Introduction
Gastric cancer (GC) is one of the most common types
of malignancies and the second leading cause of cancer-associated
mortalities worldwide (1). Despite
rapid advances in current treatment protocols incorporating
chemoradiation into surgical procedures, GC continues to be a
lethal disease with the majority of patients being diagnosed at
advanced stages at which palliative therapy is the only remaining
treatment option (2). Diagnosis at
early clinical stages and feasibility of curative surgery are the
most important factors for the successful treatment of GC. However,
nodal invasion, distant metastasis and local relapses frequently
occur even following comprehensive therapy (3). Metastasis is the major cause of
mortality; however, the mechanism underlying metastatic progression
in GC is highly complex and remains to be fully elucidated
(4,5). Thus, in order to facilitate
therapeutic intervention, it is important for clinicians to
identify specific biomarkers for the prediction of metastatic
progression and prognosis of patients with GC.
Type 2C protein phosphatase (PP2C) family proteins
are known to be involved in a wide range of physiological functions
including cellular stress response signaling, apoptosis and
regulation of the cell cycle (6).
One member of this family is the protein phosphatase
magnesium-dependent 1 delta (PPM1D). Of note, PPM1D-deficient mice
are more resistant to mammary tumor formation induced by the human
epidermal growth factor receptor 2 (Erbb2) or Harvey rat sarcoma
(Hras) oncogenes than wild-type mice with intact PPM1D (7). Mounting evidence suggests the
involvement of PPM1D in tumorigenesis. PPM1D was also shown to be
overexpressed in human primary breast, ovarian and neuroblastoma
tumors (8–10). More importantly, PPM1D is able to
complement several oncogenes, including Ras, myelocytomatosis (Myc)
and Erbb2, for cellular transformation both in vitro and
in vivo (7,8,11).
However, the expression pattern of PPM1D and its
clinical significance in GC have yet to be elucidated. The present
study aimed to investigate the expression pattern and
clinicopathological involvement of PPM1D in GC. The present study
provided evidence that PPM1D expression was significantly
upregulated in GC tissue as compared with the adjacent normal
tissue and was significantly associated with metastasis and lower
survival rates of patients with GC.
Materials and methods
Patients
Data were collected from 800 patients with GC who
received resection at The Second Affiliated Hospital of The Third
Military Medical University (Chongqing, China). The patients
included 508 males and 292 females, with a mean age of 61 years
(range, 40–86). None of the patients received chemotherapy prior to
surgery. All the patients received a R0 resection above a D1 lymph
node dissection. The present study was approved by the Ethics
Committee of The Third Military Medical University (Chongqing,
China), and informed consent was obtained from all
participants.
Assessment of PPM1D expression using
immunohistochemistry
Immunohistochemical analysis was performed according
to the manufacturer’s instructions (Dako, Carpinteria, CA, USA).
Paraffin-embedded sections (4 μm) were prepared on silane-coated
slides (Sigma, St. Louis, MO, USA), deparaffinized and incubated
with 3% H2O2 in methanol for 10 min to block
endogenous tissue peroxidase. The antigen was retrieved at 95°C for
20 min in 10 mM sodium citrate buffer. The slides were then
incubated with rabbit anti-PPM1D antibody (1:50, LifeSpan
BioSciences, Seattle, WA, USA) at 4°C overnight. Following
incubation with biotinylated secondary antibody for 30 min at room
temperature, the slides were incubated with streptavidin-peroxidase
complex for 30 min at room temperature. Immunostaining was
developed by using streptavidin peroxidase
3,3′-diaminobenzidine-chromogen detection. Rabbit immunoglobulin G
(IgG) isotope controls were used, which showed negative
staining.
Assessment of PPM1D positivity in GC
tissue samples
All the slides were evaluated by two independent
investigators who were blinded to this study. Immunoreactivity was
graded as: +++ (3); ++ (2); + (1)
and − (0). Score 0–1 was classified as PPM1D-negative and score 2–3
as PPM1D-positive. Patients were divided into two groups according
to PPM1D positivity. The correlations between clinicopathological
parameters and PPM1D expression were investigated.
Western blot analysis
GC tissue samples were analyzed using a 10%
polyacrylamide gel in a sodium dodecyl sulfate buffer by
electrophoresis. Gels were transferred onto a nitrocellulose
membrane and incubated with anti-PPM1D antibody (Abcam, Cambridge,
MA, USA). Binding of PPM1D antibody was revealed by
chemiluminescence following incubation with horseradish
peroxidise-conjugated goat anti-mouse antibody (Bio-Rad, Hercules,
CA, USA). GAPDH (Qiagen, Hilden, Germany) was used as the internal
control.
Statistical analysis
Statistical analysis was performed using the
χ2 test with SPSS 16.0 software (SPSS, Inc., Chicago,
IL, USA). Cumulative survival curves were analyzed by the
Kaplan-Meier method and statistical significance was calculated
using the log-rank test. The Cox proportional hazard model was used
in the univariate and multivariate analyses to determine prognostic
factors. A difference of P<0.05 was considered statistically
significant.
Results
PPM1D protein staining of GC tissue
samples
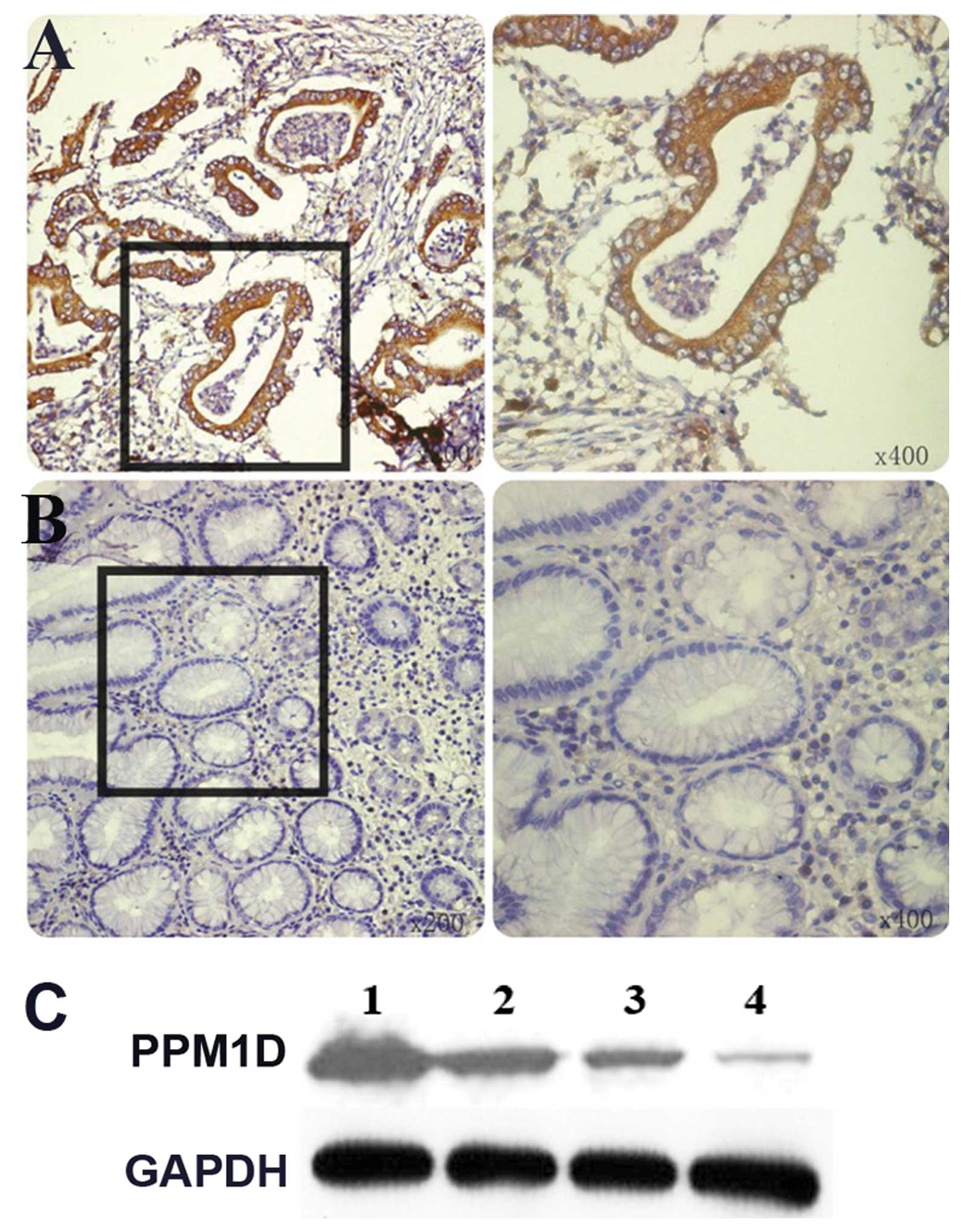
Immunohistochemistry was performed on GC tissue and
adjacent non-cancerous mucosa of 800 patients. PPM1D staining
showed a cytoplasmic staining pattern in most of the GC tissue
sections. PPM1D expression was detected in 48% (384/800) GC samples
with moderate to strong staining (Fig.
1A) versus 9% (72/800) in normal paired gastric mucosa
(Fig. 1B). Similar results were
obtained for PPM1D expression by western blot analysis (Fig. 1C).
Clinicopathological characteristics and
PPM1D positivity in GC tissue samples
The correlation between PPM1D positivity and
clinicopathological parameters with regard to patient prognosis was
analyzed (Table I). PPM1D
positivity significantly correlated with the nodal status
(p<0.001), distant metastasis (p<0.001) and vascular invasion
(p<0.001). There was no correlation between tumor histology and
PPM1D expression. The five-year survival rate of the PPM1D-positive
group was significantly lower than that of the PPM1D-negative group
(41 versus 72%; p=0.0012) (Fig.
2).
 | Table ICorrelation between clinical variables
and PPM1D positivity. |
Table I
Correlation between clinical variables
and PPM1D positivity.
| PPM1D positivity | |
|---|
|
| |
|---|
| Variables | Yes, n=356 | No, n=444 | P-value |
|---|
| Gender |
| Male | 232 | 276 | N.S. |
| Female | 124 | 168 | |
| Tumor depth |
| T1+T2 | 143 | 237 | |
| T3+T4 | 213 | 207 | N.S. |
| Nodal
involvement |
| Yes | 154 | 164 | <0.001 |
| No | 202 | 280 | |
| Distant
metastasis |
| Yes | 208 | 241 | <0.001 |
| No | 148 | 203 | |
| Vascular
invasion |
| Yes | 191 | 165 | <0.001 |
| No | 165 | 279 | |
| Stage |
| I+II | 158 | 229 | |
| III+IV | 198 | 215 | |
| Histology |
| Differentiated | 156 | 202 | N.S. |
|
Undifferentiated | 200 | 242 | |
Univariate and multivariate analyses
Results of the univariate analysis are shown in
Table II. Overall survival was
significantly correlated with the nodal status, distant metastasis,
stage, vascular invasion and PPM1D positivity. Furthermore, the
association between PPM1D positivity and survival was still
significant following regulation of other prognostic markers in
multivariate analysis [hazard ratio (HR), 6.572; 95% confidence
interval (CI), 3.108–13.471; P=0.0018] (Table III). In addition, multivariate
analysis showed that the depth of invasion (HR, 2.121; 95% CI,
1.081–4.243; P=0.021), nodal status (HR, 3.813; 95% CI,
1.526–7.907; P=0.013), distant metastasis (HR, 13.701; 95% CI,
6.091–29.772; P<0.001) and vascular invasion (HR, 1.598; 95% CI,
1.012–3.106; P=0.031) were also independent prognostic factors.
 | Table IIUnivariate analysis of prognosis in
800 patients with gastric cancer. |
Table II
Univariate analysis of prognosis in
800 patients with gastric cancer.
| Variables | HR (95% CI) | P-value |
|---|
| Depth of
invasion | 1.021
(0.401–2.021) | 0.079 |
| T1+T2 | | |
| T3+T4 | | |
| Nodal status | 3.203
(1.947–4.833) | <0.001 |
| N0 | | |
| N1+N2+N3 | | |
| Distant
metastasis | 9.197
(2.603–20.441) | <0.001 |
| Absent | | |
| Present | | |
| Stage | 2.107
(1.321–4.026) | <0.001 |
| I + II | | |
| III + IV | | |
| Vascular
invasion | 3.112
(1.667–5.394) | <0.001 |
| Absent | | |
| Present | | |
| PPM1D | 4.138
(1.698–8.619) | <0.001 |
| Positive | | |
| Negative | | |
 | Table IIIMultivariate analysis of prognosis in
800 patients with gastric cancer. |
Table III
Multivariate analysis of prognosis in
800 patients with gastric cancer.
| Variables | HR (95% CI) | P-value |
|---|
| Depth of
invasion | 2.121
(1.081–4.243) | 0.021 |
| T1+T2 | | |
| T3+T4 | | |
| Nodal status | 3.813
(1.526–7.907) | 0.013 |
| N0 | | |
| N1+N2+N3 | | |
| Distant
metastasis | 13.701
(6.091–29.772) | <0.001 |
| Absent | | |
| Present | | |
| Stage | 0.655
(0.321–1.103) | 0.43 |
| I+II | | |
| III+IV | | |
| Vascular
invasion | 1.598
(1.012–3.106) | 0.032 |
| Absent | | |
| Present | | |
| PPM1D | 6.572
(3.108–13.471) | 0.0018 |
| Positive | | |
| Negative | | |
Discussion
GC-associated mortality closely correlates with
early metastasis and strong invasion; accordingly, it is highly
important to estimate the malignant degree and metastatic tendency
of cancer in order to make timely and appropriate treatment
decisions. The present study used immunohistochemistry to analyze
the expression of PPM1D in a large group of patients with GC
(n=800). The results indicated that PPM1D positivity was
significantly higher in GC tissue samples compared with normal
gastric tissue. In addition, PPM1D positivity associated with high
expression levels of PPM1D in GC lesions was significantly
correlated with the nodal status, distant metastasis and vascular
invasion. PPM1D upregulation was an independent prognostic factor
in GC. Therefore, PPM1D may be of great value as a prognostic
marker for GC.
Previous studies have indicated that PPM1D is highly
expressed in neuroblastoma as well as pancreatic, lung, bladder,
liver, ovarian and breast cancer (9,10,12,13).
However, the expression pattern of PPM1D in GC has yet to be
elucidated. In the present study, immunohistochemical analysis
demonstrated that PPM1D was highly expressed in GC. In addition,
correlations between PPM1D expression, unfavorable prognosis and
other clinical factors in GC were determined. PPM1D was upregulated
in gastric cancer tissue compared with non-cancerous tissue,
suggesting that PPM1D overexpression is closely associated with the
progression and prognosis of GC. A number of independent studies
support the pathogenic role of PPM1D in cancer biology. The
overexpression of PPM1D contributes to malignant progression by
inactivating wild-type p53 and p38 mitogen-activated protein kinase
(MAPK) as well as decreasing p16 protein levels in human breast
tissue (14). Oncogenic PPM1D is a
prognostic marker for lung adenocarcinoma (15). Hu et al reported that high
expression of PPM1D is closely correlated with unfavorable
prognosis in pancreatic neuroendocrine tumors (16). In a follow-up study of patients
with medulloblastoma, high expression levels of PPM1D were
correlated with unfavorable prognosis (17). In the present study, a follow-up of
patients treated for GC was performed. To the best of our
knowledge, it was demonstrated for the first time that patients
with GC and positive PPM1D expression had more unfavorable outcomes
than those with negative PPM1D expression. This finding leads to
the conclusion that PPM1D is a potential factor for outcome
assessment. Furthermore, the present study has shown that the
positivity for PPM1D was an independent indicator for GC. Results
of the multivariate analysis revealed that PPM1D positivity, along
with the depth of invasion, nodal status, distant metastasis and
vascular invasion, was also an independent prognostic factor for
patients with GC.
In conclusion, the present has shown showed that
patients with GC overexpressed PPM1D, which was associated with
aggressive clinical features and unfavorable prognosis. Therefore,
PPM1D may have a significant role in the progression of GC. In
addition, this information may provide novel therapeutic and
prognostic possibilities for treating GC and improving the outcome
of patient treatments. Thus, the roles of PPM1D in cancer are
worthy of being further elucidated.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (Grant no. 81071978).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blum MA, Takashi T, Suzuki A and Ajani JA:
Management of localized gastric cancer. J Surg Oncol. 107:265–270.
2013. View Article : Google Scholar
|
|
3
|
Okamoto T, Tsuburaya A, Kameda Y, et al:
Prognostic value of extracapsular invasion and fibrotic focus in
single lymph node metastasis of gastric cancer. Gastric Cancer.
11:160–167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zagouri F, Papadimitriou CA, Dimopoulos MA
and Pectasides D: Molecularly targeted therapies in
unresectable-metastatic gastric cancer: a systematic review. Cancer
Treat Rev. 37:599–610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jang BG and Kim WH: Molecular pathology of
gastric carcinoma. Pathobiology. 78:302–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu G and Wang Y: Functional diversity of
mammalian type 2C protein phosphatase isoforms: new tales from an
old family. Clin Exp Pharmacol Physiol. 35:107–112. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bulavin DV, Phillips C, Nannenga B,
Timofeev O, Donehower LA, Anderson CW, Appella E and Fornace AJ Jr:
Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis
through p38 MAPK-mediated activation of the p16(Ink4a)-p19(Arf)
pathway. Nat Genet. 36:343–350. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Yang Y, Peng Y, Austin RJ, van
Eyndhoven WG, Nguyen KC, Gabriele T, McCurrach ME, Marks JR, Hoey
T, Lowe SW and Powers S: Oncogenic properties of PPM1D located
within a breast cancer amplification epicenter at 17q23. Nat Genet.
31:133–134. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirasawa A, Saito-Ohara F, Inoue J, Aoki
D, Susumu N, Yokoyama T, Nozawa S, Inazawa J and Imoto I:
Association of 17q21-q24 gain in ovarian clear cell adenocarcinomas
with poor prognosis and identification of PPM1D and APPBP2 as
likely amplification targets. Clin Cancer Res. 9:1995–2004.
2003.PubMed/NCBI
|
|
10
|
Saito-Ohara F, Imoto I, Inoue J, Hosoi H,
Nakagawara A, Sugimoto T and Inazawa J: PPM1D is a potential target
for 17q gain in neuroblastoma. Cancer Res. 63:1876–1883.
2003.PubMed/NCBI
|
|
11
|
Demidov ON, Kek C, Shreeram S, Timofeev O,
Fornace AJ, Appella E and Bulavin DV: The role of the MKK6/p38 MAPK
pathway in Wip1-dependent regulation of ErbB2-driven mammary gland
tumorigenesis. Oncogene. 26:2502–2506. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Loukopoulos P, Shibata T, Katoh H, Kokubu
A, Sakamoto M, Yamazaki K, Kosuge T, Kanai Y, Hosoda F, Imoto I,
Ohki M, Inazawa J and Hirohashi S: Genome-wide array-based
comparative genomic hybridization analysis of pancreatic
adenocarcinoma: identification of genetic indicators that predict
patient outcome. Cancer Sci. 98:392–400. 2007. View Article : Google Scholar
|
|
13
|
Wang P, Rao J, Yang H, Zhao H and Yang L:
Wip1 over-expression correlated with TP53/p14(ARF) pathway
disruption in human astrocytomas. J Surg Oncol. 104:679–684. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu E, Ahn YS, Jang SJ, Kim MJ, Yoon HS,
Gong G and Choi J: Overexpression of the wip1 gene abrogates the
p38 MAPK/p53/Wip1 pathway and silences p16 expression in human
breast cancers. Breast Cancer Res Treat. 101:269–278. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Satoh N, Maniwa Y, Bermudez VP, Nishimura
K, Nishio W, Yoshimura M, Okita Y, Ohbayashi C, Hurwitz J and
Hayashi Y: Oncogenic phosphatase Wip1 is a novel prognostic marker
for lung adenocarcinoma patient survival. Cancer Sci.
102:1101–1106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu W, Feng Z, Modica I, Klimstra DS, Song
L, Allen PJ, Brennan MF, Levine AJ and Tang LH: Gene amplifications
in well-differentiated pancreatic neuroendocrine tumors inactivate
the p53 pathway. Genes Cancer. 1:360–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Castellino RC, De Bortoli M, Lu X, Moon
SH, Nguyen TA, Shepard MA, Rao PH, Donehower LA and Kim JY:
Medulloblastomas overexpress the p53-inactivating oncogene
WIP1/PPM1D. J Neurooncol. 86:245–256. 2008. View Article : Google Scholar : PubMed/NCBI
|