Introduction
The development of colorectal cancer has become a
major clinical concern among patients with long-standing Crohn's
disease (CD) (1,2), and research has shown there is a higher
risk of colorectal cancer among CD patients (3,4). With
increased awareness of CD-associated cancer (CDAC), surveillance is
deemed necessary, and specific guidelines for CDAC have already
been established in Western countries (5,6). In
Japan, there is also guidelines for the therapy of inflammatory
bowel disease but a surveillance for CDAC has not been decided.
Unlike Western countries, anal canal cancer or carcinoma of the
fistula is common in Japan as CDAC, which is prone to symptoms
(2). As a result, the establishment
of the surveillance for CDAC has been late but a national study is
ongoing.
In CD patients, the principle of surgery is minimum
resection and a prophylactic excision to avoid carcinogenesis
cannot be selected. After all, surveillance for residual intestine
for cancer screening is extremely important. We present a case of
rectal carcinoma that occurred in a patient with a 25-year history
of CD.
Case report
A 37-year-old man with a 17-year history of CD
visited the outpatient clinic at our hospital complaining of
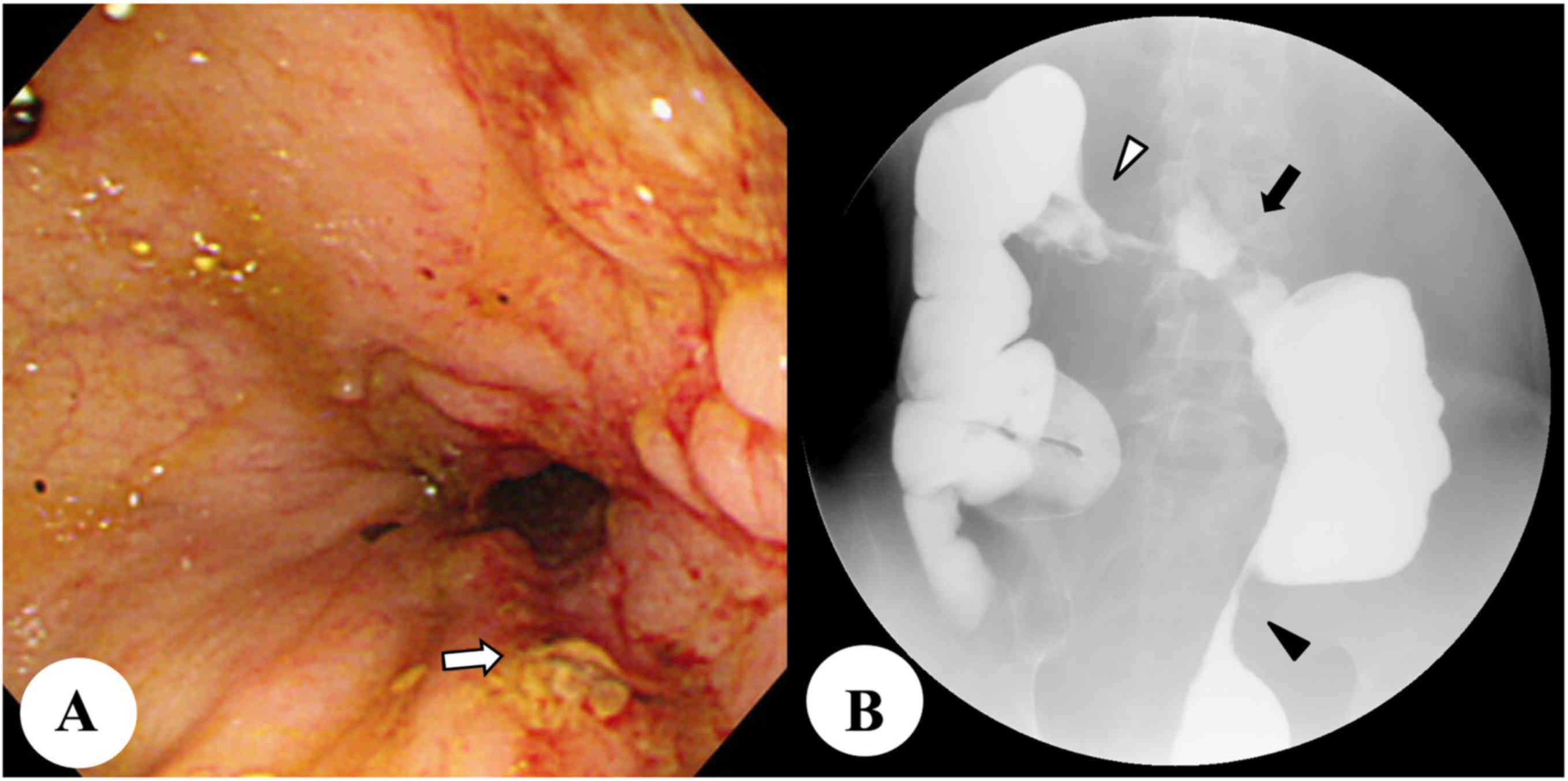
frequent diarrhea and weight loss. Colonoscopy showed severe
stenosis and the formation of an internal fistula involving the
stomach at the transverse colon, and multiple active inflammatory
lesions with a longitudinal ulcer and stenosis involving the entire
colon and rectum (Fig. 1A). Barium
enema examination revealed shrinkage of the entire colon and
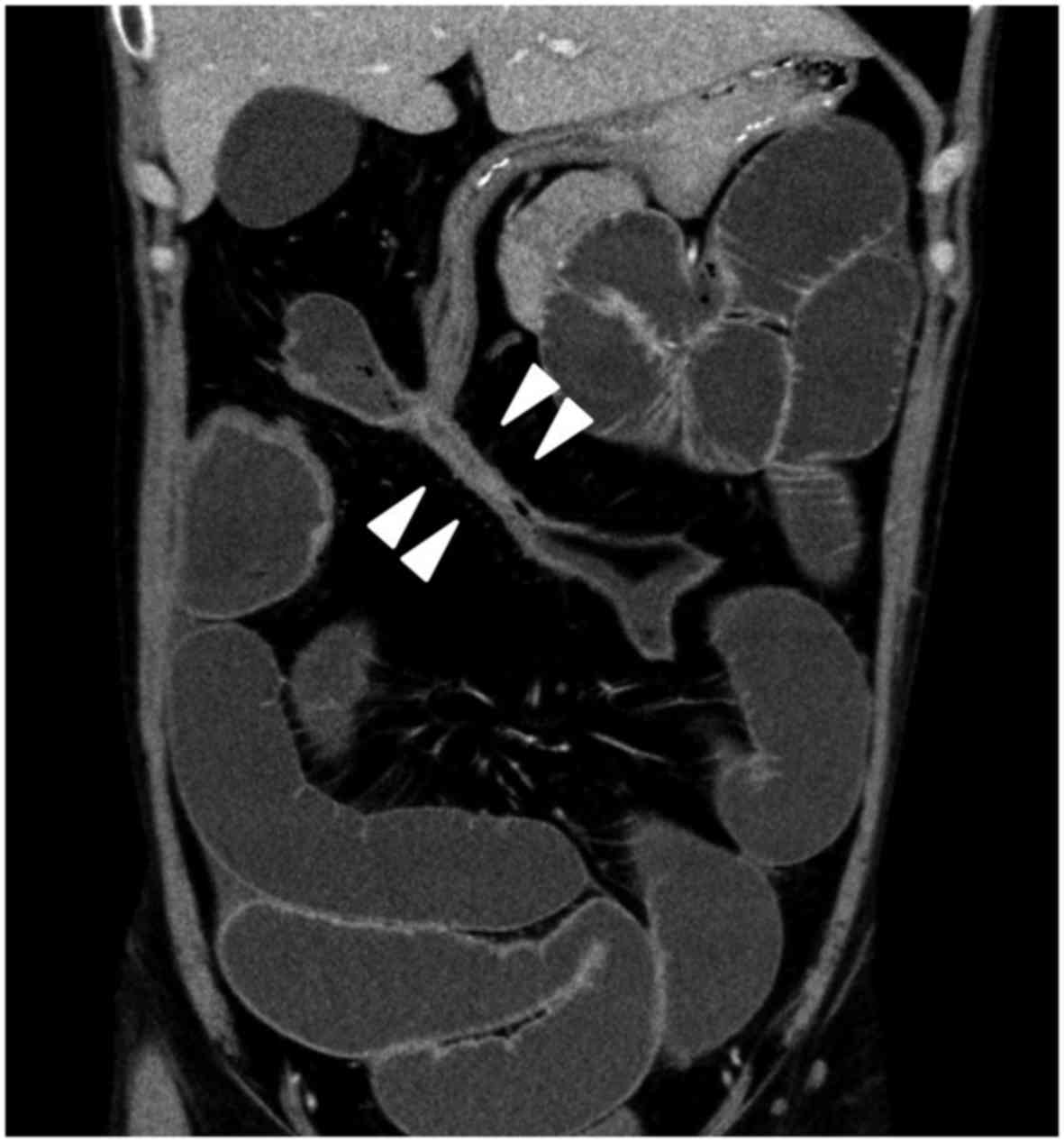
stenosis at the transverse and sigmoidal colons (Fig. 1B). Computed tomography (CT) showed
increased wall thickness across the entire colon and rectum. Two
months after pharmacological treatment, bowel obstruction developed
with dilatation of the small intestine (Fig. 2). An anal fistula and another
evidence of carcinoma were not observed. We planned transverse
colectomy with colostomy, but intraoperatively, the right-side
colon showed extreme shrinkage and the ileocecal junction was
hardly visible because it was involved in the transverse fistula.
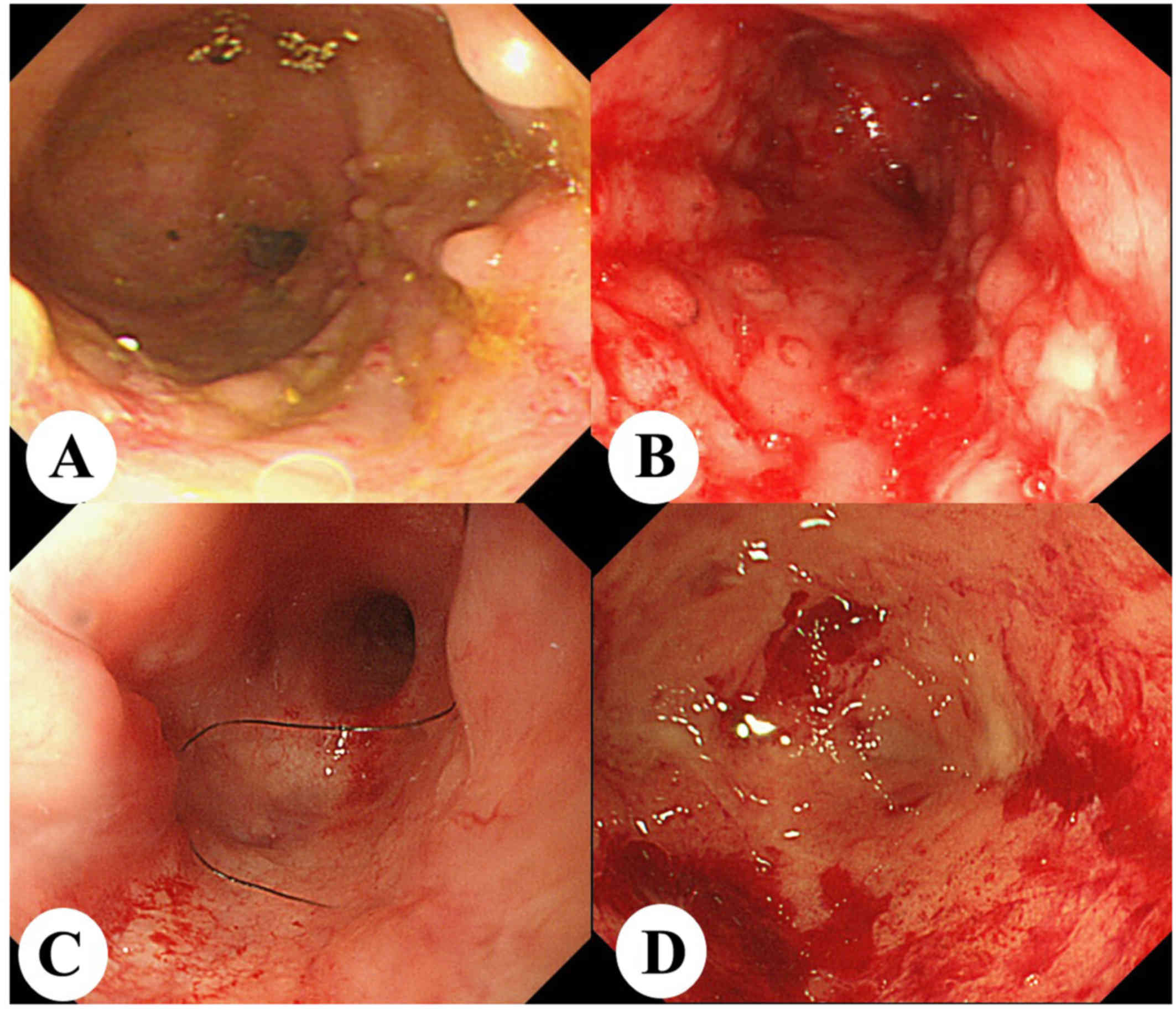
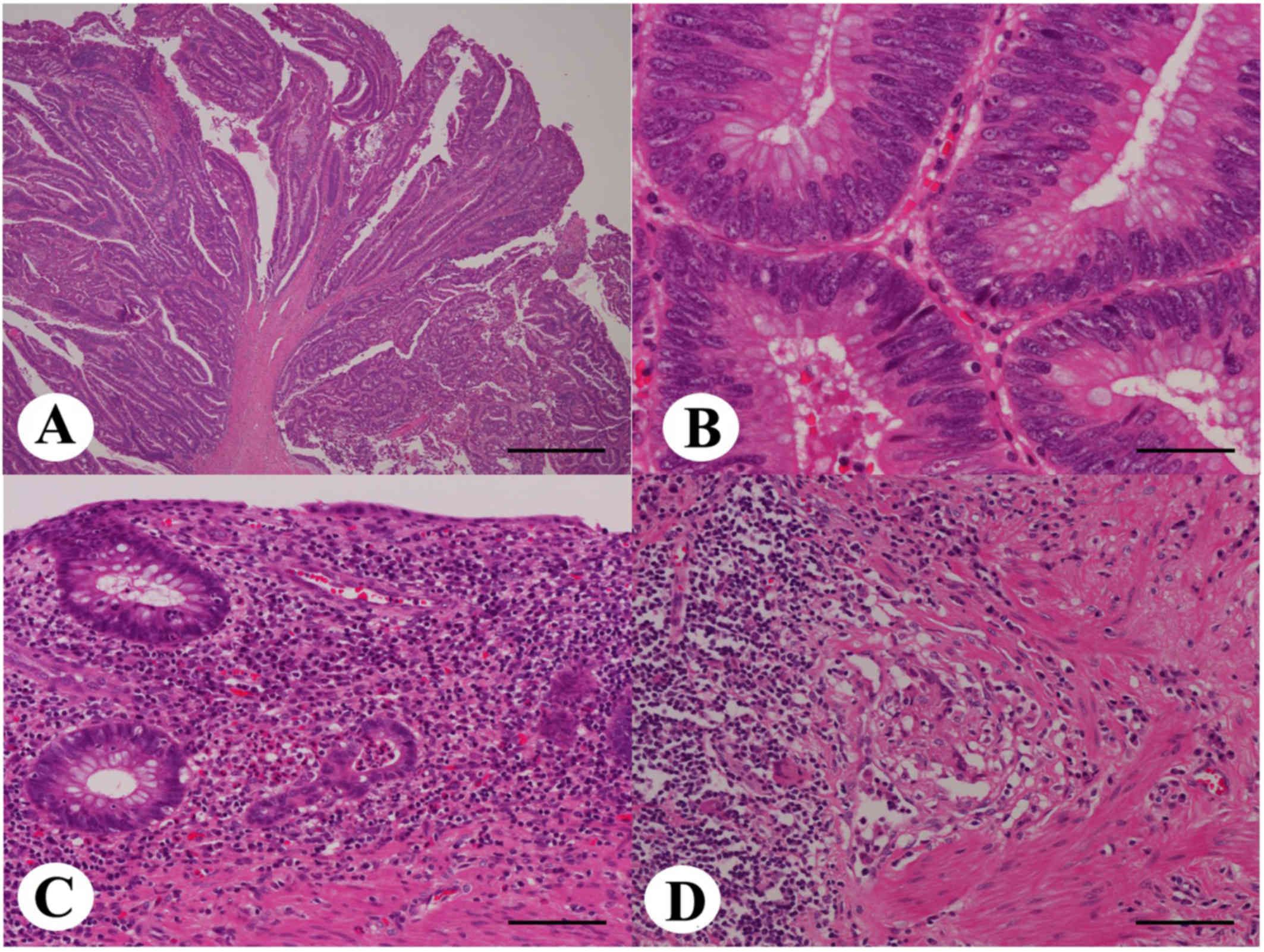
Despite severe inflammation in the rectum (Fig. 3A), we performed semi-emergent
subtotal colectomy and ileostomy, and left the inflame rectum.
Histopathological examination showed transmural inflammatory cell
infiltrates with crypt abscess and granulomatous inflammation
without any dysplasia or malignant lesions.
Postoperatively, the patient restarted medical
therapy with infliximab, antibiotics, and azathioprine, and
underwent surveillance colonoscopy at intervals of approximately
1–2 years for surveillance. Despite this pharmacological treatment,
follow-up colonoscopy two years after surgery showed moderate
inflammation in addition to erosion, stenosis, and an active ulcer
in the residual rectum (Fig. 3B);
however, dysplasia was not observed on biopsy. Active inflammation
was observed once during remission four years after surgery
(Fig. 3C), but relapsed to
mild-to-moderate inflammation two years thereafter (Fig. 3D).
Eight years after the primary operation, he
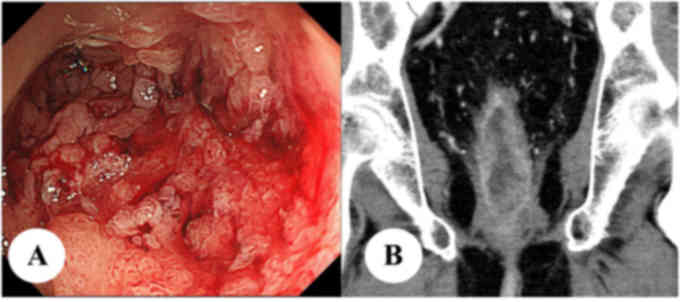
developed increased anal mucus discharge. Colonoscopy revealed
inflammatory polyposis with erosion in the lower part of the
remnant rectum (Fig. 4A), and biopsy
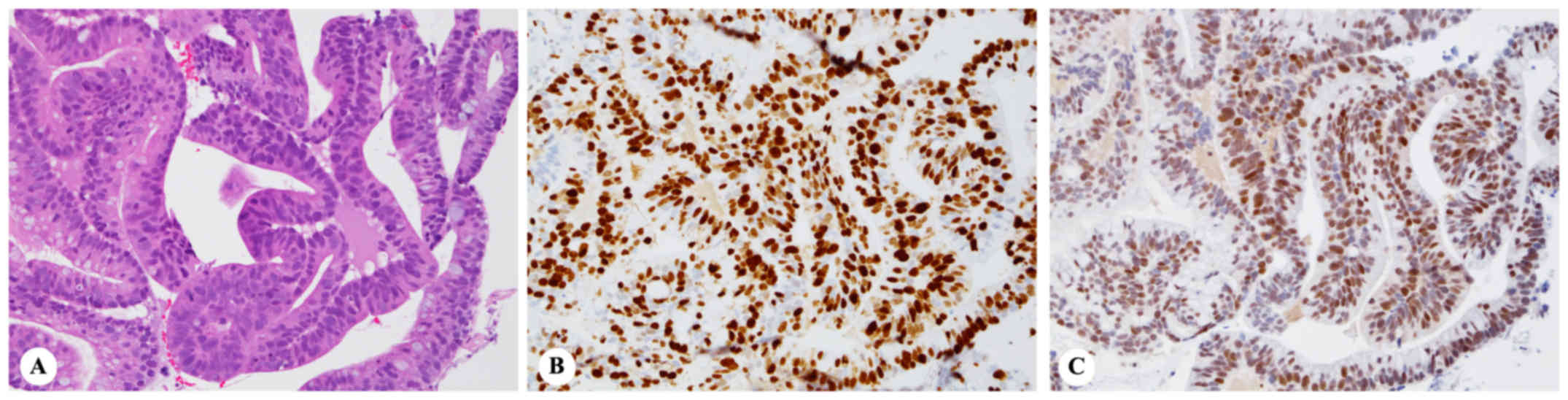
of the polypoid lesions showed adenocarcinoma histologically
(Fig. 5B). This was confirmed by
immunohistochemical findings, consisting of diffuse strong nuclear
staining of p53 (detected using BenchMark ULTRA, Roche, according
to the recommendation by the manufacturer, including incubation
with a Ready-to-Use antibody, clone DO-7, Roche, REF-No: 790-2912,
diluted, for 16 min, at 37°C, incubated, for 64 min, at 95°C)
(Fig. 5B), in addition to high Ki-67
labeling index of 82.5% (detected using Bond-III, Leica, according
to the recommendation by the manufacturer, including incubation
with a monoclonal antibody, clone MIB-1, DAKO, REF-No: M7240, ×200
diluted, for 15 min, incubated, for 20 min, at 100°C) (Fig. 5B).
Laboratory examinations revealed a mild inflammatory
reaction (white blood cell count: 5,500/µl, neutrophil count:
3,867/µl, C-reactive protein: 1.48 mg/dl). The levels of the tumor
markers, CEA and CA19-9, were 2.4 ng/dl and 11.8 U/ml,
respectively. CT and magnetic resonance imaging showed enhancement
and thickening of the remnant rectal wall with no invasion into
other organs or lymph nodes, and no distant metastasis (Fig 4B). He was diagnosed with rectal
carcinoma and underwent laparoscopic abdominoperineal resection
(APR). Using a perineal approach, an anal fistula at the right side
of the rectum was resected. Histopathological examination revealed
a well- to moderately-differentiated tubular adenocarcinoma, with a
villotubular growth pattern (Fig. 6A and
B) and with invasion into the subserosal tissue. The
pathological classification was pT3N0, pStage IIA, according to
UICC TNM Classification of MALIGNANT TUMOURS 8th for Cancer of the
Colon and Rectum (7). In addition,
chronic and active granulomatous inflammation was found in the
surrounding rectum, consisting of atrophic distorted crypts with
occasional crypt abscesses, transmural inflammatory cell
infiltrate, fibrosis, and ill-defined epithelioid cell granulomas
(Fig. 6C and D). These latter
findings were histopathologically compatible with CD after
treatment. Neither cytomegalovirus infection nor amyloid deposition
was detected either histologically or immunohistochemically. The
patient's postoperative course was uneventful, and he was
discharged on postoperative day 33. He has been without recurrence
for 12 months after APR.
Discussion
The development of colorectal cancer has become a
major complication among long-standing CD patients (1,2). The
first report describing CDAC was published in 1948 by Warren et
al (8), and several reports
discussing the epidemiology of CDAC have followed. The risk of
colorectal cancer in CD patients is 2.5 times that of the general
population (3), and the mean
duration of CD until diagnosis of colorectal cancer is 18.3 years
(4). A dysplasia to carcinoma
sequence has been associated with carcinoma in CD (5), and the risk of colorectal cancer has
been reported to gradually increases according to disease duration
in a recent meta-analysis, with an incidence of 0.4/1,000 person
years duration (pyd) in the <10 years' duration group, 0.8/1,000
pyd in the 10–20 years' duration group, and 1.2/1,000 pyd in the
>20 years' duration group (4).
More specifically in rectal cancer, the standardized relative risk
has been reported to be 1.6 (9). In
our patient, he was a high-risk patient because the disease
duration was 25 years and the inflammation in the residual rectum
had been continued. Because the principle of surgery in CD is
minimum resection, however, APR as a prophylactic excision to avoid
carcinogenesis could not be selected at the primary surgery.
Therefore, appropriate repeat colonoscopy for residual intestine
for cancer screening would be needed.
With awareness of CDAC, specific guidelines for CDAC
was established in Western countries (5,6), but it
is undeveloped in Japan. According to Western guidelines, repeat
colonoscopy over a 1- or 2-year interval is recommended for
patients who have moderate-to-severe inflammation, and more
frequent colonoscopy or colectomy is necessary for patients with
dysplasia (5). This surveillance
provides better prognosis in patients with inflammatory bowel
disease, although it is difficult for colonoscopy alone to prevent
carcinogenesis. Thus, the aim of surveillance is not to prevent the
onset of carcinoma but to identify dysplasia or carcinoma at an
early stage (10,11). However, the location of
carcinogenesis in CD is different from Western countries and Japan.
The majority of cancer location is small bowel in Western countries
(3), whereas anorectal carcinoma
including cancer in anal fistulas counts 55% of CDAC in Japan
(2). Therefore, European
surveillance protocol or guideline may not suitable for Japan
wherein anorectal observation is more important. Our patient showed
no evidence of dysplasia and therefore underwent colonoscopy,
albeit irregularly, approximately every 1–2 years after surgery.
The colonoscopy before the diagnosis of advanced carcinoma showed a
persistent moderate inflammation that had persisted for over 20
years, and the interval was 16-month. Considering this history, our
patient, who had a persistent inflammation of the residual
intestine, should have undergone annual colonoscopy for cancer
screening despite the absence of dysplasia. Thus, we recommend that
CD patients with persistent inflammation in the residual intestine
should be subjected to routine annual colonoscopy for cancer
screening after surgery.
One more important point is that the resection of
residual continuous inflammatory rectum should have been considered
in his follow-up as a prophylactic resection. In ulcerative
colitis, total proctocolectomy is generally performed to avoid
carcinogenesis and continuous inflammation (12) but there has been no evidence that a
prophylactic resection avoid carcinogenesis and improve prognosis
of CD patients. To resect the residual rectum, APR was needed which
required another surgical invasiveness and permanent artificial
anus. Moreover, the inflammation could be expected to be improved
with medical therapies and the lack of absolute surgical indication
also decreased the necessity of APR as the next surgery.
In long-standing CD patients, annual colonoscopy for
residual intestine may be considered for cancer screening, and
specific surveillance guidelines should be established.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KS, SH, TY, YO, NI and HK participated in the care
of the patients. KS, SH, TY, YO, NI, HK, TI, YM and AT were all
involved in collecting the patients' data. KS, TY, SH and AT
considered the present cases based on the past literature and
drafted the manuscript. KS was a major contributor to analyzing and
interpreting the patient data, and to writing the manuscript. YM
and TI performed the histological examination and data analysis.
SH, TY and AT revised the manuscript. AT participated in critical
revision of the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient, and patient's anonymity was preserved.
Consent for publication
Written informed consent was obtained from the
patient for the publication of any data and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
APR
|
abdominoperineal resection
|
|
CA19-9
|
carbohydrate antigen 19-9
|
|
CD
|
Crohn's disease
|
|
CDAC
|
Crohn's disease associated cancer
|
|
CEA
|
carcinoembryonic antigen
|
|
CT
|
computed tomography
|
|
INF
|
infiltration
|
|
TNF
|
tumor necrosis factor
|
References
|
1
|
Jess T, Gamborg M, Matzen P, Munkholm P
and Sørensen TI: Increased risk of intestinal cancer in Crohn's
disease: A meta-analysis of population-based cohort studies. Am J
Gastroenterol. 100:2724–2729. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shinozaki M: Crohn's disease and
intestinal cancer in Japan. J Jpn Soc Coloproctol. 61:353–363.
2008. View Article : Google Scholar
|
|
3
|
Canavan C, Abrams KR and Mayberry J:
Meta-analysis: Colorectal and small bowel cancer risk in patients
with Crohn's disease. Aliment Pharmacol Ther. 23:1097–1104. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Laukoetter MG, Mennigen R, Hannig CM,
Osada N, Rijcken E, Vowinkel T, Krieglstein CF, Senninger N,
Anthoni C and Bruewer M: Intestinal cancer risk in Crohn's disease:
A meta-analysis. J Gastrointest Surg. 15:576–583. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beaugerie L and Itzkowitz SH: Cancers
complicating inflammatory bowel disease. N Engl J Med.
372:1441–1452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Strong S, Steele SR, Boutrous M, Bordineau
L, Chun J, Stewart DB, Vogel J and Rafferty JF: Clinical Practice
Guidelines Committee of the American Society of Colon and Rectal
Surgeons: Clinical Practice Guideline for the Surgical Management
of Crohn's Disease. Dis Colon Rectum. 58:1021–1036. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
National Comprehensive Cancer Network:
NCCN Guidelines: Colon Cancer. Version 1. 2017.https://www.nccn.org/patients/guidelines/colon/files/assets/common/downloads/files/colon.pdfJanuary
9–2018
|
|
8
|
Warren S and Sommers SC: Cicatrizing
enteritis as a pathologic entity; analysis of 120 cases. Am J
Pathol. 24:475–501. 1948.PubMed/NCBI
|
|
9
|
Ekbom A, Helmick C, Zack M and Adami HO:
Increased risk of large-bowel cancer in Crohn's disease with
colonic involvement. Lancet. 336:357–359. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Friedman S, Rubin PH, Bodian C, Harpaz N
and Present DH: Screening and surveillance colonoscopy in chronic
Crohn's colitis: Results of a surveillance program spanning 25
years. Clin Gastroenterol Hepatol. 6:993–998; quiz 953–954. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cleveland Krugliak N, Colman RJ, Rodriquez
D, Hirsch A, Cohen RD, Hanauer SB, Hart J and Rubin DT:
Surveillance of IBD using high definition colonoscopes does not
miss adenocarcinoma in patients with low-grade dysplasia. Inflamm
Bowel Dis. 22:631–637. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Riddell RH, Goldman H, Ransohoff DF,
Appelman HD, Fenoglio CM, Haggitt RC, Ahren C, Correa P, Hamilton
SR, Morson BC, et al: Dysplasia in inflammatory bowel disease:
Standardized classification with provisional clinical applications.
Hum Pathol. 14:931–968. 1983. View Article : Google Scholar : PubMed/NCBI
|