 | 63.7±2.95 | 0.0037 |
| Increased
FEV1, l | 0.26±0.05 | 0.07±0.05 | 0.0145 |
| Increased
%FEV1 Improvement of >10% in FEV1 (%
predicted) (% predicted) | 12.2±2.53 | 4.21±2.16 | 0.0251 |
|
Yes | 11 (85%) | 6 (32%) |
|
| No | 2 (15%) | 13 (68%) | 0.0046 |
Surgical treatments
The surgical procedures performed were thoracotomy
in 6 (19%) and video-assisted thoracic surgery in 26 (81%). Mean
lengths of surgery were 306 min, and mean perioperative blood loss
was 309 ml.
Postoperative cardiopulmonary
complications and mortality
Postoperative cardiopulmonary complications are
shown in Table III. The incidence
of postoperative pneumonia was significantly lower in the LAMA/LABA
group than in the LAMA group (0 vs. 26%, P=0.044). There was no
significant difference between patients in the LAMA/LABA group and
the LAMA group with regard to pulmonary complications (15 vs. 42%,
P=0.109), acute respiratory failure (0 vs. 11%, P=0.227), chronic
respiratory failure (15 vs. 32%, P=0.299), cardiovascular
complications (23 vs. 16%, P=0.604), atrial fibrillation (15 vs.
16%, P=0.975). The overall surgical mortality was 0%.
 | Table III.The postoperative complications. |
Table III.
The postoperative complications.
| Complications | LABA + LAMA group
(n=13) (%) | LAMA (n=19)
(%) | P-value |
|---|
| Pulmonary
complications | 2 (15) | 8 (42) | 0.109 |
|
Pneumonia | 0 (0) | 5 (26) | 0.044 |
| Acute
respiratory failure | 0 (0) | 2 (11) | 0.227 |
| Chronic
respiratory failure | 2 (15) | 6 (32) | 0.299 |
| Cardiovascular
complications | 3 (23) | 3 (16) | 0.604 |
| Atrial
fibrillation | 2 (15) | 3 (16) | 0.975 |
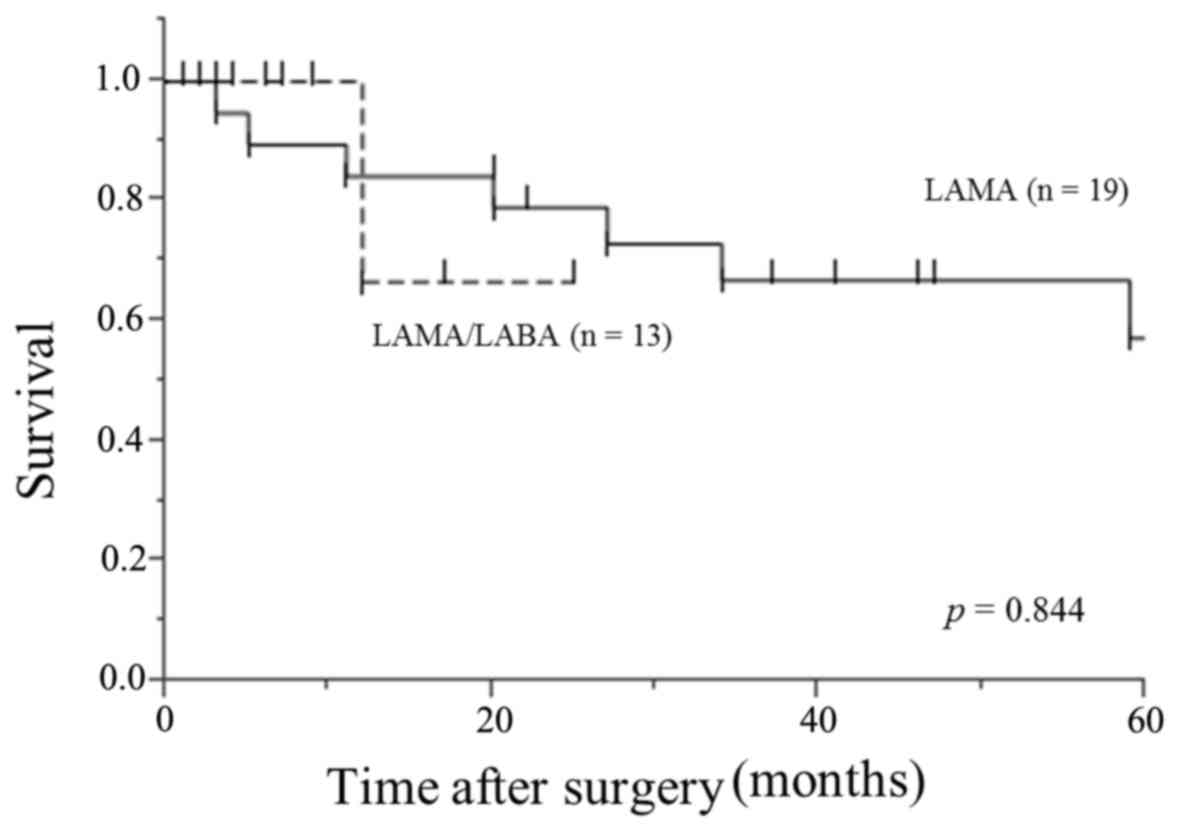
Overall survival in patients with
moderate-to-severe COPD requiring lung cancer surgery
The median follow-up after surgery was 20 months
(range, 1 to 121). The 5-year survival rate in this study was 57%.
There was no significant difference between patients in the
LAMA/LABA group and the LAMA group with regard to overall survival
(P=0.844) (Fig. 2).
Discussion
The present study had two main findings. First, in
this retrospective study of patients with moderate-to-severe COPD
requiring lung cancer surgery, inhaled LAMA/LABA therapy was
associated with greater improvements in preoperative pulmonary
function than LAMA therapy. Second, LAMA/LABA therapy significantly
reduced the incidence of postoperative pneumonia compared with LAMA
therapy.
In patients with COPD requiring lung cancer surgery,
the improvement of perioperative lung function is very crucial. In
a retrospective study of patients with mild-to-severe COPD
requiring lung cancer surgery, 2 weeks of preoperative treatment
with tiotropium significantly improved respiratory symptoms and
pulmonary function as reflected by a 226-ml increase in
FEV1 (13,14). In a prospective study of patients
with COPD requiring lung cancer surgery, 1 week of preoperative
treatment with inhaled tiotropium/formoterol/budenoside improved
pulmonary function significantly more than tiotropium/formoterol
(an increase in FEV1 of 310 vs.100 ml, respectively)
(15). In the present study, 2–4
weeks of preoperative treatment with LAMA/LABA therapy improved
pulmonary function significantly more than LAMA (an increase in
FEV1 of 260 vs. 90 ml, respectively). Moreover, in 85%
of the LAMA/LABA group, an improvement of >10% in
%FEV1 (% predicted) after treatment was achieved. These
results suggest that perioperative LAMA/LABA therapy would help to
achieve more curative resections in patients with COPD requiring
lung cancer surgery.
COPD is an independent risk factor for morbidity and
mortality in patients with lung cancer. Furthermore, patients with
COPD have a fourfold increased risk of developing postoperative
complications, mainly pulmonary complications (15,16). Leo
et al reported that up to 50% of patients with COPD develop
postoperative COPD exacerbations after lung resection (17). Therefore, the perioperative
management of patients with COPD and lung cancer is an important
issue. Recently, several studies have reported that preoperative
bronchodilators prevented postoperative complications in patients
with COPD requiring lung cancer surgery. Bolukbas et al
reported that adding budenoside to tiotropium and formoterol led to
less respiratory complications (11 vs. 43%, P=0.044) in the
postoperative period (15). Nojiri
et al reported that tiotropium prevented not only
respiratory complications (2 vs. 18%, P=0.018) but also
cardiovascular complications (16 vs. 33%, P=0.030) in patients with
COPD requiring lung cancer surgery (14) because tiotropium improved the left
ventricular diastolic function in the chronic phase after pulmonary
resection (18). However, Ueda et
al reported that preoperative treatment with tiotropium for ≥7
days improved lung function prior to thoracic surgery, but no
effect on respiratory complications was seen (19). In the present study, adding LABA to
LAMA significantly reduced the incidence of postoperative pneumonia
compared with LAMA alone (0 vs. 26%, P=0.044) and was not
associated with more cardiovascular complications in the
postoperative period (23 vs. 16%, P=0.604). We suspected that
LAMA/LABA therapy improved the lung function and, therefore,
reduced sputum retention, which accounts for the reduction in
postoperative pneumonia.
This study had several limitations. Because our data
were collected in a single center including a very limited number
of patients for a long period and reviewed retrospectively, the
study population consisted of a heterogeneous group of subjects.
LABA became commercially available later than LAMA, therefore
LABA/LAMA group patients distributed later than LAMA group
patients. In the future, a multicenter prospective study is
required for conclusion. In addition, this study is limited by the
use of pre-bronchodilator spirometry. Although the diagnosis and
staging of COPD should be based on post-bronchodilator spirometry
as the gold standard, some guidelines recommend pre-bronchodilator
spirometry because the reversibility testing is considered
impractical (20).
In conclusions, our study showed that preoperative
LAMA/LABA therapy was associated with greater improvements in
preoperative pulmonary function than LAMA; thus, more patients with
COPD who required lung cancer surgery received optimal oncological
therapy. These improvements in preoperative function tended to
reduce the incidence of pneumonia in the postoperative period.
These results may lead to not only larger improvements in
FEV1 but also less postoperative pneumonia by
encouraging the addition of inhaled LABA to LAMA in this patient
population. Perioperative LAMA/LABA therapy would help to achieve
more curative resections and improve the surgical outcomes in
patients with COPD requiring lung cancer surgery.
Acknowledgements
Not applicable.
Funding
This study was supported in part by JSPS KAKENHI
[grant no. (C) JP15K10272].
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AI designed and performed the study. TM and HO
analyzed all data and TM wrote the draft manuscript. YH, SK, YA,
KI, KS, SE, SH and AI contributed to the acquisition of data for
the present study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All participants gave individual consent to
participation and for the use of all data collected during the
study. This study was reviewed and approved by the Institutional
Review Board (M16021).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
COPD
|
chronic obstructive pulmonary
disease
|
|
LAMA
|
long-acting muscarinic antagonist
|
|
LABA
|
long-acting β2-agonist
|
|
FEV1
|
forced expiratory volume in one
second
|
|
GOLD
|
Global Initiative for Chronic
Obstructive Lung Disease
|
|
CT
|
computed tomography
|
|
SD
|
standard deviation
|
|
OS
|
overall survival
|
References
|
1
|
Mannino DM and Buist AS: Global burden of
copd: Risk factors, prevalence, and future trends. Lancet.
370:765–773. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anthonisen NR, Connett JE, Enright PL and
Manfreda J: Lung Health Study Research G. Hospitalizations and
mortality in the lung health study. Am J Respir Crit Care Med.
166:333–339. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rabe KF, Hurd S, Anzueto A, Barnes PJ,
Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R,
van Weel C, et al: Global strategy for the diagnosis, management
and prevention of chronic obstructive pulmonary disease: Gold
executive summary. Am J Respir Crit Care Med. 176:532–555. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Torres JP, Marín JM, Casanova C, Cote
C, Carrizo S, Cordoba-Lanus E, Baz-Dávila R, Zulueta JJ,
Aguirre-Jaime A, Saetta M, et al: Lung cancer in patients with
chronic obstructive pulmonary disease- incidence and predicting
factors. Am J Respir Crit Care Med. 184:913–919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Win T, Jackson A, Sharples L, Groves AM,
Wells FC, Ritchie AJ and Laroche CM: Relationship between pulmonary
function and lung cancer surgical outcome. Eur Respir J.
25:594–599. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sekine Y, Kesler KA, Behnia M,
Brooks-Brunn J, Sekine E and Brown JW: COPD may increase the
incidence of refractory supraventricular arrhythmias following
pulmonary resection for non-small cell lung cancer. Chest.
120:1783–1790. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
GOLD: Global Initiative for Chronic
Obstructive Lung Disease (GOLD) 2016 Global strategy for diagnosis,
management and prevention of COPD. http://www.goldcopd.org/
|
|
8
|
Vogelmeier C, Kardos P, Harari S, Gans SJ,
Stenglein S and Thirlwell J: Formoterol mono- and combination
therapy with tiotropium in patients with copd: A 6-month study.
Respir Med. 102:1511–1520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tashkin DP, Pearle J, Iezzoni D and
Varghese ST: Formoterol and tiotropium compared with tiotropium
alone for treatment of COPD. COPD. 6:17–25. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Noord JA, Aumann JL, Janssens E,
Smeets JJ, Verhaert J, Disse B, Mueller A and Cornelissen PJ:
Comparison of tiotropium once daily, formoterol twice daily and
both combined once daily in patients with COPD. Eur Respir J.
26:214–222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bateman ED, Ferguson GT, Barnes N,
Gallagher N, Green Y, Henley M and Banerji D: Dual bronchodilation
with QVA149 versus single bronchodilator therapy: The SHINE study.
Eur Respir J. 42:1484–1494. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Donohue JF, Maleki-Yazdi MR, Kilbride S,
Mehta R, Kalberg C and Church A: Efficacy and safety of once-daily
umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med.
107:1538–1546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobayashi S, Suzuki S, Niikawa H, Sugawara
T and Yanai M: Preoperative use of inhaled tiotropium in lung
cancer patients with untreated COPD. Respirology. 14:675–679. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nojiri T, Inoue M, Yamamoto K, Maeda H,
Takeuchi Y, Nakagiri T, Shintani Y, Minami M, Sawabata N, Okumura
M, et al: Inhaled tiotropium to prevent postoperative
cardiopulmonary complications in patients with newly diagnosed
chronic obstructive pulmonary disease requiring lung cancer
surgery. Surg Today. 44:285–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bolukbas S, Eberlein M, Eckhoff J and
Schirren J: Short-term effects of inhalative
tiotropium/formoterol/budenoside versus tiotropium/formoterol in
patients with newly diagnosed chronic obstructive pulmonary disease
requiring surgery for lung cancer: A prospective randomized trial.
Eur J Cardiothorac Surg. 39:995–1000. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lopez-Encuentra A, Astudillo J, Cerezal J,
Gonzalez-Aragoneses F, Novoa N and Sánchez-Palencia A: Bronchogenic
Carcinoma Cooperative Group of the Spanish Society of Pneumology
and Thoracic Surgery (GCCB-S): Prognostic value of chronic
obstructive pulmonary disease in 2994 cases of lung cancer. Eur J
Cardiothorac Surg. 27:8–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leo F, Venissac N, Pop D, Solli P, Filosso
P, Minniti A, Radice D, Mouroux J, Spaggiari L, Pastorino U, et al:
Postoperative exacerbation of chronic obstructive pulmonary
disease. Does it exist? Eur J Cardiothorac Surg. 33:424–429. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nojiri T, Yamamoto K, Maeda H, Takeuchi Y,
Funakoshi Y, Maekura R and Okumura M: Effects of inhaled tiotropium
on left ventricular diastolic function in chronic obstructive
pulmonary disease patients after pulmonary resection. Ann Thorac
Cardiovasc Surg. 18:206–211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ueda K, Tanaka T, Hayashi M and Hamano K:
Role of inhaled tiotropium on the perioperative outcomes of
patients with lung cancer and chronic obstructive pulmonary
disease. Thorac Cardiovasc Surg. 58:38–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Halpin D: Nice guidance for COPD. Thorax.
59:181–182. 2004. View Article : Google Scholar : PubMed/NCBI
|