Introduction
Hypereosinophilic syndrome is a rare hematological
condition characterized by the overproduction of eosinophils
(>1.5×109/l) by the bone marrow in an idiopathic
manner, which is associated with tissue infiltration and lesions to
multiple organs, with a duration of >6 months, or on two
separate occasions, excluding other causes of increased eosinophil
count, including medication, allergies, viral infection, parasitic
diseases or cancer, among others (1–3). The
term ‘hypereosinophilic syndrome’ was introduced in 1968 by Hardy
and Anderson after the evaluation of three clinical cases
associated with the production and maintenance of high levels of
eosinophils without other apparent causes (4). Since then, case reports with
descriptions of the complications have been published in the
literature, although it is not yet possible to accurately estimate
the prevalence and incidence of the disease (5).
According to new criteria, signs or symptoms of
organ involvement are not required for diagnosis, since the
patients may be initially asymptomatic and develop symptoms of
organ involvement as the disease progresses (6).
The mechanism underlying eosinophilic
hyperproliferation remains unknown, but the pathophysiology is
dependent on the deregulation of the production of eosinophils
(7). Activated eosinophils may
infiltrate various tissues, releasing a wide variety of mediators
inducing damage or dysfunction (3).
New studies are being conducted, and remarkable progress has been
achieved, including the identification of the FIP1L1-PDGFRA fusion,
the most frequent mutation found in hypereosinophilic syndrome
(1,6).
A distinction should be made between the disorder
characterized by a local increase in eosinophils without a
progressive increase of eosinophils in the peripheral blood, with
deposition in a single organ, as is the case in eosinophilic asthma
or eosinophilic dermatitis, and the hypereosinophilic syndrome,
which may affect one isolated organ or several systems, as the
production of eosinophils by the bone marrow is deregulated
(5). The systemic involvement of
this disease occurs through the activation of eosinophils, a
tendency for thrombotic events, the release of the eosinophil
granule contents and protein deposition (3,8,9).
Cardiac involvement associated with
hypereosinophilic syndrome is frequent, observed in ~50-60% of the
cases (10). In 1936, Loeffler first
described the association between eosinophilia and active carditis,
with the latter becoming known as Loeffler endocarditis, which is
characterized by eosinophilic myocarditis with a tendency for
endomyocardial fibrosis. Loeffler endocarditis, or eosinophilic
endocardial disease, or endomyocardial fibrosis, is the predominant
form of cardiac involvement. The damage to the endocardium and
myocardium is caused by toxic effects associated with the
degradation of eosinophils, and defines tissue infiltration as a
precipitating factor of local inflammation and subsequent fibrosis
(10).
In hypereosinophilic syndrome, treatment is not
always initiated at the time of diagnosis, with patients evaluated
on an individual basis in accordance with the characterization and
eosinophil count, the evidence of systemic involvement and/or the
rate of disease progression (3,5).
Therapeutic decisions are mainly based on the
presence of the FIP1L1-PDGFRA fusion. Patients who test positive
for FIP1L1-PDGFRA respond to treatment with tyrosine kinase
inhibitors, such as imatinib (4,5), whereas
patients who test negative for FIP1L1-PDGFRA should initially
receive treatment with corticosteroids, with the dose depending on
the clinical symptoms and laboratory findings. As a second choice,
the use of hydroxyurea is recommended for these patients, and
initial therapy combined with the use of corticosteroids may also
be recommended, with its efficacy depending on the successful
inhibition of eosinophil production (5,11).
The first line of treatment for patients without the
FIP1L1-PDGFRA mutation includes the administration of
corticosteroids (5), whereas when
the mutation is present, the use of imatinib is the first choice of
treatment (4,5). Cardiac involvement is frequent, and is
most commonly observed at advanced stages of endomyocardial
fibrosis (10). To the best of our
knowledge, this is the first case of a complete and sustained
response to imatinib in a patient negative for FIP1L1-PDGFRA, who
had also developed heart failure with development of isolated
mitral regurgitation, without evidence of associated restrictive
heart disease and/or evidence of associated left ventricular
hypertrophy.
Case report
A 53-year-old male patient, resident in the Middle
West region of Santa Catarina, Brazil, presented at the Emergency
Department of the Hospital Universitário Santa Terezinha (HUST) in
December 2015 with complaints of dyspnea associated with paroxysmal
nocturnal dyspnea and fatigue on moderate and mild exertion,
starting 2 months earlier and progressively worsening. When
describing medical history, the patient reported suffering from
diagnosed hypereosinophilic syndrome and receiving treatment with
hydroxyurea (2,500 mg/day) for ~6 months; he refuted the presence
of other comorbidities or the continuous use of other medication.
The patient was followed up by a local hematologist, and the
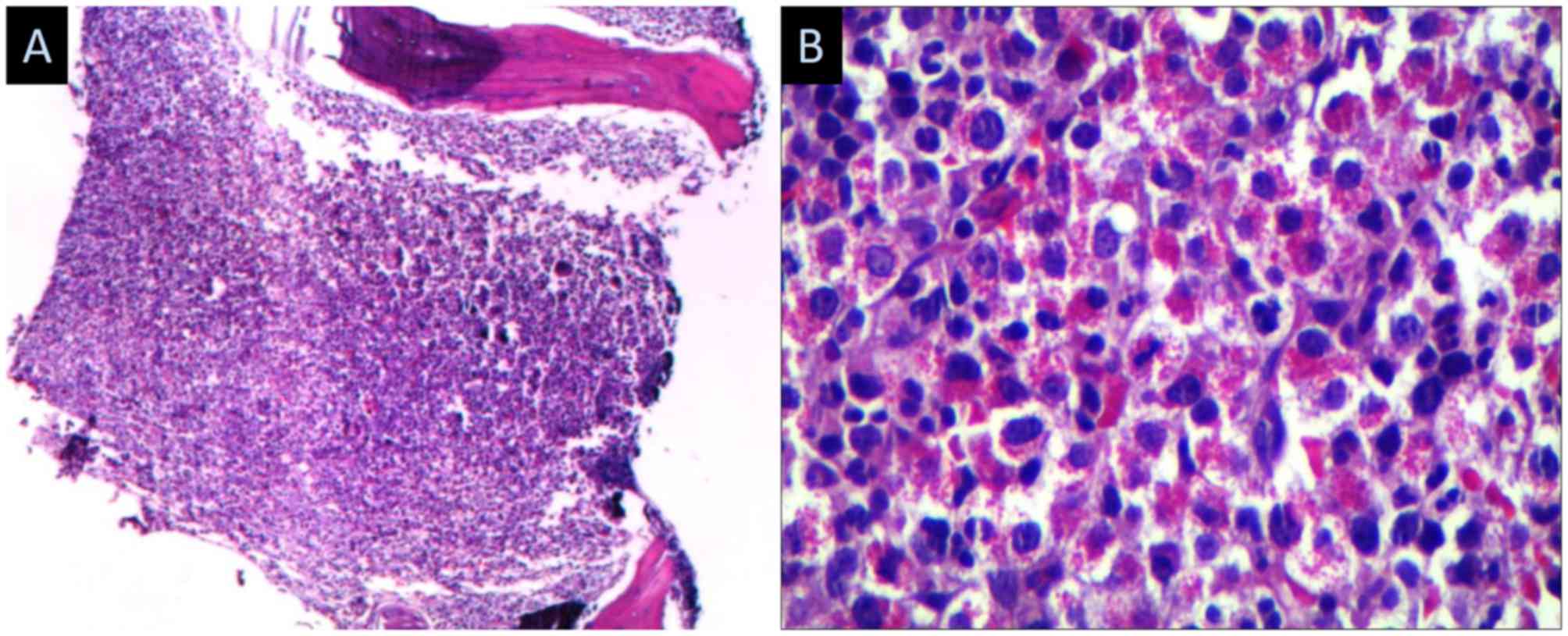
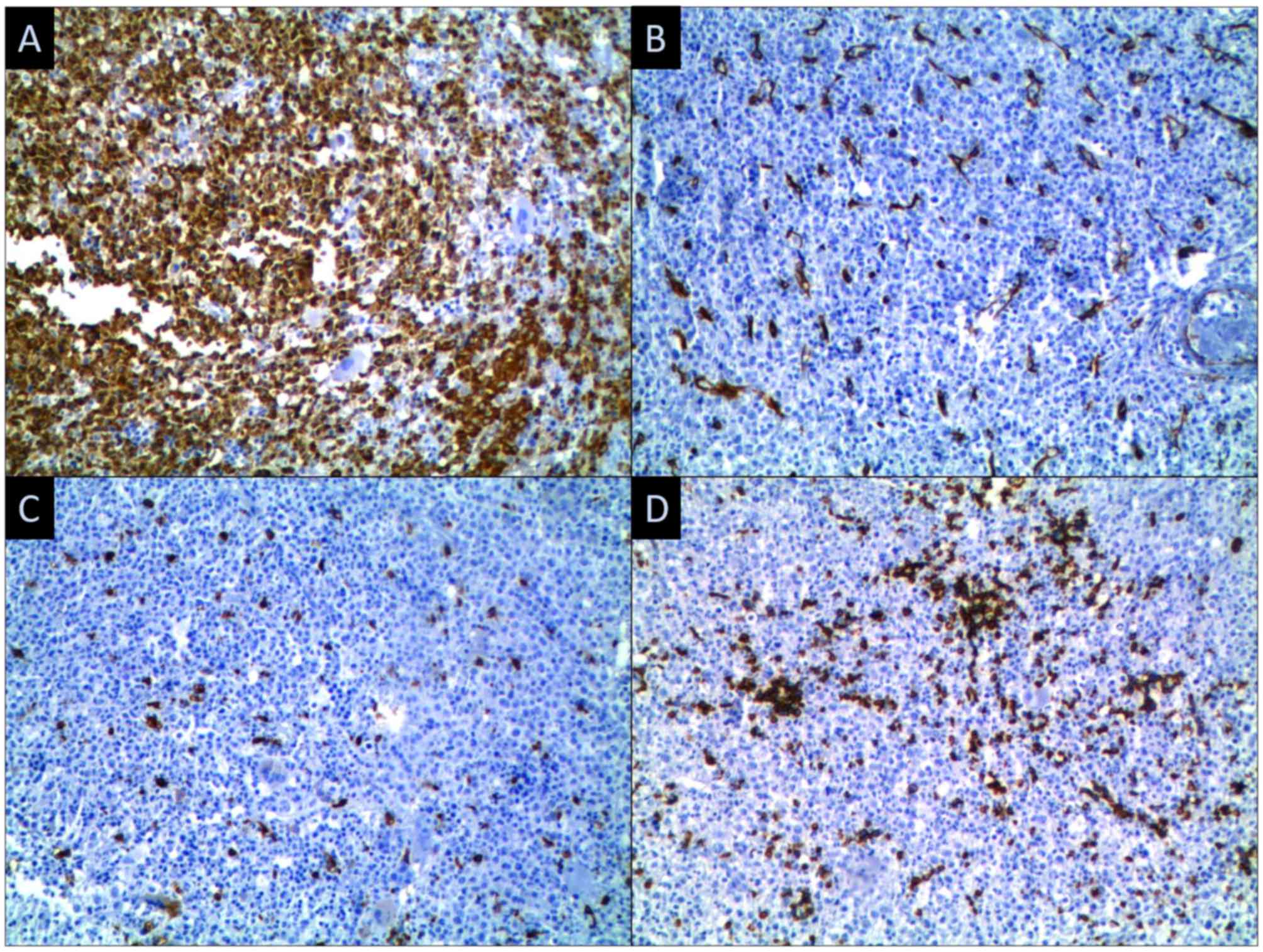
diagnosis was made in 2012 through a bone marrow biopsy (Figs. 1 and 2), a BCR/ABL test and a FIP1L1-PDGFRA test;
he denied having any complications of the underlying disease until
then. During the clinical examination, jugular swelling, bilateral
hepatojugular reflux, diffuse crepitations on pulmonary
auscultation, systolic murmur in the mitral valve area on cardiac
auscultation and edema of the lower limbs were observed.
A chest X-ray performed on admission revealed signs
of pulmonary congestion and pleural effusion on the right side; the
laboratory analysis revealed normal renal function, a normal
electrolyte profile (sodium, potassium, magnesium and calcium),
hemoglobin 10.3 g/dl, hematocrit 28.7%, 12,610/ml total leukocytes
with 67.9% (8,562/ml) eosinophils, D-dimer 825 ng/ml (normal range,
190–440 ng/ml) and brain natriuretic peptide 187 pg/ml (normal,
<100 pg/ml).
After the laboratory and radiological evaluations,
the diagnosis of acute decompensated heart failure with unknown
etiology was established. An echocardiogram performed in June 2012
did not reveal structural alterations, with a preserved ejection
fraction of the left ventricle, and a segmental and global
assessment of the left ventricle without contractility alterations.
The patient was admitted to the Cardiology Department for
treatment, with simultaneous investigation of the cause underlying
the clinical decompensation. A computed tomographic angiography of
the chest was performed, which showed no evidence of arterial
thromboembolism. Abdominal ultrasonography revealed evidence of
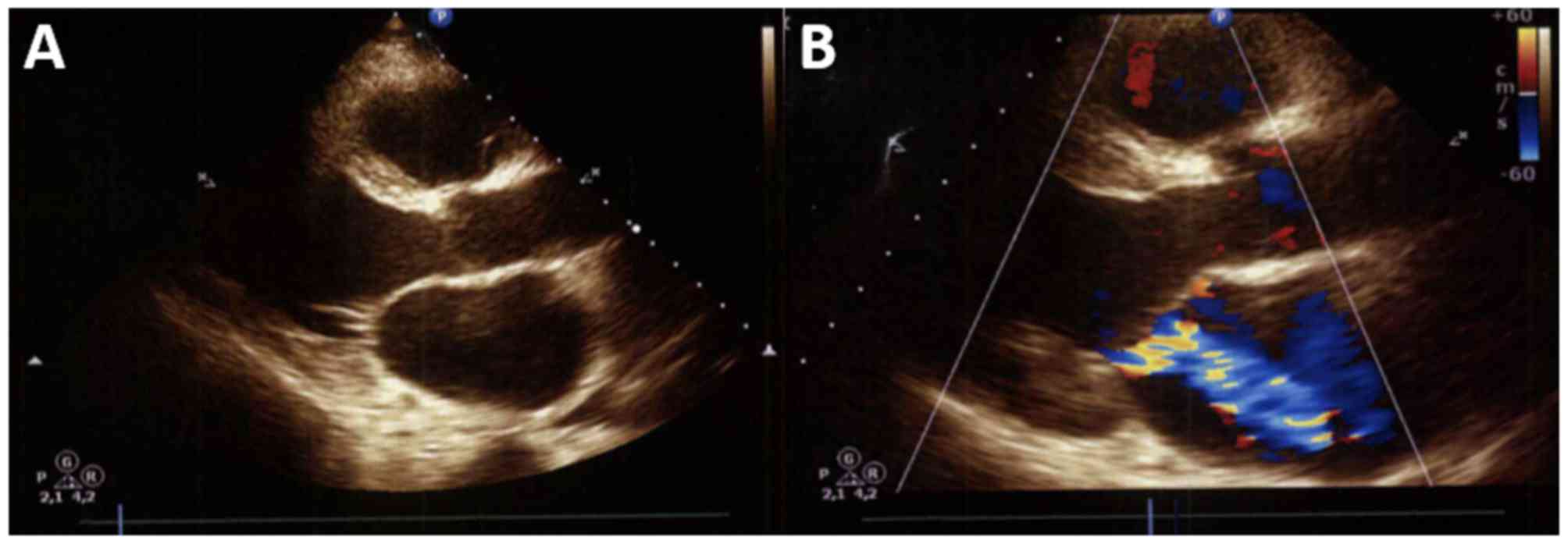
splenomegaly, and the transthoracic echocardiogram revealed severe
mitral insufficiency with valve retraction, with a regurgitant
volume of 56 ml per beat and a regurgitant orifice area of 55
mm2 (Fig. 3).
Daily examinations were performed during
hospitalization, which revealed eosinophil levels between 8,562 and
20,150/ml, with variations on a daily basis without changing the
dosage of hydroxyurea. The patient was discharged after 9 days of
hospitalization with optimization of the clinical treatment and
without symptoms.
At ~7 days after discharge, the patient visited the
Emergency Department of HUST with signs of decompensated heart
failure, and was again admitted to the Cardiology Department. The
patient was reevaluated by an assistant hematologist during the
second hospitalization, and the previously performed cytogenetic
bone marrow examination showing negativity for BCR/ABL and
FIP1L1-PDGFRA was reviewed. After reviewing the treatments
performed until that time, treatment with imatinib was recommended
due to the rapid cardiac and splenic involvement. Within 24 h of
imatinib therapy, changes in the laboratory tests were apparent,
with an eosinophil count of 1,789/ml. By the second day, the
eosinophil count was 184/ml. The patient was discharged after 5
days of hospitalization following the significant response to
imatinib and compensation of the cardiac symptoms.
After 2 weeks, the patient returned to the hospital
with an exacerbation of heart failure, with the signs and symptoms
of systemic congestion despite the optimization of treatment with
oral diuretics. Tests performed on admission revealed the
stabilization of the eosinophil count at 21/ml. An optimization of
clinical treatment was performed, and subsequent to hemodynamic
stabilization, the patient was referred to the cardiac surgery
department for replacement of the mitral valve.
The patient returned for follow-up at 8 months after
surgery and 9 months from the onset of therapy with imatinib. The
patient displayed no cardiovascular symptoms and eosinophil levels
were maintained at 40/ml. On the last visit in August 2017, after
19 months of imatinib therapy, the patient presented as stable at
HUST, without megaly or adenopathy, using 100 mg/day imatinib, with
a hemoglobin value of 13.4, hematocrit of 41.8, 6,352
leucocytes/ml; (0% banded neutrophils, 67.2% segmented neutrophils,
0.6% basophils, 0.8% eosinophils, 10.6% lymphocytes and 20.8%
monocytes) and 103,700 platelets/ml.
Discussion
Hypereosinophilic syndrome is a rare disorder with
an unknown incidence rate, which was estimated to be 0.035 per
100,000 individuals in the United States (12). The diagnosis of idiopathic
hypereosinophilic syndrome is made following the exclusion of other
factors that may lead to increased eosinophil count (1–3,5). In 2008, the World Health Organization
proposed a classification based on the subtypes of
hypereosinophilia causes, e.g., neoplastic causes and FIP1L1-PDGFRA
abnormalities (13). In
non-neoplastic cases and following the exclusion of secondary
causes, patients are tested for familial hypereosinophilia; if this
is excluded, the diagnosis of idiopathic hypereosinophilic syndrome
is confirmed (5). In the present
case study, a patient with hypereosinophilic syndrome was examined
via bone marrow biopsy and BCR/ABL and FIP1L1-PDGFRA status
evaluations twice (2012 and 2015), and was found to be negative in
both examinations.
The treatment of patients who test negative for
FIP1L1-PDGFRA is based on the use of corticosteroids as a first
line of therapy (14), with
treatment success in 81–85% of the cases (2), including the improvement of
eosinophilia and symptom relief (14). The second line of treatment typically
includes hydroxyurea (2); its
combination with corticosteroids is recommended, but, despite the
reduction in eosinophilia, there is no proven benefit against the
natural progression of the disease (15), whereas severe side effects may lead
to treatment discontinuation (14).
The patient reported use of both therapies for the treatment of his
condition; however, despite the partial reduction in the eosinophil
count, the disease progressed, with significant cardiac dysfunction
and limitations of daily activities.
Treatment with imatinib would be the first
therapeutic option for patients testing positive for the
FIP1L1-PDGFRA fusion (15); however,
previous case studies have demonstrated benefits in
FIP1L1-PDGFRA-negative patients as well. In 2007, a study included
72 patients, of whom 63 received imatinib at doses of 100–400 mg
per day. Of those patients, 36 were negative for FIP1L1-PDGFRA. A
complete response was observed in 4 FIP1L1-PDGFRA-negative patients
after 1 month of treatment, and in 1 negative patient after 3
months, totaling an initial response rate of 14%, but with a loss
of response after 1–15 months of treatment (16). Among 15 patients who tested negative
for FIP1L1-PDGFRA, 6 (40%) experienced disease relapse after 4–8
months of imatinib treatment at a dose of 400 mg per day (17). In another study with 188 patients, 68
(36%) of whom received imatinib at the maximum dose of 400 mg per
day, 43 patients tested negative for FIP1L1-PDGFRA, of whom only 10
(23%) exhibited a complete (n=6) or partial (n=4) response. By
contrast, 88% (15) of
FIP1L1-PDGFRA-positive patients exhibited a good response to
treatment (14). Another study
divided patients with FIP1L1-PDGFRA into two classes according to
the presence of >4 criteria suggestive of myeloid neoplasms. In
that study, 16 patients negative for FIP1L1-PDGFRA without any
criteria of myeloid neoplasms received imatinib, and none of those
patients exhibited a clinical or laboratory response to treatment
(18).
In the case described herein, the patient had a
complete response with 100 mg imatinib after <48 h of treatment,
with clinical improvement, suggesting that imatinib may achieve
good results in certain cases of FIP1L1-PDGFRA fusion-negative
hypereosinophilic syndrome. It also suggests a cardiotoxic effect
of imatinib, which may have been the triggering factor for the
clinical decompensation, despite the progressive laboratorial
improvement.
This syndrome may be responsible for the dysfunction
of numerous organs, including cardiac involvement in up to 60% of
the cases (6,10). Typically, cardiac involvement in
hypereosinophilic syndrome preferentially affects the endocardium
and goes through three stages: i) Acute necrosis (initial stage),
which is usually asymptomatic and is associated with the
infiltration of the myocardium by eosinophils; ii) thrombotic
stage, which manifests by progressive damage to the endocardium and
may be associated with thrombotic events; and finally, iii) the
fibrosis stage, in which the condition progresses to restrictive
cardiomyopathy and severe fibrosis, which may cause valvular
regurgitation (2,10,19).
In the case presented herein, there was an
exacerbation of the heart failure symptoms with evidence of
isolated mitral regurgitation, without evidence of associated
restrictive cardiomyopathy or left ventricular hypertrophy. Such a
clinical presentation is uncommon, as the symptoms of heart failure
usually appear during the third stage of disease progression. Two
cases of mitral valve involvement in the thrombotic stage were
previously described, likely induced by thrombus formation in the
endothelium, causing valvular damage (20,21).
In conclusion, according to gathered references, the
cardiac involvement in the present case is uncommon, as the
clinical changes suggest that the patient had concomitant
myocardial dysfunction and fibrosis; however, for unknown reasons,
isolated mitral valve involvement was the initial form of cardiac
involvement in our patient, which has never been previously
described in the literature to the best of our knowledge. The
patient in question exhibited disease progression with cardiac and
splenic involvement, despite receiving the standard therapy
recommended for FIP1L1-PDGFRA-negative patients and stable results
in the laboratory tests. Due to the limited treatment options, the
use of imatinib was selected despite the absence of the
FIP1L1-PDGFRA fusion mutation, with a remarkable response at 24 h
after treatment initiation, and maintenance of the low eosinophil
levels during the outpatient treatment. However, more studies are
required and additional novel treatment options should be tested to
further improve the quality of life of patients suffering from
hypereosinophilic syndrome.
Acknowledgements
The authors would like to thank the staff of the
Hospital Universitário Santa Terezinha.
Availability of data and materials
The datasets generated during and/or analyzed during
the current study are available from the corresponding author on
reasonable request.
Funding
No funding was received.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Ethics Committee (Comitê de Ética em Pesquisa Unoesc/HUST; approval
no. 160968).
Consent for publication
Consent for publication was obtained from the
patient.
Authors' contributions
ASDB: Study concept and design; acquisition of data;
analysis and interpretation of data; drafting of the manuscript;
critical revision of the manuscript. RC: Analysis and
interpretation of data; drafting of the manuscript; critical
revision of the manuscript. ESM: Analysis and interpretation of
data; drafting of the manuscript; critical revision of the
manuscript. ARB: Study concept and design; analysis and
interpretation of data; drafting of the manuscript; critical
revision of the manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Roufosse F: Management of
hypereosinophilic syndromes. Immunol Allergy Clin N Am. 35:561–575.
2015. View Article : Google Scholar
|
|
2
|
Cools J, DeAngelo DJ, Gotlib J, Stover EH,
Legare RD, Cortes J, Kutok J, Clark J, Galinsky I, Griffin JD, et
al: A tyrosine kinase created by fusion of the PDGFRA and FIP1L1
genes as a therapeutic target of imatinib in idiopathic
hypereosinophilic syndrome. N Engl J Med. 348:1201–1214. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klion AD: How I treat hypereosinophilic
syndromes. Blood. 126:1069–1077. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hardy WR and Anderson RE: The
hypereosinophilic syndromes. Ann Intern Med. 68:1220–1229. 1968.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mankad R, Bonnichsen C and Mankad S:
Hypereosinophilic syndrome: Cardiac diagnosis and management.
Heart. 102:100–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cogan E and Roufosse F: Clinical
management of the hypereosinophilic syndromes. Expert Rev Hematol.
5:275–289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ronchi Junior I, Vasconcelos Krebs CN,
Pietrovicz J, Nocera VB, Pedri LE, Fouani MM, Lopes GL, Loidi de
Santana WM, Akiyoshi C and Henriques C: Síndrome hipereosinofílica
idiopática. Relato de caso e revisão de literatura. Rev Bras Clin
Med. 8:177–182. 2010.
|
|
8
|
Kalac M, Quintás-Cardama A, Vrhovac R,
Kantarjian H and Verstovsek S: A critical appraisal of conventional
and investigational drug therapy in patients with hypereosinophilic
syndrome and clonal eosinophilia. Cancer. 110:955–964. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brito-Babapulle F: The eosinophilias,
including the idiopathic hypereosinophilic syndrome. Br J Haematol.
121:203–223. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Benezet-Mazuecos J, Marcos-Alberca P,
Farré J, Orejas M, de la Fuente A and Prieto E: Images in
cardiovascular medicine. Early differential resolution of right and
left ventricular obliteration in Löffler endocarditis after
chemotherapy and anticoagulation. Circulation. 114:e635–e637. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Curtis C and Ogbogu P: Hypereosinophilic
syndrome. Clinic Rev Allerg Immunol. 50:240–251. 2016. View Article : Google Scholar
|
|
12
|
Crane MM, Chang CM, Kobayashi MG and
Weller PF: Incidence of myeloproliferative hypereosinophilic
syndrome in the United States and an estimate of all
hypereosinophilic syndrome incidence. J Allergy Clin Immunol.
126:179–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bain BJ, Gilliland DG, Horny HP, et al:
Myeloid and lymphoid neoplasms with eosinophilia and abnormalities
of PDGFRA, PDGFRB or FGFR1. World Health Organization
classification of tumors of hematopoietic and lymphoid tissues:
68–73. Lyon. France; IARC Press; 2008
|
|
14
|
Ogbogu PU, Bochner BS, Butterfield JH,
Gleich GJ, Huss-Marp J, Kahn JE, Leiferman KM, Nutman TB, Pfab F,
Ring J, et al: Hypereosinophilic syndrome: A multicenter,
retrospective analysis of clinical characteristics and response to
therapy. J Allergy Clin Immunol. 124:1319–1325.e3. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gotlib J: World Health
Organization-defined eosinophilic disorders: 2015 update on
diagnosis, risk stratification, and management. Am J Hematol.
90:1078–1089. 2015. View Article : Google Scholar
|
|
16
|
Baccarani M, Cilloni D, Rondoni M,
Ottaviani E, Messa F, Merante S, Tiribelli M, Buccisano F, Testoni
N, Gottardi E, et al: The efficacy of imatinib mesylate in patients
with FIP1L1-PDGFRalpha-positive hypereosinophilic syndrome. Results
of a multicenter prospective study. Haematologica. 92:1173–1179.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Metzgeroth G, Walz C, Erben P, Popp H,
Schmitt-Graeff A, Haferlach C, Fabarius A, Schnittger S, Grimwade
D, Cross NC, et al: Safety and efficacy of imatinib in chronic
eosinophilic leukaemia and hypereosinophilic syndrome: A phase-II
study. Br J Haematol. 143:707–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khoury P, Desmond R, Pabon A,
Holland-Thomas N, Ware JM, Arthur DC, Kurlander R, Fay MP, Maric I
and Klion AD: Clinical features predict responsiveness to imatinib
in platelet-derived growth factor receptor-alpha-negative
hypereosinophilic syndrome. Allergy. 71:803–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weller PF and Bubley GJ: The idiopathic
hypereosinophilic syndrome. Blood. 83:2759–2779. 1994.PubMed/NCBI
|
|
20
|
Madhwal S, Goldberg J, Barcena J, Guha A,
Gogate P, Cmolik B and Elgudin Y: Unusual cause of acute mitral
regurgitation: Idiopathic hypereosinophilic syndrome. Ann Thorac
Surg. 93:974–977. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim NK, Kim CY, Kim JH, Jang SY, Bae MH,
Lee JH, Yang DH, Park HS, Cho Y and Chae SC: A hypereosinophilic
syndrome with cardiac involvement from thrombotic stage to fibrotic
stage. J Cardiovasc Ultrasound. 23:100–102. 2015. View Article : Google Scholar : PubMed/NCBI
|