Introduction
Members of the AP-2 family of transcription factors
have important roles in several cellular processes, including
apoptosis, migration and differentiation. Furthermore, AP-2
transcription factors have been implicated in carcinogenesis, a
process where the normal program of cell growth and differentiation
is disrupted. The human AP-2 family of transcription factors
consists of five members, AP-2α, -β, -γ, -δ and -ɛ. The C-terminal
half of the AP-2 proteins contains a basic domain and a
helix-span-helix motif that mediates DNA binding and dimerization.
This portion of the AP-2 protein is highly conserved among all AP-2
family members. The N-terminal half of AP-2 proteins contains the
transactivation domain. The transactivation domains of AP-2
proteins are significantly more divergent, although certain
critical residues and a PY motif (XPPXY) are conserved (1–6). A
number of functional AP-2 binding sites, consensus to a palindromic
core sequence, 5′-GCCN3GGC-3′, have been identified in
cellular and viral enhancers, and preferred binding to the sequence
motifs GCCN3GGC, GCCN4GGC and
GCCN3/4GGG was observed in an in vitro binding
site selection assay (7,8). The PY motif and critical residues in
the transactivation domain, which are considered necessary for
transactivation, were divergent in Ap-2δ. The unique protein
sequence and functional features of Ap-2δ implicate other
underlying mechanisms, besides tissue-specific AP-2 gene
expression, for the specific control of target gene activation.
Despite the considerable sequence similarities and
overlap in the expression of AP-2 family members, it has been
described that each of the five AP-2 genes exhibits a distinct
expression pattern in mouse embryos, suggesting that each of them
may have a different role in development (9). In addition, the knockout of
individual AP-2 members in mice results in specific developmental
defects (10,11). Consequently, identification of the
specific targets of each member is highly important in further
understanding the function of AP-2.
Heme oxygenase-1 (HMOX1) has emerged as a key
cytoprotective gene and enzyme in numerous experimental and
clinical contexts. The HMOX1 gene is under complex regulation and
is markedly upregulated by heme, the physiological substrate for
the HMOX1 protein, by iron and other transition metallic ions, and
by oxidative and heat stress and other stressful perturbations
(12–14). Regulation of the HMOX1 gene
expression is associated in part with alterations in the levels of
several transcription factors, including Bach1 and nuclear
factor-erythroid 2-related factor 2 (Nrf2) (15–18).
Ap-2δ was identified as a new activating transcription factor of
HMOX1. However, the mechanism underlying the regulation of HMOX1
expression by Ap-2δ remained to be elucidated. The present study
aimed to investigate the mechanism by which AP-2δ mediates HMOX1
gene expression.
Materials and methods
Plasmid constructs
cDNA encoding human AP-2δ (GenBank Accession Number
AY028376) was generated by PCR from human brain cDNA library
(Invitrogen Life Technologies, Carlsbad, CA, USA). The coding
region of AP-2δ cDNA was amplified by PCR using primers
(5′-GGGGTACCATGTCAACTACCTTTCCGGGAC-3′ and
5′-CCCTCGAGGGTCTGTCTTTTCTGTTTTGCCCTC-3′) containing HindIII
and EcoRI restriction sites and then it was subcloned into
the vector pcDNA4C (maintained in our laboratory) to generate the
construct pcDNA4C-AP-2δ. The primers were obtained from Biomics
Biotechnologies (Nantong, China). The constructs were verified by
DNA sequencing (Biomics Biotechnologies).
Cell culture and transient
transfection
AD293 cells and NIH3T3 cells obtained from Shanghai
Institute of Cell Biology and Biochemistry (Shanghai, China), were
cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal
bovine serum (Gibco-BRL, Carlsbad, CA, USA). The cells were split
on 60 mm dishes at 1×106/dish. Following 24 h, the cells
were transfected using Lipofectamine 2000 (Invitrogen Life
Technologies) according to the manufacturer’s instructions. Each 60
mm dish was transfected with 10 μg of pcDNA4C-AP-2δ. An empty
vector, pcDNA4C, was used as a negative control for the
transfection assays.
Luciferase activity assay
The pGL3-basic-HMOXI reporter constructs containing
the 1360 bp upstream sequences of the human HMOXI gene (GenBank
Accession Number BC001491), were inserted into the
pGL3-basic-vector (Promega Corporation, Madison, WI, USA). To
generate HMOX1-1360luc plasmids, PCR was performed using the
following oligonucleotides: Sense primer, 5′-CCGCTC
GAGGAATACAGTAGCGTGGTCAC-3′ and antisense primer,
5′-CCGCTCGAGATGCCAGGCCTGAAAGC CAT-3′. The 1,100 bp (sense primer:
5′-CCGCTCGAGATGCCAGGCCTGAAAGCCAT-3′),850 bp (sense primer:
5′-CCGCTCGAGAGAGTGTCCCACGCATTCCA-3′), 637 bp (sense primer:
5′-CCGCTCGAGCAGGTCAGTTGTAGGGA TGAACC-3′), 460 bp (sense primer:
5′-CCGCTCGAGGTTC CTGATGTTGCCCACCA-3′), 206 bp (sense primer:
5′-CCGCTCGAGCTATGGCCAGACTTTGTTTCC-3′) and 113 bp (sense primer:
5′-CCCAAGCTTTCGAGAGGAGGCA GGCGTT-3′) fragments were isolated
following digestion of the PCR products with Xho I and
HindIII, and were subcloned to the pGL3-basic vector,
resulting in HMOX1-1100luc, HMOX1-850luc, HMOX637luc, HMOX1-460luc,
HMOX1-206luc and HMOX1-113luc plasmids, respectively. The same
anti-sense primer (5′-CCCAAGCTTTCGAGAG GAGGCAGGCGTT-3′) was used
for all deletional mutant luciferase reporters. The primers were
obtained from Biomics Biotechnologies. The upstream and junction
regions of these constructs were confirmed by sequence analysis
(Biomics Biotechnologies).
Base substitutions at the AP-2 binding sites were
generated in the context of HMOX1-206luc and HMOX1-113luc using the
Transformer™ Site-Directed Mutagenesis kit (Clontech Laboratories,
Inc., Mountain View, CA, USA). HMOX1-206lucm mutated
from 5′-ccgccccgggccagtgtg-3′ to
5′-ccgccccgaatcagtgtg-3′ and
HMOX1-113lucm mutated from 5′-cagctgttccgcctggccca-3′ to
5′-cagctgttattcctggccca-3′ (underlined
bases represent mutated sequences) according to the manufacturer’s
instructions. Constructs with correct mutations were confirmed by
direct sequence analysis (Biomics Biotechnologies).
A total of 24 h following transfection, the cells
were seeded into 96-well plates (8×103 viable
cells/well) and allowed to attach overnight. A total of 200 ng of
pGL3-HMOX1-5′ untranslated region (UTR) or pGL3-mut HMOX1-5′UTR
plus 80 ng pRL-SV40 (Promega Corporation) were transfected in
combination with pcDNA4C-AP-2δ or empty vector pcDNA4C (final
concentration, 80 nM) using Lipofectamine 2000 (Invitrogen Life
Technologies) according to the manufacturer’s instructions.
Luciferase activity was measured 48 h following transfection using
the Dual Luciferase Reporter Assay system (Promega Corporation).
Firefly luciferase activity was normalized to renilla luciferase
activity for each transfected well. Three independent experiments
were performed in triplicate.
Electromobility shift assays (EMSA)
Double-stranded AP-2 oligonucleotides end-labeled
with biotin were obtained from Promega Corporation. The
biotin-labled sequences were as follows: HMOX1-1:
5′-AGTTCCTGATGTTGCCCACCAGGCTATTGCTCTGAGCAGC-3′, HMOX1-2:
5′-CTCCTCTCCACCCCACACTGGCCCGGGGCGGGCTGGGCGC-3′. The optimized
biotin-labled AP-2 binding sequence was as follows:
5′-GAACTGACCGCCTGAGGCGCGTGTGCAGAG-3′. The four overlapping and
biotin-labeled sections were as follows: HMOX1-3 (−206 to −149 bp
of HMOX1 5′UTR), HMOX1-4 (−161 to −104 bp of HMOX1 5′UTR), HMOX1-5
(−113 to −56 bp of HMOX1 5′UTR) and HMOX1-6 (−65 to −1 bp of HMOX1
5′UTR), respectively. Binding was performed at room temperature in
a reaction mixture that included 2 μl of Ap-2δ protein directly
from nuclear extracts, 20 fmol labeled AP-2 oligonucleotide, 4%
glycerol, 1.0 mM MgCl2, 0.5 mM EDTA, 0.5 mM
dithiothreitol, 50 mM NaCl, 10 mM Tris (pH 7.5) and 0.05 mg/ml
poly(deoxyinosinic-deoxycytidylic) acid. The products were
fractionated on 4% non-denaturing polyacrylamide gels at room
temperature. Binding specificity was determined using 200-fold
concentrations of unlabeled AP-2 oligonucleotide during the binding
reactions. To characterize the specificity of the DNA binding of
Ap-2δ, competition assays were performed by competing a
biotin-labeled optimized AP-2 oligonucleotide. For comparison, the
human AP-2α protein was translated in vitro and then
subjected to competition assays with the same ratios of optimized
AP-2 oligonucleotides. All of the experiments were repeated three
times.
Results
Exogenous expression of AP-2 robustly
transactivates HMOX1 activities in AD 293 and NIH3T3 cell
lines
Through bioinformatics analysis, it was identified
that the 5′ proximal promoter regions of HMOX1 genes contain two
consensus AP-2 motifs. To examine whether exogenous expression of
AP-2δ activates HMOX1 promoter activity, AP-2δ expression vector
was co-transfected with the reporter construct HMOX1-1360luc to
AP-2δ-positive (AD293) and AP-2δ-negative (NIH3T3) cell lines.
To obtain further direct evidence that the HMOX1
gene is a target of AP-2δ, the binding site of AP-2δ in the 5′UTR
of HMOX1 was investigated. Several deletional mutant luciferase
reporters were constructed (HMOX1-1100luc, HMOX1-850luc,
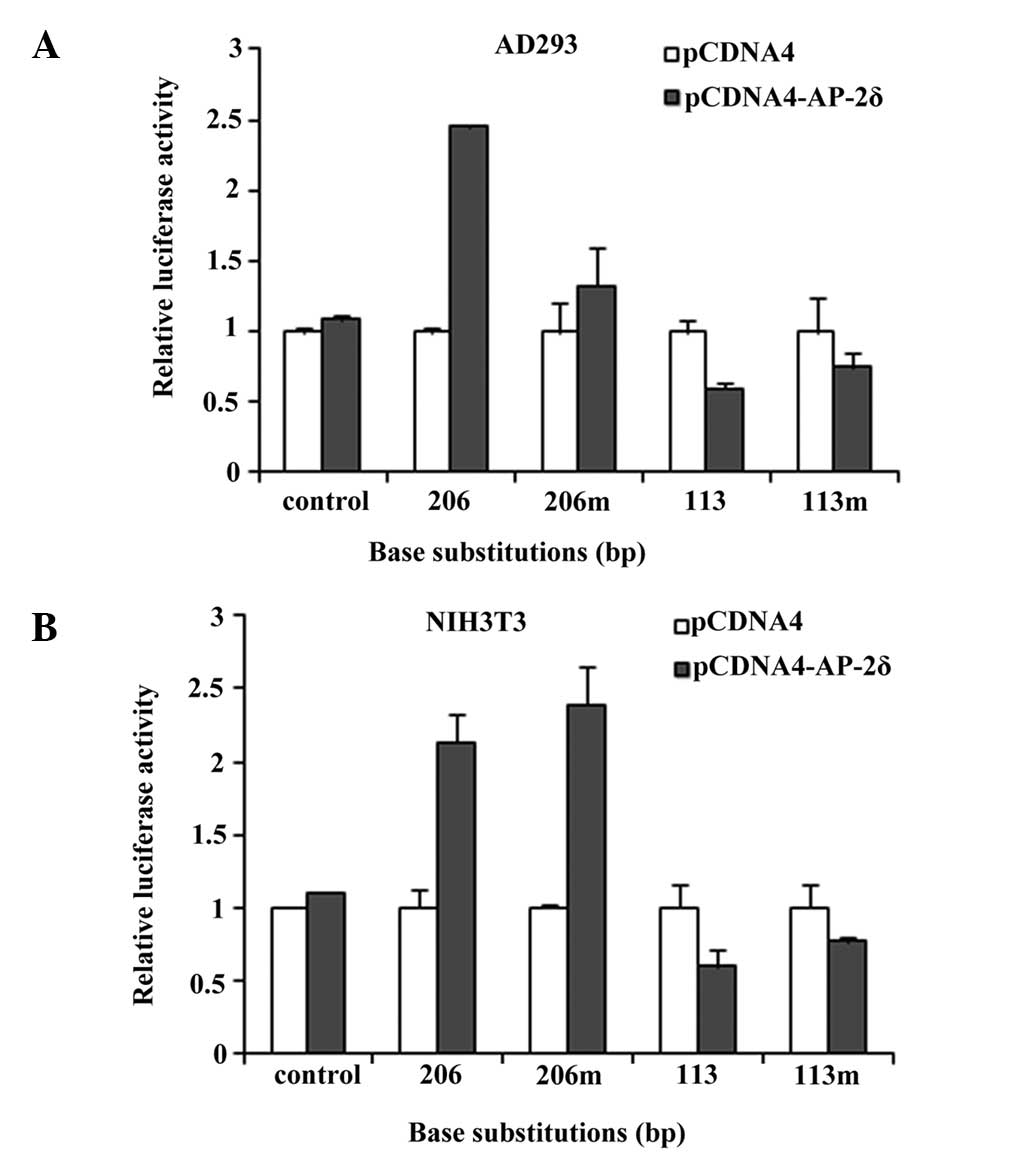
HMOX-637luc, HMOX1-460luc, HMOX1-206luc and HMOX1-113luc; Fig. 1). Correspondingly, two mutant
reporters (HMOX1-206lucm and HMOX1-113lucm)
were also generated, in which the sequences in AP-2 consensus
binding sites were changed (Fig.
3). Each of these constructs were co-transfected with
PcDNA4C-AP-2α and pcDNA4C-AP-2δ plasmid into the AD293 and NIH3T3
cells. An empty vector, pcDNA4C, was used as a negative control for
the tranfection assays.
The assays demonstrated that the luciferase activity
in the HMOX1-1360luc-transfected cells was significantly increased
by three-fold compared with the luciferase activity in the negative
control cells, suggesting that AP-2δ increased the luciferase
activity of HMOX1 in the two cell lines (Fig. 2). Therefore, it was concluded that
the inserted fragment of HMOX1 (position −1 to −1360 bp of HMOX1
5′UTR) was the target of AP-2δ.
To examine which fragment was the regulatory
sequence, the various HMOX1-luc constructs containing different
lengths of HMOX1 upstream sequences for their transcriptional
activation in response to exogenous AP-2 expression were
investigated (Fig. 1). Deletions
starting from the −1360 kb to the −206 kb site did not change the
AP-2-responsive promoter activation of the HMOX1 gene, which was
activated more than once, both in the AD293 and NIH3T3 cell lines.
However, transcriptional activation of HMOX1-113luc in response to
exogenous AP-2 expression was diminished by 1-fold, evidently
suggesting that the HMOX1 promoter from −206 to −1 may contain
multiple AP-2-responsive sites.
Furthermore, two mutant reporters
(HMOX1-206lucm and HMOX1-113lucm) were
generated, in which the sequences in AP-2 consensus binding sites
were changed (Fig. 3). The AP-2δ
responsiveness of the HMOX1 promoter was affected only marginally,
if at all, by this mutation in the cell lines (Fig. 4). These data led to the hypothesis
that transcriptional activation of the HMOX1 gene by AP-2δ may
require additional cis-elements, rather than traditional AP-2
consensus binding sites.
Sequence-specific DNA binding
Ap-2 proteins have been reported to bind to a highly
conserved GC-rich consensus sequence present in numerous cellular
and viral promoters and enhancers. An in vitro binding site
selection assay identified GCCN3/4GGC and GCCN3/4GGG as the
preferred sequence motifs bound by Ap-2 transcription factors
(7,8). Two multiple protein-binding sites
were identified in the HMOX1 upstream promoter regions, which were
HMOX1-1, 5′-agttcctgatgttgcccaccaggctattgctctgagcagc-3′
(underlined bases represent similar AP-2-binding sequence) and
HMOX1-2, 5′-ctcctctccaccccacactggcccggggcgggctgggcgc-3′
(underlined bases represent consensus AP-2-binding sequence).
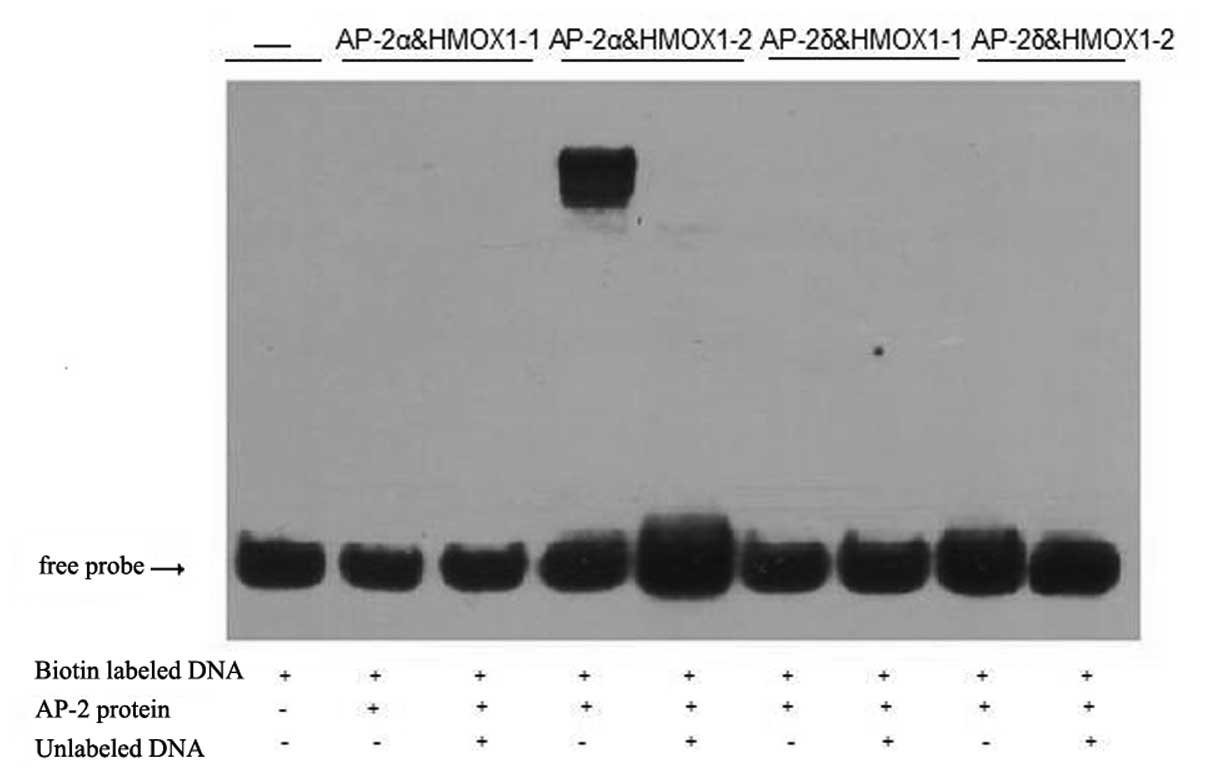
Domain HMOX1-2 of the HMOX1 promoter includes a consensus
AP-2-binding motif (5′-GCCN3GGC-3′). Domain HMOX1-1 includes a
similar AP-2-binding sequence (5′-GCCN5GGC-3′). Initially, empty
pcDNA4C, Ap-2δ and AP-2α genes were translated in vitro,
producing equivalent amounts of protein, and then gel shift assays
were performed using the AP-2 binding sequence derived from the
HMOX1 upstream promoter. These studies revealed consistently
shifted bands with domain HMOX1-2 when the AP-2α protein was used,
but weak and inconsistent shifts were observed when Ap-2δ was
employed (Fig. 5).
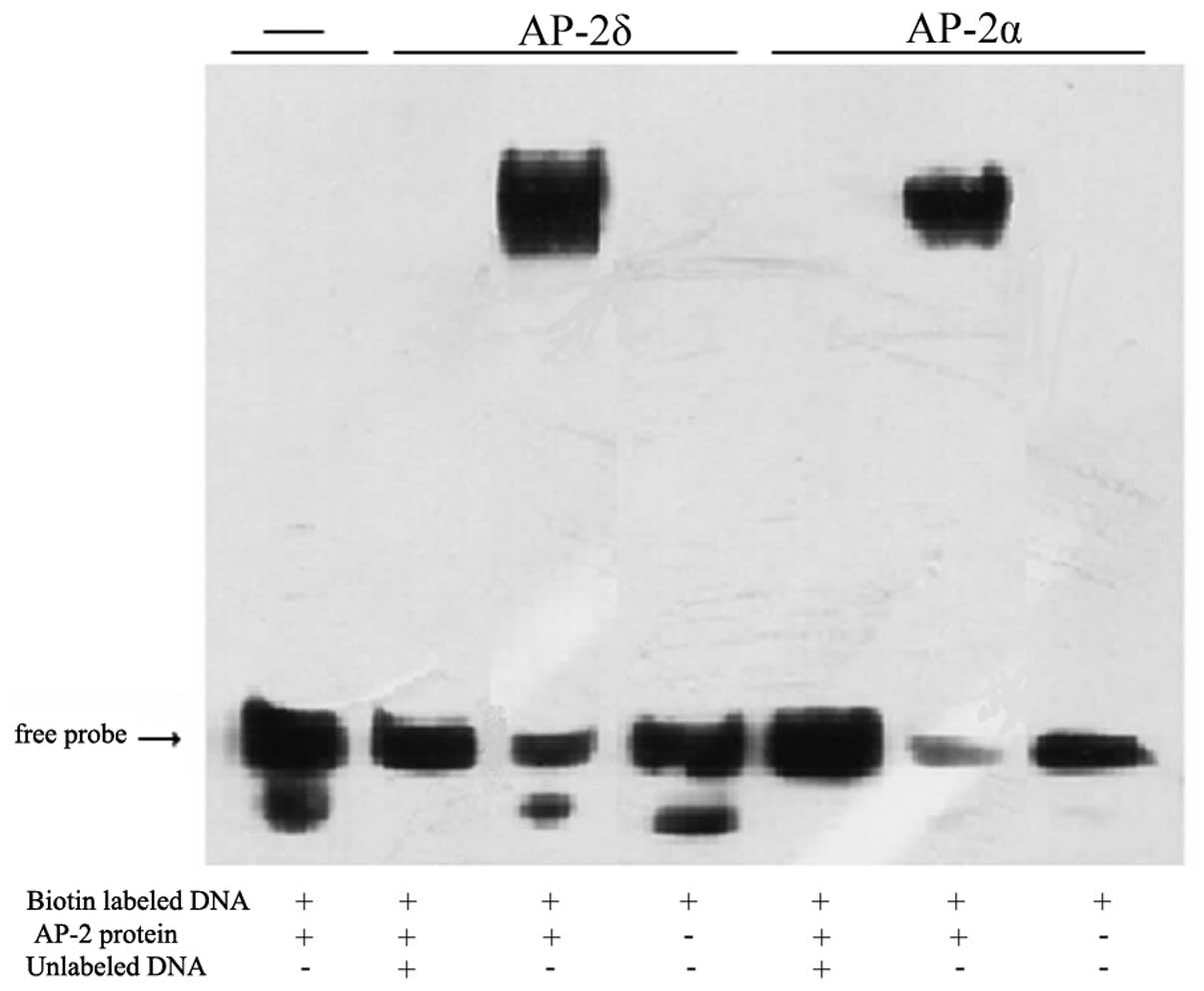
Previous studies have demonstrated the importance of
the flanking sequence of the core 5′-GCCNNNGCC-3′ sequence. It was
identified that the presence of a guanosine at the −1 position on
either DNA strand significantly reduced AP-2 binding. In addition,
the study reported that TGA was the preferred sequence for the
central 3 bp between the palindromic GCC and GGC motifs (19). The two HMOX1 AP-2 binding sequences
did not conform to those rules, so the gel shifts were repeated
using an oligonucleotide with an optimized sequence
(5′-GAACTGACCGCCTGAGGCGCGTGTGCAGAG-3′). Under these conditions,
Ap-2δ protein induced a strong and consistently shifted band. To
document DNA binding specificity, 200-fold amounts of unlabeled
optimized AP-2 oligonucleotide were added in competition. Cold AP-2
oligonucleotide significantly reduced the intensity of the shifted
band comprised of the biotin-labeled AP-2 oligonucleotide/Ap-2δ
protein complex (Fig. 6). These
results showed that Ap-2δ protein binds to the optimized AP-2
consensus sequence with specificity. In addition to the HMOX1
results, it appeared that Ap-2δ exhibited a higher DNA sequence
preference than AP-2α under in vitro conditions.
The luciferase assays demonstrated that the HMOX1
promoter from −206 to −1 may contain multiple AP-2-responsive
sites. It was observed that the sequence from −206 to −1 did not
contain the optimized sequence 5′-GCCTGAGCC-3′, which indicates the
existence of other AP binding sites. To examine the binding sites,
the 206 bp were divided into four overlapping sections and
biotin-labeled. The four sections were HMOX1-3 (−206bp to −149bp),
HMOX1-4 (−161bp to −104bp), HMOX1-5 (−113bp to −56bp) and HMOX1-6
(−65bp to −1bp), respectively. For comparison, an empty vector,
pcDNA4C, was used as a negative control and human AP-2α protein was
translated in vitro as a positive control. From Fig. 7, HMOX1-3, HMOX1-5 and HMOX1-6 did
not generate any detectable complexes with the recombinant AP-2δ
protein. AP-2δ protein induced a consistently shifted band with
HMOX1-4. A total of 200-fold amounts of unlabeled optimized AP-2
oligonucleotide significantly reduced the intensity of the shifted
band, comprised of the biotin-labeled AP-2 oligonucleotide/AP-2δ
protein complex.
Discussion
The AP-2 family members define a distinct class of
transcription factors characterized by a highly conserved
C-terminal basic region and helix-span-helix motif, which are
critical for DNA binding functions (20). In the present study, it was
identified that AP-2 transcription factors have a close to
identical binding specificity (21). AP-2δ, however, binds weakly to the
conserved GCCN3/4GGC sequence in vitro, providing the first
example of target sequence specificity among the AP-2 family. It
was identified that the AP-2δ protein binds to an optimized AP-2
consensus sequence with specificity. In addition to the HMOX1
results, it also appeared that AP-2δ exhibited a higher DNA
sequence preference than AP-2α under in vitro conditions.
Based on these results, AP-2δ was able to transactivate luciferase
expression in cell culture using the HMOXI promoter which did not
contain the optimized AP-2-binding oligonucleotide. This was
expected, as it has been previously demonstrated that upon
overexpression in cell culture, AP-2 proteins perform
transactivation using DNA sequences for which they have relatively
weak affinity (22). The binding
specificity of AP-2 proteins is considered to reside within the
basic region (23). Therefore, it
is noteworthy that four residues in that domain, which are
completely conserved among all other AP-2 proteins, differ in AP-2δ
(Asp209, Leu210, Lys235 and Ile252). These substitutions, often
conservative, may provide clues about contact points between AP-2
with its DNA binding sequence.
By contrast, N-terminal sequences of the AP-2
transactivation domains are weakly conserved compared with the
basic and helix-spanhelix regions (23). This sequence divergence is
hypothesized to result in the varying transactivation efficiency
observed among the AP-2 family members, presumably through altered
co-activator interactions. Certain residues in the transactivation
domain are highly conserved, and alterations in these amino acids
often have marked adverse effects on transactivation (24,25).
These residues are highly conserved in the human AP-2 proteins;
however, they are present as Thr86 and Ser91 in AP-2δ. The
effectiveness of AP-2δ in transactivation, despite those
evolutionary substitutions, suggests that AP-2δ may form complexes
with a substantially different set of co-activators than the other
AP-2 proteins. This provides another level of control for the
induction or repression of gene transcription.
Although the five human AP-2 proteins are highly
similar in their C-terminal halves, they appear to vary in function
during development. This diversity, despite their structural
similarity, may arise in various ways. Homologous AP-2 genes are
expressed in spatially and temporally distinct patterns (26,27).
In one study, PCR analysis demonstrated that AP-2δ was expressed at
high levels in adult thymus, prostate, small intestine, skeletal
muscle, placenta, brain and testis tissues. Subsequent studies
demonstrated that AP-2δ may significantly regulate certain genes,
including RPL29, CAPNS1, HMOX1, PDGFC and RPL29 (28).
In the present study, the regulatory function of
AP-2δ on HMOX1 expression was examined. HMOX1 is a multitask enzyme
that has an important role in the regulation of cell proliferation,
differentiation, oxidative status and apoptosis, thereby affecting
immune responses, inflammatory reaction and angiogenesis (13,14).
Therefore, its significance is notably wider than only haem
elimination. HMOX1 is one of the most highly induced genes in cells
exposed to stressful conditions, and hundreds of experiments have
demonstrated that the products of HMOX1 activity are involved in
the maintenance of cell homoeostasis (29). The importance of HMOX1 in
cardiovascular diseases and cancer progression has been commonly
accepted, particularly when considering the variability of HMOX1 in
the human population, resulting from HMOX1 promoter polymorphism
(30). Regulation of HMOX1 gene
expression is associated in part with alterations in the levels of
several transcription factors. In the present study, Ap-2δ was
identified as a new activating transcription factor of HMOX1.
Further investigations will focus on examining the regulatory
function of AP-2δ on HMOX1 in vivo. Future studies are
expected to provide more data elucidating the complex interactions
of HMOX1 within this elaborate gene-regulatory system.
Acknowledgements
The present study was supported by the Fundamental
Research Funds for the Central Universities (no. 222201314027) and
Fundamental Research Funds for the Central Universities (no.
wf1114045).
References
|
1
|
Wenke AK and Bosserhoff AK: Roles of AP-2
transcription factors in the regulation of cartilage and skeletal
development. FEBS J. 277:894–902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dietz KJ, Vogel MO and Viehhauser A:
AP2/EREBP transcription factors are part of gene regulatory
networks and integrate metabolic, hormonal and environmental
signals in stress acclimation and retrograde signalling.
Protoplasma. 245:3–14. 2010. View Article : Google Scholar
|
|
3
|
Bisgrove DA, Monckton EA and Godbout R:
Involvement of AP-2 in regulation of the R-FABP gene in the
developing chick retina. Mol Cell Biol. 17:5935–5945.
1997.PubMed/NCBI
|
|
4
|
Ebert SN, Ficklin MB, Her S, et al:
Glucocorticoid-dependent action of neural crest factor AP-2:
stimulation of phenylethanolamine N-methyltransferase gene
expression. J Neurochem. 70:2286–2295. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maconochie M, Krishnamurthy R, Nonchev S,
et al: Regulation of Hoxa2 in cranial neural crest cells involves
members of the AP-2 family. Development. 126:1483–1494.
1999.PubMed/NCBI
|
|
6
|
Batsché E, Muchardt C, Behrens J, Hurst HC
and Crémisi C: RB and c-Myc activate expression of the E-cadherin
gene in epithelial cells through interaction with transcription
factor AP-2. Mol Cell Biol. 18:3647–3658. 1998.PubMed/NCBI
|
|
7
|
Lin HH, Chen YH, Chiang MT, Huang PL and
Chau LY: Activator protein-2α mediates carbon monoxide-induced
stromal cell-derived factor-1α expression and vascularization in
ischemic heart. Arterioscler Thromb Vasc Biol. 33:785–794.
2013.
|
|
8
|
Debiève F, Depoix Ch and Hubinont C:
Transcription factor AP2 regulates human inhibin α subunit gene
expression during in vitro trophoblast differentiation. Mol Hum
Reprod. 17:702–709. 2011.PubMed/NCBI
|
|
9
|
Maconochie M, Krishnamurthy R, Nonchev S,
et al: Regulation of Hoxa2 in cranial neural crest cells involves
members of the AP-2 family. Development. 126:1483–1494.
1999.PubMed/NCBI
|
|
10
|
Schorle H, Meier P, Buchert M, Jaenisch R
and Mitchell PJ: Transcription factor AP-2 essential for cranial
closure and craniofacial development. Nature. 381:235–238. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Hagopian-Donaldson S, Serbedzija
G, et al: Neural tube, skeletal and body wall defects in mice
lacking transcription factor AP-2. Nature. 381:238–241. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grochot-Przeczek A, Dulak J and Jozkowicz
A: Haem oxygenase-1: non-canonical roles in physiology and
pathology. Clin Sci (Lond). 122:93–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu X, Fan WG, Li DP, Kung H and Lin MC:
Heme oxygenase-1 system and gastrointestinal inflammation: a short
review. World J Gastroenterol. 17:4283–4288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu ML, Layne MD and Yet SF: Heme
oxygenase-1 in environmental toxin-induced lung disease. Toxicol
Mech Methods. 22:323–329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hou WH, Rossi L, Shan Y, Zheng JY,
Lambrecht RW and Bonkovsky HL: Iron increases HMOX1 and decreases
hepatitis C viral expression in HCV-expressing cells. World J
Gastroenterol. 15:4499–4510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiang Y, Liu G, Yang L and Zhong JL:
UVA-induced protection of skin through the induction of heme
oxygenase-1. Biosci Trends. 5:239–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirota A, Kawachi Y, Itoh K, Nakamura Y,
et al: Ultraviolet A irradiation induces NF-E2-related factor 2
activation in dermal fibroblasts: protective role in UVA-induced
apoptosis. J Invest Dermatol. 124:825–832. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun J, Hoshino H, Takaku K, et al:
Hemoprotein Bach1 regulates enhancer availability of heme
oxygenase-1 gene. EMBO J. 21:5216–5224. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mohibullah N, Donner A, Ippolito JA and
Williams T: SELEX and missing phosphate contact analyses reveal
flexibility within the AP-2(alpha) protein: DNA binding complex.
Nucleic Acids Res. 27:2760–2769. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Williams T and Tjian R: Analysis of the
DNA-binding and activation properties of the human transcription
factor AP-2. Genes Dev. 5:670–682. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wankhade S, Yu Y, Weinberg J, Tainsky MA
and Kannan P: Characterization of the activation domains of AP-2
family transcription factors. J Biol Chem. 275:29701–29708. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McPherson LA and Weigel RJ: AP2alpha and
AP2gamma: a comparison of binding site specificity and
trans-activation of the estrogen receptor promoter and single site
promoter constructs. Nucleic Acids Res. 27:4040–4049. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Williams T and Tjian R: Characterization
of a dimerization motif in AP-2 and its function in heterologous
DNA-binding proteins. Science. 251:1067–1071. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmidt M, Huber L, Majdazari A, Schütz G,
Williams T and Rohrer H: The transcription factors AP-2β and AP-2α
are required for survival of sympathetic progenitors and
differentiated sympathetic neurons. Dev Biol. 355:89–100. 2011.
|
|
25
|
Li X, Glubrecht DD and Godbout R: AP2
transcription factor induces apoptosis in retinoblastoma cells.
Genes Chromosomes Cancer. 49:819–830. 2010.PubMed/NCBI
|
|
26
|
Moser M, Rüschoff J and Buettner R:
Comparative analysis of AP-2 alpha and AP-2 beta gene expression
during murine embryogenesis. Dev Dynam. 208:115–124. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chazaud C, Oulad-Abdelghani M, Bouillet P,
et al: AP-2.2, a novel gene related to AP-2, is expressed in the
forebrain, limbs and face during mouse embryogenesis. Mech Dev.
54:83–94. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun L, Huang S, Wu Q, et al:
Identification of genes differentially regulated by transcription
factor, AP-2delta. Front Biosci. 12:1699–1706. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu XM, Peyton KJ and Durante W:
Physiological cyclic strain promotes endothelial cell survival via
the induction of heme oxygenase-1. Am J Physiol Heart Circ Physiol.
304:H1634–H1643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jozkowicz A, Was H and Dulak J: Heme
oxygenase-1 in tumors: is it a false friend? Antioxid Redox Signal.
9:2099–2117. 2007. View Article : Google Scholar : PubMed/NCBI
|