Introduction
In mammals, the cardiac pacemaking current (If)
naturally originates in the sinoatrial node (SAN) due to its
spontaneous firing of action potentials (APs). The cardiac If is
one of the most noteworthy features of the SAN myocytes, as it has
an important role in generating and modulating cardiac rhythmic
activity through slow diastolic depolarization (1–4). The
cardiac If has an unusual characteristic in that it is activated by
membrane hyperpolarization within a voltage rage, which is also why
it is known as the ‘funny’ current. The If is a mixed-cation
current that is carried by both Na+ and K+,
and is controlled by the direct binding of intracellular cyclic
adenosine monophosphate, which accounts for the activation and
inhibition of the cardiac If by β-adrenergic and muscarinic M2
receptor stimulation, respectively (5). Four hyperpolarization-activated
cyclic nucleotide-gated (HCN) channel members, HCN1-4, have been
detected in the heart and are members of a superfamily of
voltage-dependent K+ and cyclic nucleotide gated (CNG)
channels, and combine to form tetrameric channels. The earliest
studies demonstrated that HCN4 is the major isoform for the
mediation of the sympathetic stimulation of pacemaker activity that
exists in the SAN (6–9). In addition, a previous study
demonstrated that the ‘funny’ current in non-pacemaker
cardiomyocytes may affect membrane excitability and predispose the
human heart to atrial and ventricular arrhythmias (10–12).
These findings suggested that the If may have a role in causing
ectopic automaticity, particularly in several pathological
conditions. However, the mechanisms underlying its action are yet
to be fully elucidated.
Berberine (Fig. 1),
a benzodioxoloquinolozine alkaloid occurring in numerous plants of
the genera Berberis and Coptis, has been used in Traditional
Chinese Medicine for many centuries. The chemical name of berberine
is 5,6-dihydro-9, 10-dimethoxy-benzo[g]-1, 3-benzodioxolo [5,6-α]
quinolizinium (13). It has been
demonstrated that berberine exerts a protective effect against
cardiac arrhythmias and has positive inotropic actions. Previous
studies have also revealed that berberine decreases the maximal
velocity of depolarization (Vmax) and prolongs the AP duration
(APD) and effective refractory period (ERP) in cardiac myocytes and
Purkinje fibers (14,15). It has been suggested that berberine
exerts class III antiarrhythmic effects in the cardiac muscle of
mammals in vitro. However, several experiments on cellular
electrophysiology have demonstrated that berberine is a multi-ion
channel blocker, with blockade actions on numerous currents,
including the cardiac ATP-sensitive K+
(KATP), the delayed rectifier K+ current
(IK), inward rectifier K+ current
(IKl), L-type Ca2+ (ICa-L) and the
Na+-Ca2+ exchange current (16–20).
To the best of our knowledge, to date there have been no studies
investigating the action of berberine on cardiac If. Therefore, the
present study aimed to investigate the effects of berberine on the
SAN of rabbits and hHCN4-mediated currents that are present in
cardiac tissue. The heterologously expressed hHCN4 currents in
xenopus oocytes were examines, and it was sought to examine the
mechanisms underlying these effects. The present study provided
insight into the ionic mechanisms responsible for the possible
antiarrhythmic effects of berberine.
Materials and methods
Ethical considerations
Animals used in the present study were treated in
accordance with the Guide for the Care and Use of Laboratory
Animals regulated by the Administrative Regulation of Laboratory
Animals of Hubei Province and all experimental methods were
approved by the Animal Research Committee of the First Clinic
College of Wuhan University (Wuhan, Hubei, China).
Preparation of SAN tissues and AP
recordings
Healthy rabbits of both sexes, weighing 1.5–2.5 kg
and ~6–8 weeks old, were anaesthetized with 30 mg/kg sodium
pentobarbital intravenously. Following exsanguination, the hearts
were rapidly removed and immersed in cold (0–4°C) oxygenated
Ca2+-free tyrode solution containing 135 mM NaCl, 5.4 mM
KCl, 1 mM MgCl2×6H2O, 0.33 mM
NaH2PO4, 10 mM hydroxyethyl
piperazineethanesulfonic acid (HEPES) and 10 mM glucose (4°C; pH
7.4). The SA node preparations, bounded by the crista terminalis,
the superior and inferior vena cava and the interatrial septum,
were carefully dissected out to be pinned in the experimental
chamber. The preparations were continuously superfused with
modified tyrode solution at a rate of 10 ml/min and a temperature
of 37±0.5°C until ~1 h prior to the recordings. The composition of
modified tyrode solution was as follows: 140 mM NaCl, 5.4 mM KCl,
1.8 mM CaCl2, 0.5 mM MgCl2×6H2O, 5
mM HEPES and 10 mM glucose (pH 7.4).
Following 1 h of recovery, the transmembrane
potentials were recorded with glass microelectrodes filled with 3
mM KCl (10–15 MΩ) connected to a high input impedance amplifier
(Dua 773; World Precision Instruments, Sarasota, FL, USA). The
signal was digitalized and collected using specific software
(AcqKnowledge 4.1; BIOPAC Systems, Inc., Norfolk, UK). Spontaneous
APs from the pacemaker cells were recorded for 20–30 min in control
conditions. In this experiment, three concentrations of berberine
(0.3, 3 and 30 μM) were added and the spontaneous AP firing rate
was measured every 5 min for 1–2 h. In addition, changes in the AP
amplitude and duration (APA, APD50 and
APD90), the spontaneous firing frequency, the maximal
diastolic potential (MDP) and the diastolic depolarization rate
(DDR) were determined at the end of the drug exposure period.
HCN channels expressed in xenopus
oocytes
In vitro transcription and functional
expression in xenopus oocytes
Wild-type human HCN4 (hHCN4) cDNA inserted into the
pcDNA3 vector were provided by Professor A. Ludwig and J. Stieber
(Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen,
Germany). Complementary RNAs (cRNAs) which were used for injection
into oocytes were prepared with the mMESSAGEmMACHINE® T7
kit (Ambion, Austin, TX, USA) following linearization of the
expression constructs with XbaI (Takara, Kyoto, Japan). The RNA
quality was examined by gel electrophoresis and the RNA
concentration was quantified by ultraviolet spectroscopy (UV-2201;
Shimadzu Corporation, Kyoto, Japan).
Voltage clamp assay of xenopus
oocytes
Xenopus frogs were anesthetized by cooling on
crushed ice for 30–40 min. Ovarian lobes were digested with 1 mg/ml
type IA collagenase (Sigma Chemicals, St. Louis, MO, USA) in
Ca2+-free ND96 solution for 30 min to remove follicle
cells. Stage IV and V xenopus oocytes were injected with 30 nl (1
μg/μl) of hHCN4 cRNAs per oocyte using a Nanoject microdispenser
(Nanoliter 2000; World Precision Instruments, Sarasota, FL, USA)
and then cultured in ND96 solution supplemented with 100 U/ml
penicillin, 100 U/ml streptomycin and 2.5 mM pyruvate at 17°C for
2–3 days prior to their use in the voltage clamp experiments. The
ND96 solution contained 96 mM NaCl, 2 mM KCl, 1.8 mM
CaCl2, 2 mM MgCl2, 5 mM HEPES, titrated to pH
7.40 with NaOH. The recordings were performed 2–9 days following
injection. A standard two-microelectrode voltage-clamp technique
was used to record the currents at 21–23°C. The glass
microelectrodes were filled with 3 M KCl solution to obtain a
resistance of 1–3 MΩ. The oocytes were clamped with a standard
two-microelectrode voltage-clamp amplifier (DAGAN CA-1B; Dagan
Corporation, Minneapolis, MN, USA) and PLAMP software (Axon
Instruments, Foster City, CA, USA). Oocytes injected with hHCN4
cRNA were superfused with ND96 solution at a rate of 2.0 ml/min.
The control currents were recorded repeatedly at 1 min intervals,
with drug application continuing until the control currents
achieved a stable level.
Drugs and reagents
Collagenase type I, zaterbradine, CsCl, HEPES and
4-aminopyridine were purchased from Sigma Chemicals. Pronase E was
obtained from Roche (Basel, Switzerland). Berberine hydrochloride
was obtained from the Yichang Humanwell Pharmaceutical Co., Ltd
(Yichang, Hubei, China) as base powders and dissolved in distilled
water. To maintain the drug and ion concentrations constant, the
perfusion rate was strictly controlled using the perfusion device
BPS-4 (ALA Scientific Instruments, Inc, Westbury, NY, USA) and a
constant-flow pump.
Data acquisition and statistical
analysis
All data were stored on a computer hard disk and
analyzed off-line using Clampfit 10.0 (Axon Instruments) and Origin
8.0 software (Origin Laboratory, Northampton, MA, USA). The
amplitudes of HCN-mediated currents were defined as the
time-dependent components (Istep) at the end of the hyperpolarizing
pulses or peak tail currents (Itail) at the beginning of the
depolarizing pulses. To construct the I–V correlations, the
currents were normalized to their own maximum current measured
prior to drug treatment and then plotted as a function of the test
potential (Vt). The voltage dependency of the HCN current
activation was determined by analysis of the Itail measured at
depolarizing potentials. All tail current amplitudes from each
individual oocyte were normalized to their own Imax, plotted as a
function of Vt and fitted with a Boltzmann function:
I/Imax=1/[1+exp(Vt-V1/2)/k)] to determine the values of
the half-point (V1/2) and the slope (k). The time
constants for HCN current activation or deactivation (τactivation
or τdeactivation) at different Vt were determined using standard
exponential curve fitting. Activating or deactivating currents were
fitted to a single exponential function: I(t)=Ae−t/τ+C.
The concentration-effect curves were fitted using the Hill equation
in the form f=1/[1+(IC50/D)n], where f represents the
increase in HCN currents, expressed as a percentage change from the
control values, IC50 was the half-maximum inhibitory
concentration of berberine, D was the concentration of berberine
and n was the Hill coefficient.
The data are presented as the mean ± standard
deviation. The Student’s t-test was used for statistical analysis
of the paired observations, and an analysis of variance was
performed to test the difference among the groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
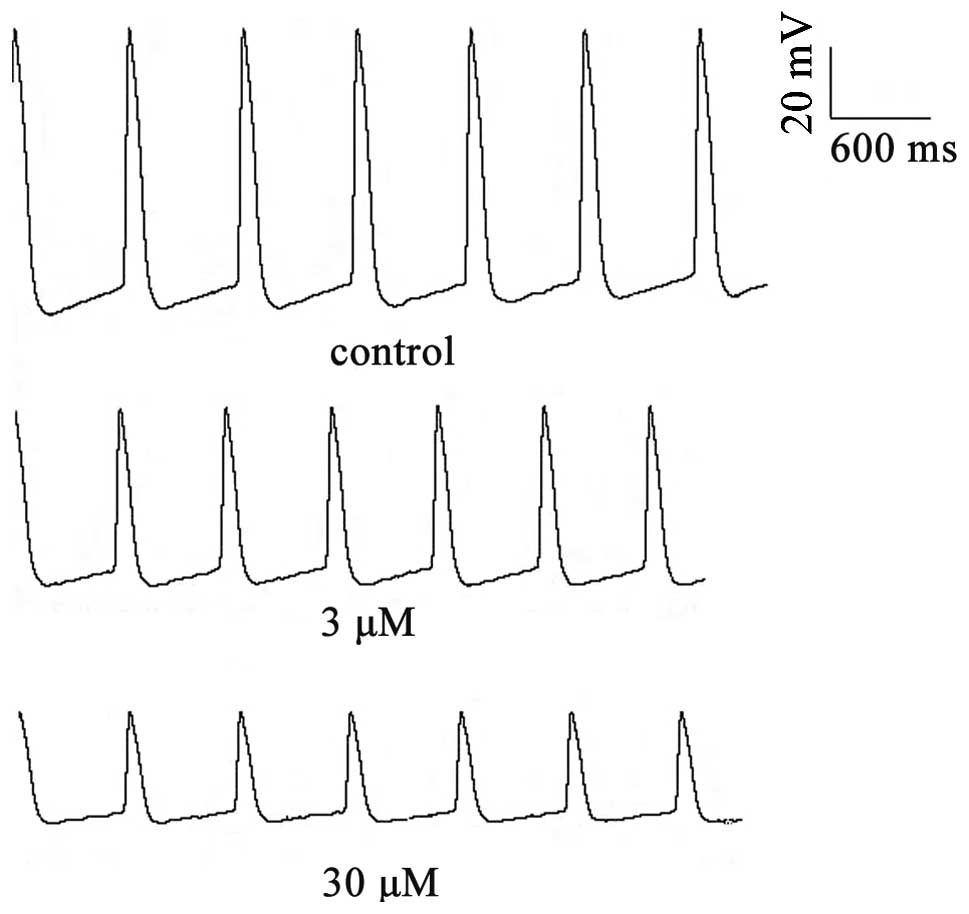
Effects of berberine on the spontaneous
APs of rabbit SNA tissues
To examine the effects of berberine on the
spontaneous APs in rabbit SAN tissues, transmembrane potentials
were recorded by a standard microelectrode technique. Berberine
application had depressant effects on spontaneous activity, as
demonstrated in Fig. 2. The
effects of berberine (3 or 30 μM) on the AP parameters in the
normal SA node pacemaker cells are shown in Table I. Compared with the control group,
berberine (3 or 30 μM) significantly decreased the DDR and rate of
pacemaker firing (RPF), and the changes in the RPF induced by
berberine paralleled those in the DDR. Meanwhile, the amplitude of
the AP and the maximal diastolic potential decreased as a result of
berberine treatment. The above effects occurred following 5–10 min
of berberine superfusion and reached their peak within 15–20
min.
 | Table IEffects of berberine on spontaneous AP
characteristics in rabbit sinoatrial node preparations. |
Table I
Effects of berberine on spontaneous AP
characteristics in rabbit sinoatrial node preparations.
| n | MDP (mV) | APA (mV) | DDR (mV/s) | APD50
(ms) | APD90
(ms) | RPF (bpm) |
|---|
| Control | 6 | −54.4±4.1 | 59.5±8.1 | 27.3±5.2 | 111.6±16.8 | 153.0±14.9 | 112.8±10.5 |
| 3 μM | 6 | −32.5±3.4b | 43.4±4.4a | 18.3±2.5b | 126.9±34.6 | 161.9±41.8 | 95.1±14.7a |
| 30 μM | 6 | −21.0±1.7b | 39.3±2.4b | 13.6±1.2b | 133.9±14.5 | 180.0±18.6a | 86.4±9.8b |
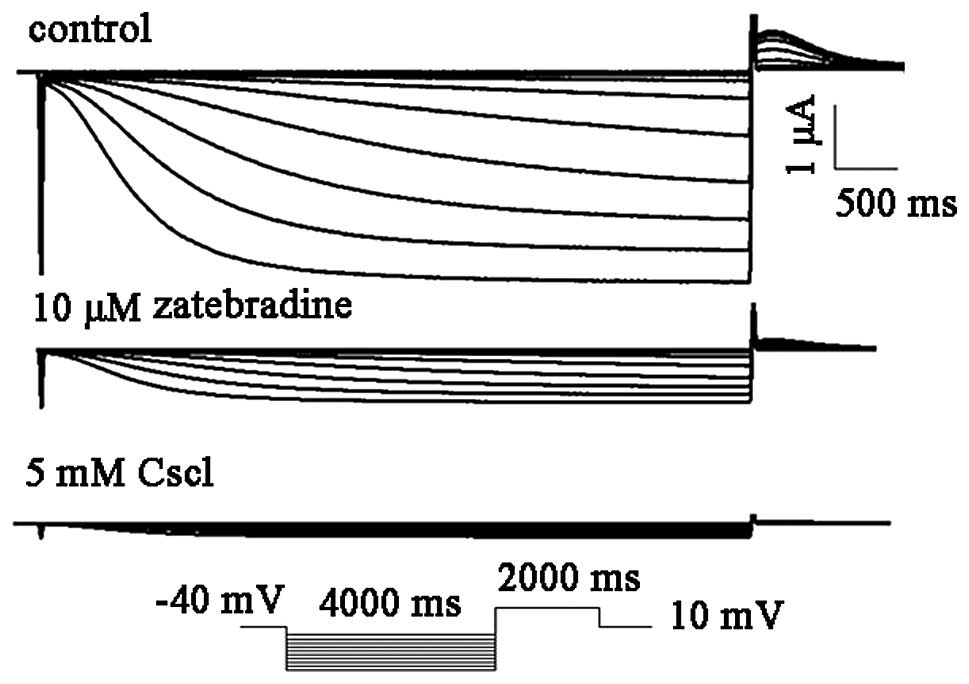
Electrophysiological properties of hHCN4
channels heterologously expressed in xenopus oocytes
Xenopus oocytes were utilized as a heterologous
expression system and the actions of berberine on the expression of
hHCN4 were analyzed. For the voltage-clamp recordings, the hHCN4
current was elicited by hyperpolarization pulses of 4,000 ms from a
holding potential of −60 to −150 mV in 10 mV decrements at 0.1 Hz
and then clamped back to 10 mV for 2,000 ms (Fig. 2). Thereafter, selective and
non-selective f-channel blockers, zatebradine and CsCl, were
utilized to confirm the biophysical properties of the HCN channel.
The HCN4 (n=4) currents were readily and completely blocked by 5 mM
CsCl. By contrast, 10 μM zatebradine markedly inhibited the hHCN4
currents by 82.1±8.4% (n=4; Fig.
3).
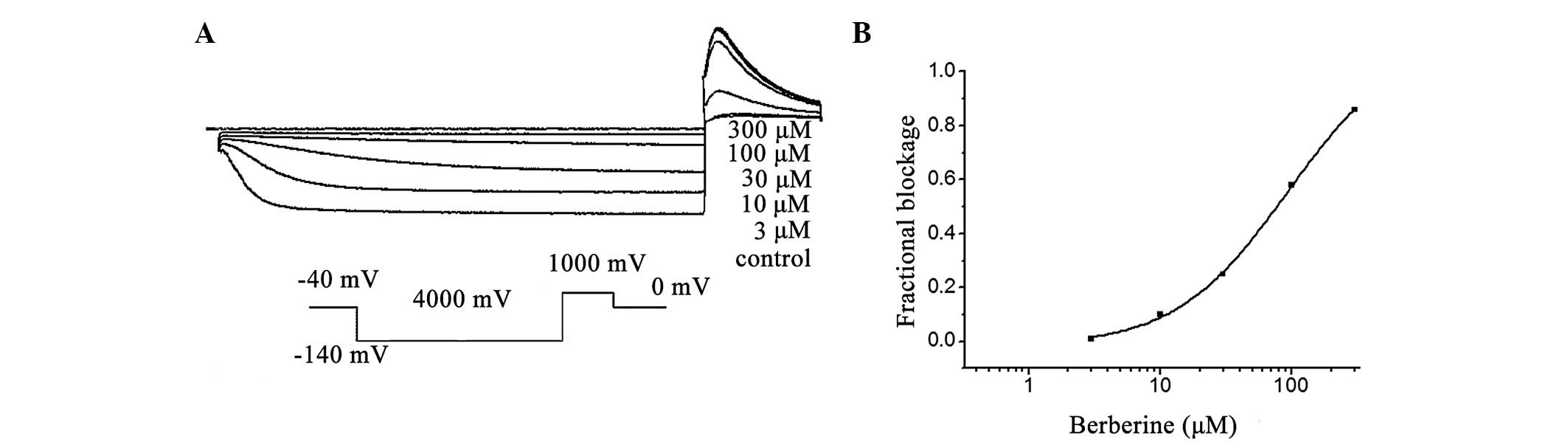
Concentration-dependent blockage of hHCN
currents by berberine
In the present study, it was identified that hHCN4
currents were inhibited by berberine in a concentration-dependent
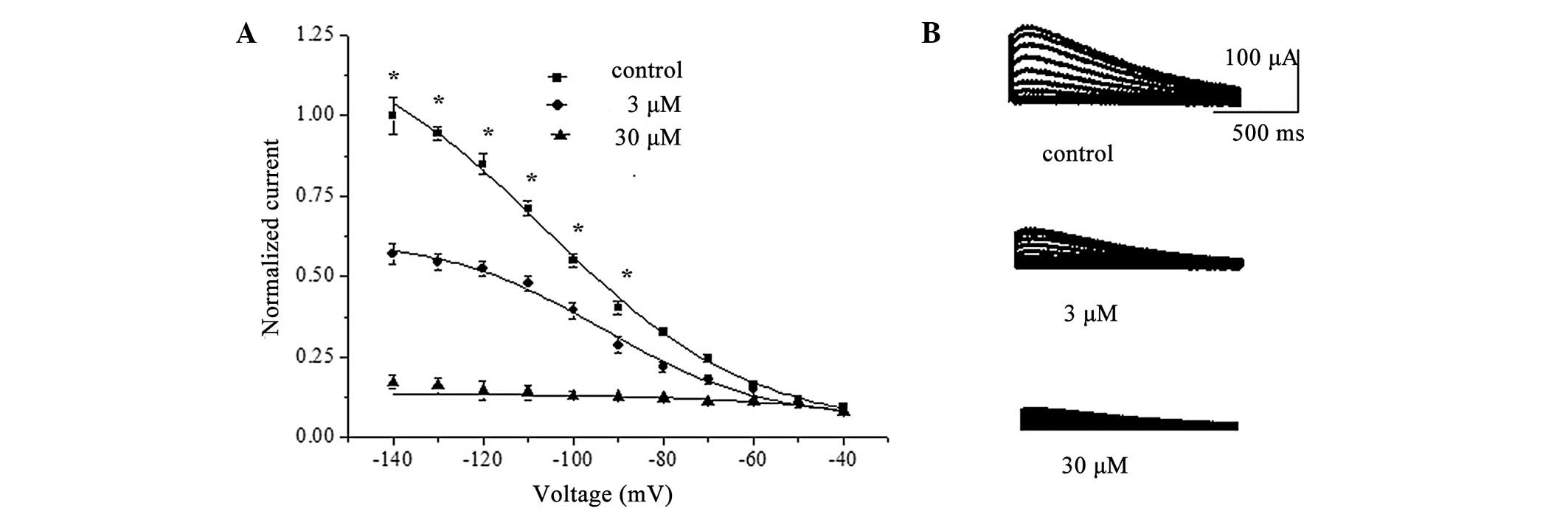
manner at the investigated test potential of −110 mV (Fig. 4A). Superfusion of berberine (1 to
300 μM) reduced the normalized Istep at voltages ranging from −100
to −140 mV, with the most pronounced effects observed at the more
hyperpolarized voltages (n=8; P<0.05). Fig. 4 also depicts the correlation
between the decreased fraction of the hHCN4 current and the
concentration of berberine at −120 mV, with an IC50
value of 32.3±2.1 μM and a Hill coefficient of 1.5±0.2 (n=8;
Fig. 4B).
Berberine increases the HCN4 current
values of τactivation and τdeactivation and slows the kinetics of
the hHCN4 channel
In addition to the inhibitory effect of berberine on
current amplitude in HCN4 channels demonstrated above, berberine
also modulated HCN4 channel current kinetics. The representative
traces of the hHCN4 current and the expanded traces of the outward
tail current prior to (control) and following 3 or 30 μM berberine
treatment are illustrated in Fig.
5A, and the amplitudes of the measured tail currents were
normalized to the peak value, plotted as a function of test
voltage, and fitted with a Boltzmann function to obtain the
isochronal voltage dependence of HCN4-channel activation.
Superfusion of berberine (3 or 30 μM) reduced the normalized Itail
at voltages ranging from −90 to −140 mV, with more pronounced
effects at the more hyperpolarized voltages (n=8; P<0.05;
Fig. 5B). Furthermore, the average
value for V1/2 was −102.7±1.9 mV under the control
conditions and −93.8±1.7 mV (n=8; P<0.05) or −80.1±2.4 mV (n=8;
P<0.05) following the addition of berberine (3 or 30 μM). The
slope factor (k) of the activation curve was decreased from
19.0±1.9 to 14.7±1.8 mV and 12.5±2.3 mV in the presence of
berberine at 3 and 30 μM, respectively.
In accordance with the more negative Vt caused by
berberine treatment, the activation of HCN4 channels was
significantly easier. In the presence of berberine, τactivation was
significantly increased in the potential channel from −140 mV to
−90 mV, and with Vt becoming more negative, this change was more
evident. The values of τactivation were markedly increased by
berberine at 3 and 30 μM, from 504.6±39.8 ms (n=8) to 588.4±21.7 ms
(3 μM, n=8, P<0.05) and 1176.4±57.3 ms (30 μM, n=8, P<0.05),
respectively, at a Vt of −110 mV. The time constant for the current
deactivation at 10 mV corresponding to a test potential of −120 mV
was 504.6±39.8 ms (n=8) in the control and 588.4±21.7 ms (3 μM,
n=8, P>0.05) and 1176.4±57.3 ms (30 μM, n=8, P<0.05) in the
presence of berberine at 3 and 30 μM, respectively (Fig. 6).
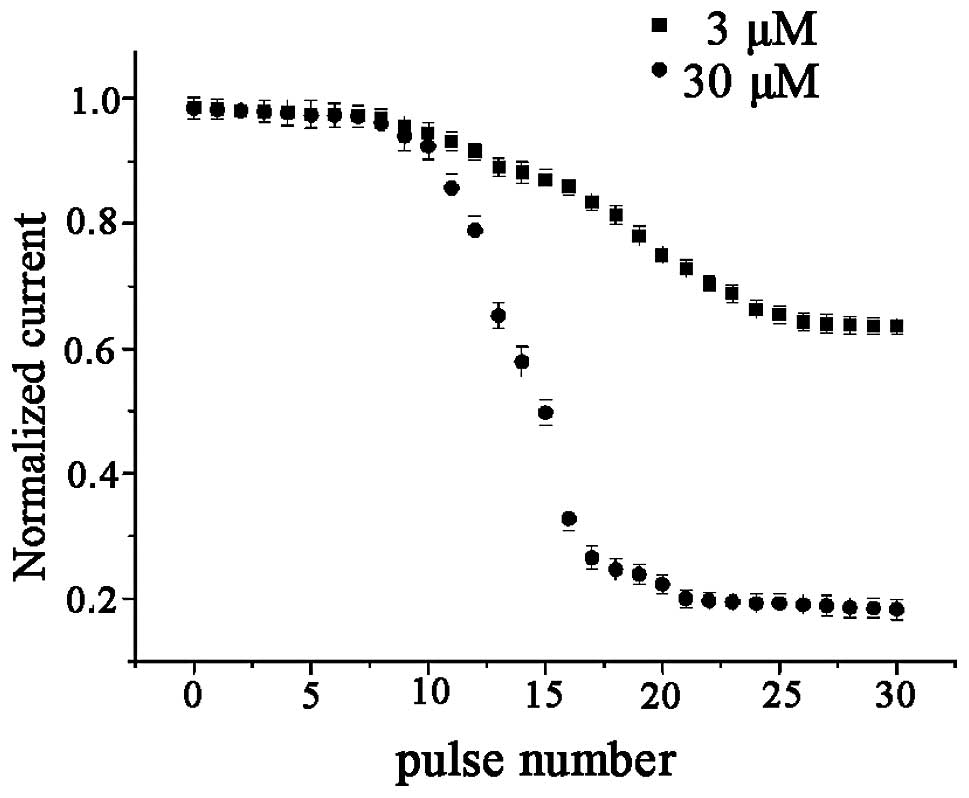
Use-dependent blockage of hHCN4 currents
by berberine in xenopus oocytes
To study the use-dependent blockage of the hHCN4
current by berberine, a standard activation/deactivation protocol
of 2,000 ms pulse from a holding potential of −30 mV to −100 mV
followed by a 1,000 ms depolarizing pulse to 0 mV at a rate of 0.25
Hz was used. The HCN currents, measured at the end of −100 mV, were
plotted as a function of the pulse number (Fig. 7). During the train pulse
stimulation there was no decline in the amplitude of the hHCN4
currents in the absence of berberine. In the absence of berberine,
hHCN4 currents generated by 30 pulse burst stimulations were
essentially identical. Further studies demonstrated that hHCN4
currents appeared to exhibit a gradual decline following
superfusion with berberine (1–300 μM), until the amplitude of HCN
currents reached a steady state. Furthermore, compared with the
control conditions, superfusion with 30 μM berberine may have no
marked blocking effect on the amplitude of hHCN4 currents at the
first pulse stimulation, which may be considered as the control
pulse. Following berberine application at the holding potential, a
significant decline in the amplitudes of the hHCN4 currents
occurred. From the results, it was identified that the higher
concentration of the drug (30 μM) had faster kinetics of blockage
than the lower concentration (3 μM).
Discussion
In the present study, it was identified that the
partial depression of If by berberine was parallel to the decrease
of the slope of DDR and the reduction of the firing rate of the SAN
cells by the intracellular microelectrode technique. This indicated
that the negative chronotropic effect of berberine was mainly
through its inhibition of the If. Next, the effects of berberine on
the hHCN4 channels expressed in xenopus oocytes, which were able to
generate the If of the SAN, were characterized by the standard
two-microelectrode voltage-clamp technique. The results were as
follows: i) Berberine decreased the rate of spontaneous RPF and the
DDR of the SAN pacemaker cells; ii) hHCN4 channel blockage by this
drug was concentration-dependent; iii) berberine markedly shifted
the activation and deactivation curve of the hHCN4 currents towards
more negative potentials and markedly slowed the kinetics of the
activation and deactivation of hHCN4 channels; iv) berberine
blocked the hHCN4 channel current in a use-dependent manner.
Previous studies have indicated that berberine
decreases the frequency of the spontaneous contractions of rabbit
sinoatrial cells and guinea pig right atria in a
concentration-dependent manner (15,21),
Riccioppo (15) hypothesized that
this decrease in the spontaneous contraction frequency was
accompanied by a depression of the phase 4 depolarization, without
significant changes in the other parameters of the nodal AP.
However, the present study identified that berberine potently
decreased the spontaneous firing and increased the AP duration of
SAN pacemaker cells in rabbits. Next, the study focused on the
action of berberine on the most prevalent members of the HCN family
in cardiac SA node cells, the hHCN4 subunits, which were
heterologously expressed in xenopus oocytes. Individual HCN
subunits have six transmembrane segments (S1–S6). The highly
positively charged S4 domain is the putative voltage sensor, and
the P domain between the S5 and S6 domains acts as the ion
conducting pore and selectivity filter C (22–25).
The allosteric hypothesis proposed by Altomare et al
(26) suggested that the
probability of a channel opening increased every time one voltage
sensor switched to the activated state. The results of the present
study demonstrated that berberine principally affected the
activation of the HCN4 channel, which decreased the probability of
channel opening. This may be one reason why berberine is able to
inhibit the HCN channel current. By contrast, the fully activated
current relation of native HCN channels was linear and reversed at
potentials compatible with permeability to both Na+ and
K+, with a preference for a PNa/PK
ratio ranging from 0.25 to 0.41 (5). Therefore, K+ current
inhibition by berberine may lead to the reduction of the HCN
channel current.
In addition, it was hypothesized that the effect of
berberine on the hHCN4 currents in xenopus oocytes may occur in a
use-dependent manner. By the application of a train voltage pulse
stimulation, it was observed that the inhibitory action of
berberine on the HCN current was progressively strengthened, until
reaching a steady state. There was incomplete blockage during the
first pulse and incomplete recovery during the interval between the
pulses until a steady state was reached. This demonstrated that
through repeated stimulation, inhibition of the HCN4 current by
berberine increased, i.e., it reduced the number of channel
openings per unit of time. The repeated stimulation led to the
channels combining with the drug in an inactive state.
In recent studies, it has been well established
through the cardiac-specific and inducible knockout model of HCN4
and HCN4 channel mutation models, that the HCN4 current provides a
fundamental contribution to basal heart rate maintenance and
modulation, as its removal led to basal bradycardia and a markedly
reduced response to sympathetic stimulation. Furthermore, HCN4
ablation in the model by Baruscotti et al (25) caused progressive development of
deep bradycardia (~50% of the original rate), as recorded by
telemetry, eventually leading to an atrioventricular (AV) block and
heart arrest in ~5 days. These data revealed that the expression of
HCN4 in the SAN is a direct determinant of the heart rate and that
removal of cardiac HCN4 channels from pacing tissue is lethal.
Clinical trials and animal studies have suggested a number of
beneficial effects of berberine on cardiovascular performance. In
one study, berberine prevented ischemia-induced ventricular
tachyarrhythmias, enhanced the force of cardiac contractions and
decreased peripheral vascular resistance and blood pressure
(27). Previously, it has been
noted that paroxysmal fibrillation may be triggered by ectopic
firing foci located in the pulmonary veins and that slow diastolic
depolarization and HCN4 proteins have been observed in the
pulmonary veins (28,29). Other studies observed that, in
cardiac hypertrophy and heart failure, HCN4 is upregulated in the
atrial and ventricular myocardium and may therefore contribute to
ectopic beat formation and enhanced electrical activity (10). Therefore, the overexpression of If
may be an important trigger of arrhythmogenic activity in the
hypertrophied heart (30–33). Therefore, inhibition of the
pacemaker current by berberine in these extranodal areas may
contribute to its well known antiarrhythmic actions.
Acknowledgements
This study was supported by ‘the Fundamental
Research Funds for the Central Universities’ (grant no.
201130202020022). The authors are grateful to Professor A. Ludwig
and Professor J. Stieber (Friedrich-Alexander-Universität
Erlangen-Nürnberg, Erlangen, Germany) for generously providing the
hHCN4 clones.
References
|
1
|
DiFrancesco D: Pacemaker mechanisms in
cardiac tissue. Annu Rev Physiol. 55:455–472. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DiFrancesco D: The role of the funny
current in pacemaker activity. Circ Res. 106:434–446. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Robinson RB and Siegelbaum SA:
Hyperpolarization-activated cation currents: from molecules to
physiological function. Annu Rev Physiol. 65:453–480. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baruscotti M, Bucchi A and Difrancesco D:
Physiology and pharmacology of the cardiac pacemaker (‘funny’)
current. Pharmacol Ther. 107:59–79. 2005.
|
|
5
|
Accili EA, Proenza C, Baruscotti M and
DiFrancesco D: From funny current to HCN channels: 20 years of
excitation. News Physiol Sci. 17:32–37. 2002.PubMed/NCBI
|
|
6
|
Shi W, Wymore R, Yu H, et al: Distribution
and prevalence of hyperpolarization-activated cation channel (HCN)
mRNA expression in cardiac tissues. Circ Res. 85:e1–e6. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thollon C, Bedut S, Villeneuve N, et al:
Use-dependent inhibition of hHCN4 by ivabradine and relationship
with reduction in pacemaker activity. Br J Pharmacol. 150:37–46.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ludwig A, Zong X, Jeglitsch M, Hofmann F
and Biel M: A family of hyperpolarization-activated mammalian
cation channels. Nature. 393:587–591. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaupp UB and Seifert R: Molecular
diversity of pacemaker ion channels. Annu Rev Physiol. 63:235–257.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sartiani L, Cerbai E and Mugelli A: The
funny current in cardiac non-pacemaker cells: functional role and
pharmacological modulation. Modern Pacemakers Present and Future.
32:595–610. 2011.
|
|
11
|
Hoppe UC and Beuckelmann DJ:
Characterization of the hyperpolarization-activated inward current
in isolated human atrial myocytes. Cardiovasc Res. 38:788–801.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stillitano F, Sartiani L, DePaoli P,
Mugelli A and Cerbai E: Expression of the
hyperpolarization-activated current, I(f), in cultured adult rat
ventricular cardiomyocytes and its modulation by hypertrophic
factors. Pharmacol Res. 57:100–109. 2008. View Article : Google Scholar
|
|
13
|
Imanshahidi M and Hosseinzadeh H:
Pharmacological and therapeutic effects of Berberis vulgaris and
its active constituent, berberine. Phytother Res. 22:999–1012.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dai DZ: Vulnerable substrate and multiple
ion channel disorder in a diseased heart will be new targets for
antiarrhythmic therapy. Acta Pharmacol Sin. 21:289–295. 2000.
|
|
15
|
Riccioppo Neto F: Electropharmacological
effects of berberine on canine cardiac Purkinje fibers and
ventricular muscle and atrial muscle of the rabbit. Br J Pharmacol.
108:534–537. 1993.PubMed/NCBI
|
|
16
|
Wang YX, Zheng YM and Zhou XB: Inhibitory
effects of berberine on ATP-sensitive K+ channels in
cardiac myocytes. Eur J Pharmacol. 316:307–315. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sánchez-Chapula J: Increase in action
potential duration and inhibition of the delayed rectifier outward
current IK by berberine in cat ventricular myocytes. Br J
Pharmacol. 117:1427–1434. 1996.PubMed/NCBI
|
|
18
|
Xu SZ, Zhang Y, Ren JY and Zhou ZN:
Effects of berberine of L- and T-type calcium channels in guinea
pig ventricular myocytes. Zhongguo Yao Li Xue Bao. 18:515–518.
1997.PubMed/NCBI
|
|
19
|
Wang YX and Zheng YM: Ionic mechanism
responsible for prolongation of cardiac action-potential duration
by berberine. J Cardiovasc Pharmacol. 30:214–222. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li BX, Yang BF, Zhou J, Xu CQ and Li YR:
Inhibitory effects of berberine on IK1, IK,
and HERG channels of cardiac myocytes. Acta Pharmacol Sin.
22:125–131. 2001.
|
|
21
|
Shaffer JE: Inotropic and chronotropic
activity of berberine on isolated guinea pig atria. J Cardiovasc
Pharmacol. 7:307–315. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Santoro B and Tibbs GR: The HCN gene
family: molecular basis of the hyperpolarization-activated
pacemaker channels. Ann NY Acad Sci. 868:741–764. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Biel M, Schneider A and Wahl C: Cardiac
HCN channels: structure, function, and modulation. Trends
Cardiovasc Med. 12:206–212. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wahl-Schott C and Biel M: HCN channels:
structure, cellular regulation and physiological function. Cell Mol
Life Sci. 66:470–494. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baruscotti M, Bucchi A, Viscomi C, et al:
Deep bradycardia and heart block caused by inducible
cardiac-specific knockout of the pacemaker channel gene
Hcn4. PNAS. 108:1705–1710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Altomare C, Terragni B, Brioschi C, et al:
Heteromeric HCN1-HCN4 channels: a comparison with native pacemaker
channels from the rabbit sinoatrial node. J Physiol. 549:347–359.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lau CW, Yao XQ, Chen ZY, Ko WH and Huang
Y: Cardiovascular actions of berberine. Cardiovasc Drug Rev.
19:234–244. 2001.PubMed/NCBI
|
|
28
|
Chen YJ, Chen SA, Chang MS and Lin CI:
Arrhythmogenic activity of cardiac muscle in pulmonary veins of the
dog: implication for the genesis of atrial fibrillation. Cardiovasc
Res. 48:265–273. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haïssaguerre M, Jaïs P, Shah DC, et al:
Spontaneous initiation of atrial fibrillation by ectopic beats
originating in the pulmonary veins. N Engl J Med. 339:659–666.
1998.PubMed/NCBI
|
|
30
|
Cerbai E, Barbieri M and Mugelli A:
Occurrence and properties of the hyperpolarization-activated
current If in ventricular myocytes from normotensive and
hypertensive rats during aging. Circulation. 94:1674–1681. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fernández-Velasco M, Goren N, Benito G, et
al: Regional distribution of hyperpolarization-activated current
(If) and hyperpolarization-activated cyclic nucleotide-gated
channel mRNA expression in ventricular cells from control and
hypertrophied rat hearts. J Physiol. 553:395–405. 2003.
|
|
32
|
Stilli D, Sgoifo A, Macchi E, et al:
Myocardial remodeling and arrhythmogenesis in moderate cardiac
hypertrophy in rats. Am J Physiol Heart Circ Physiol.
280:H142–H150. 2001.PubMed/NCBI
|
|
33
|
Zorn-Pauly K, Schaffer P, Pelzmann B, et
al: If in left human atrium: a potential contributor to atrial
ectopy. Cardiovasc Res. 64:250–259. 2004. View Article : Google Scholar : PubMed/NCBI
|