Introduction
Unexplained pain has been reported as a common
complaint of the elderly, and age-related patterns in pain
prevalence are complex (1,2). Neuropathic pain, one of the most
common types of chronic pain, results from the abnormal processing
of sensory input due to damage caused by disorders of the nervous
system, such as spinal cord injury, and increases with advancing
age (3,4).
c-Fos is a cellular proto-oncogene, belonging to the
immediate early gene family. c-Fos has been used as a relative
marker of neuronal activity in the brain following numerous types
of brain insult (5–7). Furthermore, c-Fos expression in the
spinal cord is considered to be a neurotoxic biomarker which has
been detected in the dorsal spinal neurons, following nociceptive
stimulation (8–12) and repeated swim stress (13).
Previous studies regarding age-related physiological
changes, have focussed on spinal cord-specific changes. A loss of
myelin and axonal involution have been observed in the aged rat
spinal cord (14), and expression
levels of substance P, a major neurotransmitter of primary afferent
nociceptive fibers, and somatostatin have been shown to decrease in
the spinal cord of the aged rat (15,16).
Little is currently known about the changes in c-Fos expression in
the spinal cord during the process of normal aging.
The present study compared the age-related changes
in the immunoreactivity of c-Fos in the spinal cords of the young
adult and aged Beagle dog and C57BL/6J mouse. Both the Beagle and
C57VL/6J mice are considered to be good animal models to study
aging (17–21).
Materials and methods
Experimental animals
Clinically and neurologically healthy male Beagle
dogs and male C57BL/6J mice were used in the present study. Young
adult dogs, aged 2–3 years, and aged dogs, aged 10–12 years were
used (n=7/group); alongside young adult mice, at 6 months, and aged
mice, at 24 months (n=14/group). The animals were maintained in
conventional housing under adequate temperature (23°C) and humidity
(60%) conditions, with a 12 h light/12 h dark cycle, and free
access to food and water.
Animal handling and care followed the guidelines of
the current international laws and policies [National Institute of
Health (NIH) Guide for the Care and Use of Laboratory Animals, NIH
Publication no. 85-23, 1985, revised 1996], and the experimental
protocol was approved by the Institutional Animal Care and Use
Committee of Kangwon National University (approval no.
KW-130424-3). All of the experiments were conducted to minimize the
number of animals used, and to avoid animal suffering.
Tissue processing for histology
For histochemical analysis, the young adult and aged
dogs and mice (n=7 in each group) were anesthetized with a mixture
of Zoletil 50 (8 mg/kg; Virbak Korea, Seoul, Korea) Xylazine (2
mg/kg; Bayer Korea, Seoul Korea) and pentobarbital sodium (40
mg/kg; JW Phar. Co., Ltd., Seoul, Korea), respectively.
Anaesthetization was followed by a transcardial perfusion with 0.1
M phosphate-buffered saline (PBS, pH 7.4; Sigma-Aldrich, St. Louis,
MO, USA), followed by 4% paraformaldehyde (Samchun Chemicals,
Pyeongtaek, Korea) in 0.1 M phosphate buffer (pH 7.4). The cervical
(C6-C8) and lumbar (L5-L6) spinal cord regions were harvested from
the animals and postfixed, in the same fixative, for 12 h. The
spinal cord tissues were cryoprotected by infiltration with 30%
sucrose (Junsei Chemical Co., Ltd., Tokyo, Japan) overnight.
Subsequently, the frozen tissues were serially sectioned at 30 μm
using a cryostat (Leica, Wetzlar, Germany) and the sections were
then placed into six-well plates containing PBS.
Fluoro-Jade B (F-J B) histofluorescence
staining
F-J B histofluorescence staining procedures were
conducted according to previous methods (22). Briefly, the sections were immersed
in a solution of 80% ethanol containing 1% sodium hydroxide,
followed by immersion in 70% ethanol. The sections were then
transferred into a solution of 0.06% potassium permanganate, prior
to staining with a 0.0004% F-J B solution (Histochem, Jefferson,
AR, USA). The sections were placed on a slide warmer (~50°C), and
examined using an epifluorescent microscope (Carl Zeiss,
Oberkochen, Germany) with blue (450–490 nm) excitation light and a
barrier filter. This method has been previously reported as being
useful in the detection of neuronal degeneration, as degenerating
neurons brightly fluoresce in comparison to background fluorescence
(23).
Immunohistochemistry for NeuN and
c-Fos
Immunohistochemistry for NeuN and c-Fos was
performed under the same conditions in both the dogs and the mice
of different age groups, in order to determine whether the degree
of immunohistochemical staining was accurate. The sections were
sequentially treated with 0.3% H2O2 and 10%
normal donkey serum (Vector Laboratories, Burlingham, CA, USA). The
sections were then incubated with diluted mouse anti-NeuN (1:1,000;
Chemicon International, Temecula, CA, USA) and goat anti-c-Fos
(1:100; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA)
antibodies. The sections were subsequently exposed to biotinylated
horse anti-mouse or rabbit anti-goat antibodies and streptavidin
peroxidase complex (1:200; Vector Laboratories Inc., Burlingame,
CA, USA). The immunocomplexes were visualized by staining with
3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich, St. Louis,
MO, USA) in 0.1 M Tris-HCl buffer (pH 7.2) and mounted onto
gelatin-coated slides. The sections were mounted in Canada balsam
(Kanto Chemical Co., Inc., Portland, OR, USA) following
dehydration. In order to establish the specificity of the
immunostaining, a negative control test was carried out with
pre-immune serum in place of a primary antibody. The negative
control test resulted in the absence of immunoreactivity in all
structures.
Data analysis
All measurements were performed double blind, in
order to ensure objectivity, and the measures of both the control
and experimental samples were carried out under the same
conditions. NeuN-immunoreactive neurons in the young adult and aged
dogs and mice were quantified from 10 sections/each animal, using
Optimas 6.5 image analyzing system (CyberMetrics, Scottsdale, AZ,
USA) equipped with a computer-based charge coupled device camera.
The cell counts were obtained by averaging the counts from the
sections taken from each animal. The data rpesented are
representative of a percentage of the young adult group.
Ten sections per animal were selected to
quantitatively analyze c-Fos immunoreactivity. Digital images of
the spinal cord were captured using an Axio M1 light microscope
(Carl Zeiss) equipped with a digital camera (Axiocam; Carl Zeiss),
connected to a PC monitor. The staining intensity of the
c-Fos-immunoreactive structures was evaluated on the basis of a
relative optical density (ROD), which was obtained after the
transformation of the mean gray level using the following formula:
ROD = log (256/mean gray level). The ROD of the complete field was
measured, and the brightness and contrast of each image file was
calibrated using Adobe Photoshop version 8.0 (Adobe Systems
Incorporated, San Jose, CA, USA). The images were then analyzed
using the NIH Image 1.59 software (National Institutes of Health,
Bethesda, MD, USA). The values of the background staining were
obtained and subtracted from the immunoreactive intensities. The
data was presented as %, with the young adult group designated as
100%.
Western blot analysis for c-Fos
To confirm the changes in the c-Fos expression
levels in the cervical spinal cord region between the young adult
and aged mice, the mice spinal cord tissues (n=7 in each group)
were used for western blot analysis. Briefly, the tissues were
homogenized in 50 mM PBS (pH 7.4), containing ethylene glycol
tetraacetic acid (pH 8.0), 0.2% NP-40, 10 mM
ethylenediaminetetraacetic acid (pH 8.0), 15 mM sodium
pyrophosphate, 100 mM β-glycerophosphate, 50 mM sodium fluoride,
150 mM sodium chloride, 2 mM sodium orthovanadate, 1 mM
phenylmethanesulfonyl fluoride, and 1 mM dithiothreitol (DTT; Santa
Cruz Biotechnology, Inc.). Following centrifugation at 16,000 × g
for 20 min, the protein concentration of the supernatants was
determined using a Micro Bicinchoninic Acid Protein Assay kit
(Pierce Biotechnology, Rockford, IL, USA). Aliquots containing 20
μg of total protein were boiled in a loading buffer containing 150
mM Tris (pH 6.8), 3 mM DTT, 6% SDS, 0.3% bromophenol blue, and 30%
glycerol. Subsequently, the aliquots were loaded onto a
polyacrylamide gel, and separated by electrophoresis, after which
the blots were transferred to nitrocellulose membranes (Pall
Corporation, Port Washington, NY, USA). The membranes were
incubated with 5% non-fat dry milk in PBS containing 0.1% Tween-20,
followed by an incubation with the primary antibody for 2 h. The
membranes were then incubated with peroxidase-conjugated donkey
anti-goat immunoglobulin G (Sigma-Aldrich). An Enhanced
Chemiluminescence kit (Pierce Biotechnology) was used to visualize
the blots. Western blot analysis was repeated three times.
Following exposure of the membranes, the blots were scanned and
densitometric analysis for the quantification of the bands was
performed using Scion Image software (Scion Corporation, Frederick,
MD, USA), which was used to determine the ROD. A ratio of the ROD
was calibrated as a percentage, with the young adult group
designated as 100%.
Statistical analysis
The difference of the mean ROD between the groups
was statistically analyzed using a Student t-test. A P<0.05 was
considered to indicate a statistically significant difference.
Results
NeuN-immunoreactive neurons
NeuN-immunoreactive neurons were shown to be
distributed throughout the grey matter of the cervical and lumbar
spinal cord regions in the young adult and aged dogs (Fig. 1A–1D). There were no significant
differences in the number of NeuN-immunoreactive neurons between
the young adult and aged dogs, however the number of
NeuN-immunoreactive neurons was shown to be slightly decreased in
the cervical and lumbar regions of the aged spinal cord, as
compared with the number in the young adult spinal cord (data not
shown).
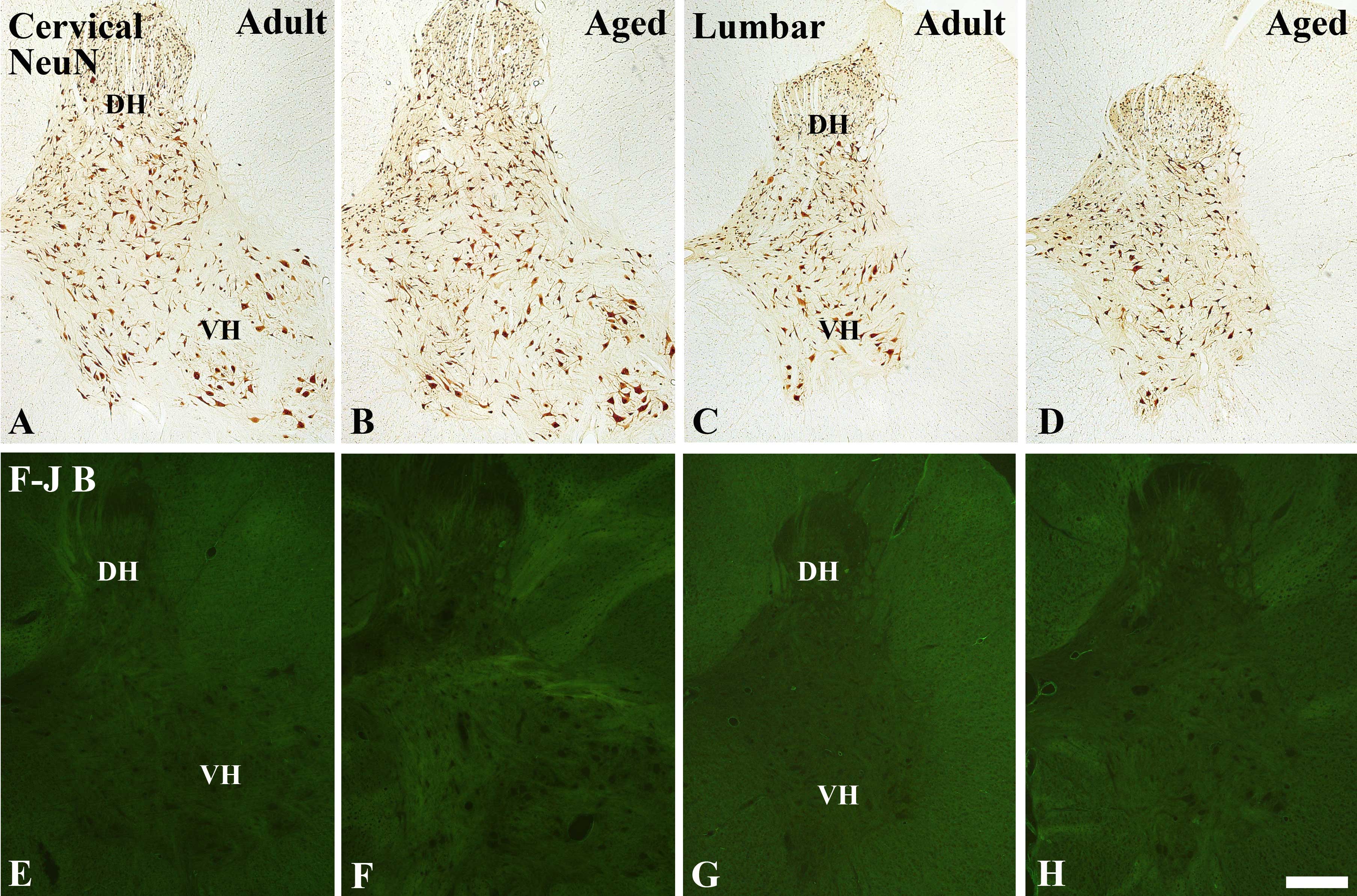 | Figure 1(A–D) Immunohistochemical staining for
NeuN immunoreactivity and (E–H) Fluoro-Jade B histofluorescence in
the (A, B, E and F) cervical and (C, D, G and H) lumbar spinal cord
regions of the (A, C, E and G) young adult and (B, D, F and H) aged
dogs. There were no significant differences in neuronal loss in the
young adult or aged dogs. DH, dorsal horn; VH, ventral horn.
Magnification, ×5; Scale bar = 500 μm. |
Similarly to the dogs, the number of
NeuN-immunoreactive neurons in the cervical and lumbar spinal cord
regions was not significantly different between the young adult and
aged mice (data not shown).
F-J B positive cells
To examine the extent of neuronal degeneration of
the spinal cord, F-J B staining was performed in the young adult
and aged dogs and mice. F-J B positive cells were not observed in
the cervical and lumbar spinal cord regions of the young adult and
aged dogs (Fig. 1E–H) or mice
(data not shown).
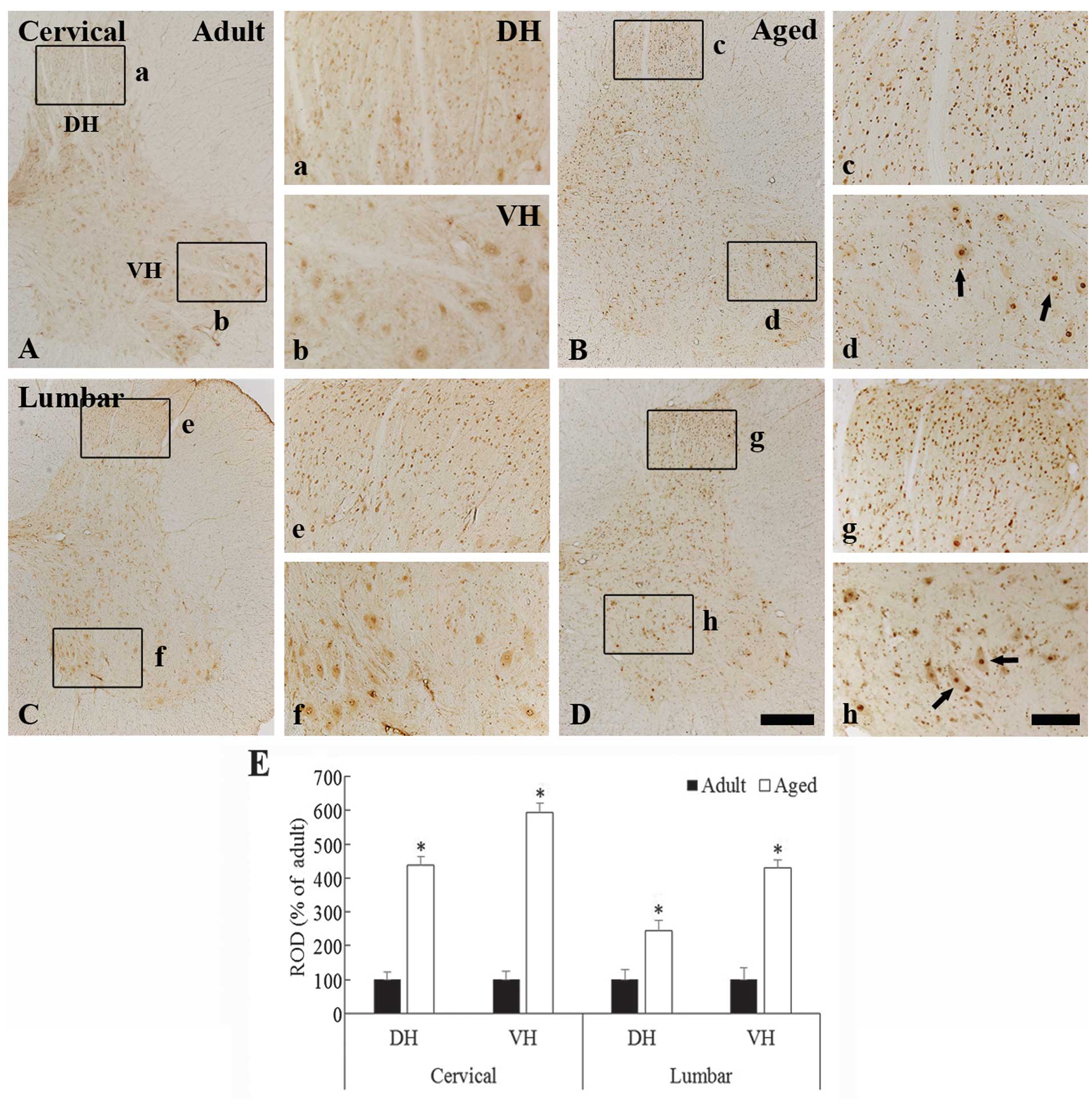
c-Fos immunoreactivity
In the young adult dogs, moderate c-Fos
immunoreactivity was observed in the neurons in the whole grey
matter of the cervical and lumbar spinal cord regions (Fig. 2A, 2b, 2C and 2f). The c-Fos
immunoreactivity was generally found to be contained within the
nuclei of the spinal neurons (Fig. 2b
and 2f). In the aged dogs, the c-Fos expression pattern was
similar to that in the young adult dogs (Fig. 2B, 2c, 2d, 2D, 2g, and 2h); however,
the c-Fos immunoreactivity in the nuclei of the neurons in the aged
dogs was significantly higher as compared with that in the young
adult dogs (Fig. 2c, 2d, 2g, 2h and
2E) (P<0.05).
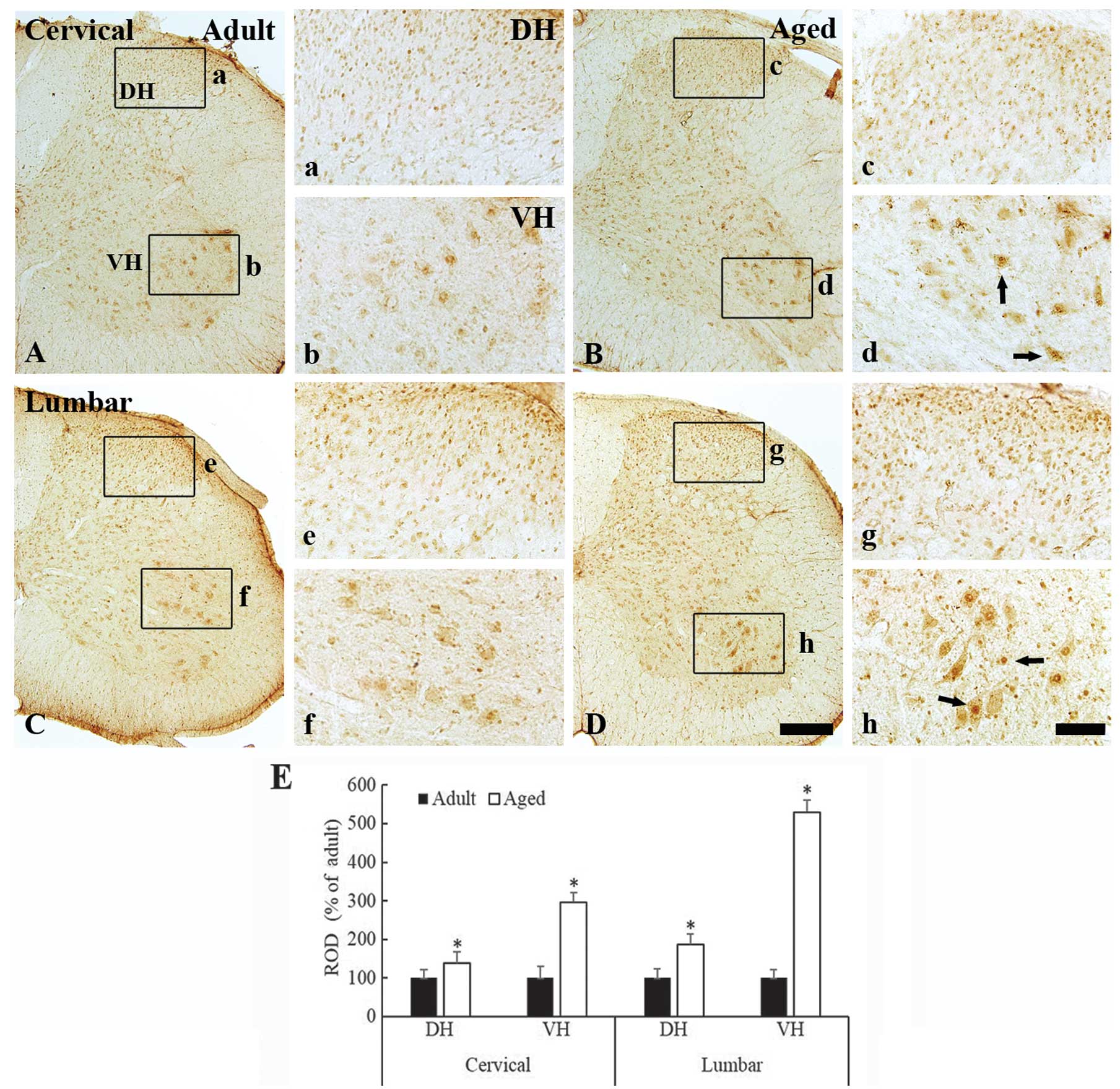
c-Fos immunoreactivity was also observed in the
nuclei of the spinal neurons of the cervical and lumbar spinal cord
regions in both the young adult and aged mice (Fig. 3A–3D). Similarly to the aged dogs,
c-Fos immunoreactivity was significantly increased in the aged mice
as compared with the young adult mice. This increase in
immunoreactivity was observed in both the dorsal (Fig. 3c and 3g) and the ventral horn
(Fig. 3d and 3h) of the cervical
and lumbar spinal cord regions (Fig.
3E) (P<0.05).
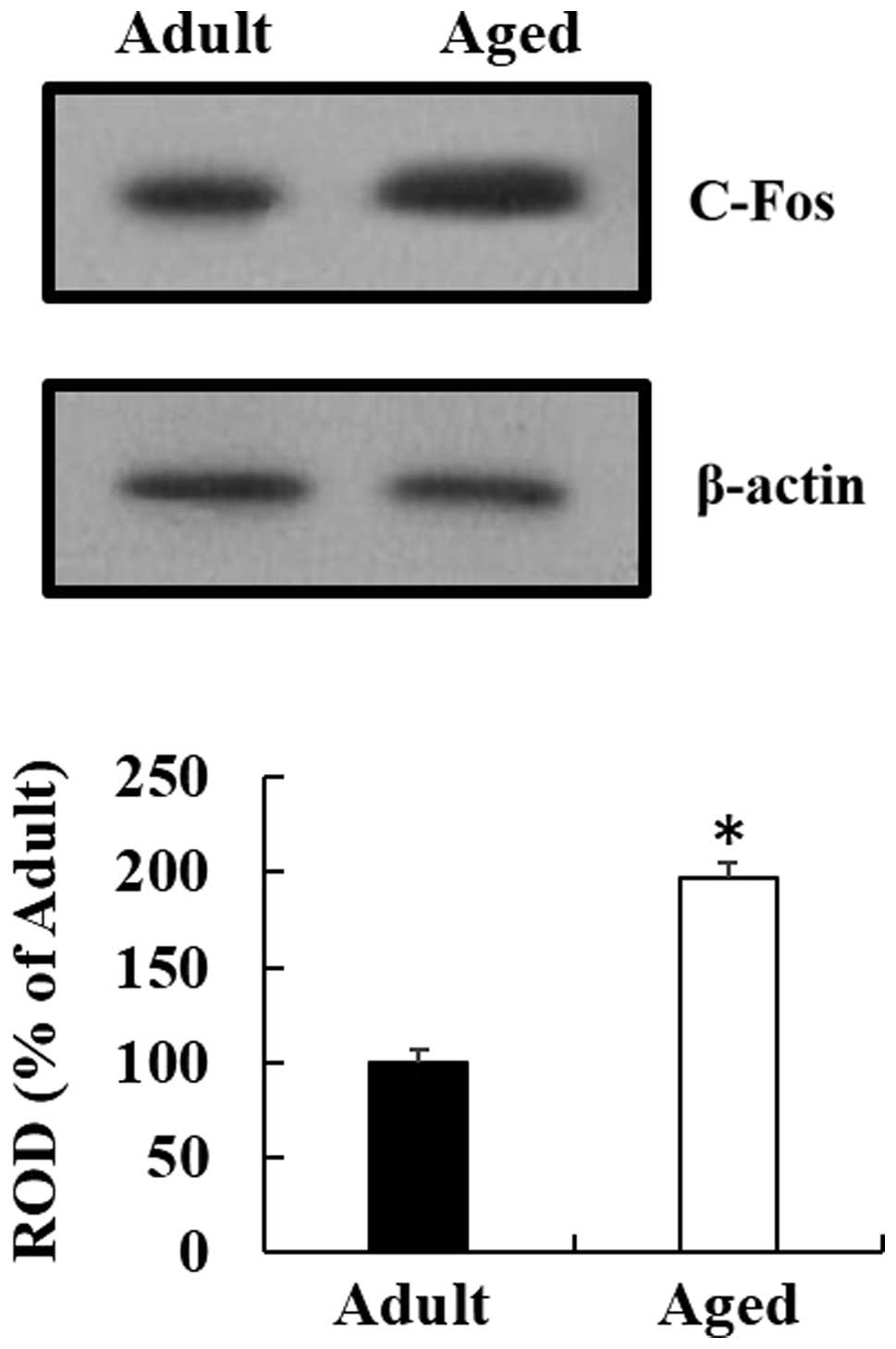
c-Fos protein levels
Western blot analysis indicated that the pattern of
change in the c-Fos protein expression levels, in the cervical
spinal cord region of the aged mice, was similar to that observed
by immunohistochemical analysis. c-Fos protein expression in the
cervical spinal cord region of the aged mice was significantly
increased, as compared with the expression in the young adult mice
(Fig. 4) (P<0.05).
Discussion
Age-related pain is associated with a poor quality
of life, physical disability and an increased risk of mortality
(24,25). In the present study, c-Fos
immunoreactivity in the spinal cord was compared between young
adult and aged dogs and mice. Changes to the distribution and
damage/death of spinal neurons were also investigated in both the
young adult and aged spinal cords.
There was no significant difference observed in the
number of NeuN-immunoreactive neurons between the spinal cords of
the young adult and aged dogs and mice. In addition, F-J B positive
degenerating neurons were not detected in the spinal cord of either
the young adult or aged animals. This finding is consistent with
findings from our previous studies, which reported that no distinct
neuronal loss was observed in the aged spinal cord of German
shepherd dogs (26,27).
The present study found that weak c-Fos
immunoreactivity was observed in the dorsal and ventral spinal
neurons of the young adult dogs and mice, whereas the c-Fos
immunoreactivity was significantly increased in the aged dogs and
mice as compared with the young adult groups. The present findings
support previous research that showed that low levels of c-Fos
immunoreactivity was observed in young adult animals (28–30).
However, other studies have previously reported that the basal
expression of c-Fos in the dorsal horn was decreased in the aged
rat spinal cord (31), which is
not consistent with the findings of the present study. This
discrepancy may result from differences in experimental
methods.
It has previously been reported that c-Fos and
astrocytes become activated by a combined stress of non-thermal
irradiation and the toxic effects of picrotoxin in the rat brain
(32). Activated astrocytes are
associated with several neuropathic and cancer pain states, through
the release of neurotoxic substances including pro-inflammatory
cytokines (33–35). In addition, it has previously been
reported that pro-inflammatory cytokines, including interferon-γ,
and interleukins (IL)-1β and -2, were markedly increased, without
any significant neuronal loss, in the spinal cord of the aged dog
(26,36). It has also been reported that
increasing age can enhance the expression levels of tumor necrosis
factor-α, the IL-6 family of cytokines, chemokine receptor-2 and
pro-inflammatory chemokines following ischemic stroke in the aged
rat (37). Therefore, based on
these data and the results of the present study, c-Fos expression
in the spinal cord may be triggered by different stress factors,
including chronic inflammatory activity.
In conclusion, the present study observed that c-Fos
immunoreactivity in the aged dog and mouse spinal cords was
markedly increased, as compared with young adult animals. This
finding implicates that the increase in the expression of c-Fos in
the aged dog spinal cord may be associated with aging-related
changes in the aged spinal cord.
Acknowledgements
The authors would like to thank Mr. Seung Uk Lee for
his technical help in this study. This research was supported by
the National Research Foundation of Korea funded by the Ministry of
Education, Science and Technology (2010-0010580) and by the ICT
R&D program of MSIP/IITP (10033634).
References
|
1
|
Gibson SJ and Helme RD: Age-related
differences in pain perception and report. Clin Geriatr Med.
17:433–456. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Helme RD and Gibson SJ: The epidemiology
of pain in elderly people. Clin Geriatr Med. 17:417–431. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Werhagen L, Budh CN, Hultling C and
Molander C: Neuropathic pain after traumatic spinal cord injury -
relations to gender, spinal level, completeness, and age at the
time of injury. Spinal Cord. 42:665–673. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schmader KE: Epidemiology and impact on
quality of life of postherpetic neuralgia and painful diabetic
neuropathy. Clin J Pain. 18:350–354. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coggeshall RE: Fos, nociception and the
dorsal horn. Prog Neurobiol. 77:299–352. 2005.PubMed/NCBI
|
|
6
|
Munglani R, Hudspith MJ, Fleming B, et al:
Effect of pre-emptive NMDA antagonist treatment on long-term Fos
expression and hyperalgesia in a model of chronic neuropathic pain.
Brain Res. 822:210–219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu Y and Westlund KN: Effects of baclofen
on colon inflammation-induced Fos, CGRP and SP expression in spinal
cord and brainstem. Brain Res. 889:118–130. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abbadie C, Besson JM and Calvino B: c-Fos
expression in the spinal cord and pain-related symptoms induced by
chronic arthritis in the rat are prevented by pretreatment with
Freund adjuvant. J Neurosci. 14:5865–5871. 1994.PubMed/NCBI
|
|
9
|
Hwang HJ, Lee HJ, Kim CJ, Shim I and Hahm
DH: Inhibitory effect of amygdalin on lipopolysaccharide-inducible
TNF-alpha and IL-1beta mRNA expression and carrageenan-induced rat
arthritis. J Microbiol Biotechnol. 18:1641–1647. 2008.PubMed/NCBI
|
|
10
|
Liu CR, Duan QZ, Wang W, et al: Effects of
intrathecal isoflurane administration on nociception and Fos
expression in the rat spinal cord. Eur J Anaesthesiol. 28:112–119.
2011. View Article : Google Scholar
|
|
11
|
Wu J, Hu Q, Huang D, Chen X and Chen J:
Effect of electrical stimulation of sciatic nerve on synaptic
plasticity of spinal dorsal horn and spinal c-fos expression in
neonatal, juvenile and adult rats. Brain Res. 1448:11–19. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zeng X, Huang H and Hong Y: Effects of
intrathecal BAM22 on noxious stimulus-evoked c-fos expression in
the rat spinal dorsal horn. Brain Res. 1028:170–179. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Quintero L, Cuesta MC, Silva JA, et al:
Repeated swim stress increases pain-induced expression of c-Fos in
the rat lumbar cord. Brain Res. 965:259–268. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Terao S, Sobue G, Hashizume Y, Shimada N
and Mitsuma T: Age-related changes of the myelinated fibers in the
human corticospinal tract: a quantitative analysis. Acta
Neuropathol. 88:137–142. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hukkanen M, Platts LA, Corbett SA,
Santavirta S, Polak JM and Konttinen YT: Reciprocal age-related
changes in GAP-43/B-50, substance P and calcitonin gene-related
peptide (CGRP) expression in rat primary sensory neurones and their
terminals in the dorsal horn of the spinal cord and subintima of
the knee synovium. Neurosci Res. 42:251–260. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ranson RN, Priestley DJ, Santer RM and
Watson AH: Changes in the substance P-containing innervation of the
lumbosacral spinal cord in male Wistar rats as a consequence of
ageing. Brain Res. 1036:139–144. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Head E, Nukala VN, Fenoglio KA, Muggenburg
BA, Cotman CW and Sullivan PG: Effects of age, dietary, and
behavioral enrichment on brain mitochondria in a canine model of
human aging. Exp Neurol. 220:171–176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
González-Martínez Á, Rosado B, Pesini P,
et al: Plasma β-amyloid peptides in canine aging and cognitive
dysfunction as a model of Alzheimer’s disease. Exp Gerontol.
46:590–596. 2011. View Article : Google Scholar
|
|
19
|
Barańczyk-Kuźma A, Usarek E,
Kuźma-Kozakiewcz M, et al: Age-related changes in tau expression in
transgenic mouse model of amyotrophic lateral sclerosis. Neurochem
Res. 32:415–421. 2007. View Article : Google Scholar
|
|
20
|
Garbuzova-Davis S, Haller E, Saporta S,
Kolomey I, Nicosia SV and Sanberg PR: Ultrastructure of blood-brain
barrier and blood-spinal cord barrier in SOD1 mice modeling ALS.
Brain Res. 1157:126–137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coksaygan T, Magnus T, Cai J, et al:
Neurogenesis in Talpha-1 tubulin transgenic mice during development
and after injury. Exp Neurol. 197:475–485. 2006. View Article : Google Scholar
|
|
22
|
Candelario-Jalil E, Alvarez D, Merino N
and León OS: Delayed treatment with nimesulide reduces measures of
oxidative stress following global ischemic brain injury in gerbils.
Neurosci Res. 47:245–253. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmued LC and Hopkins KJ: Fluoro-Jade B:
a high affinity fluorescent marker for the localization of neuronal
degeneration. Brain Res. 874:123–130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kendig H, Browning CJ and Young AE:
Impacts of illness and disability on the well-being of older
people. Disabil Rehabil. 22:15–22. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thomas E, Peat G, Harris L, Wilkie R and
Croft PR: The prevalence of pain and pain interference in a general
population of older adults: cross-sectional findings from the North
Staffordshire Osteoarthritis Project (NorStOP). Pain. 110:361–368.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chung JY, Choi JH, Lee CH, et al:
Comparison of ionized calcium-binding adapter molecule
1-immunoreactive microglia in the spinal cord between young adult
and aged dogs. Neurochem Res. 35:620–627. 2010. View Article : Google Scholar
|
|
27
|
Ahn JH, Choi JH, Kim JS, et al: Comparison
of immunoreactivities in 4-HNE and superoxide dismutases in the
cervical and the lumbar spinal cord between adult and aged dogs.
Exp Gerontol. 46:703–708. 2011.PubMed/NCBI
|
|
28
|
Lawrence J, Stroman PW, Bascaramurty S,
Jordan LM and Malisza KL: Correlation of functional activation in
the rat spinal cord with neuronal activation detected by
immunohistochemistry. Neuroimage. 22:1802–1807. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bullitt E: Expression of c-fos-like
protein as a marker for neuronal activity following noxious
stimulation in the rat. J Comp Neurol. 296:517–530. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu YP and Ling EA: Expression of Fos in
the spinal motoneurons labelled by horseradish peroxidase following
middle cerebral artery occlusion in rat. Brain Res Bull.
45:571–576. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim JM, Lee KW, Chung YH, Shin CM, Baik SH
and Cha CI: c-Fos basal immunoreactivity decreases in rat spinal
cord during normal ageing. Neuroreport. 10:585–588. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carballo-Quintás M, Martínez-Silva I,
Cadarso-Suárez C, et al: A study of neurotoxic biomarkers, c-fos
and GFAP after acute exposure to GSM radiation at 900 MHz in the
picrotoxin model of rat brains. Neurotoxicology. 32:478–494. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun YN, Luo JY, Rao ZR, Lan L and Duan L:
GFAP and Fos immunoreactivity in lumbo-sacral spinal cord and
medulla oblongata after chronic colonic inflammation in rats. World
J Gastroenterol. 11:4827–4832. 2005.PubMed/NCBI
|
|
34
|
Hald A, Nedergaard S, Hansen RR, Ding M
and Heegaard AM: Differential activation of spinal cord glial cells
in murine models of neuropathic and cancer pain. Eur J Pain.
13:138–145. 2009. View Article : Google Scholar
|
|
35
|
Watkins LR, Milligan ED and Maier SF:
Glial activation: a driving force for pathological pain. Trends
Neurosci. 24:450–455. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee DH, Ahn JH, Park JH, et al: Comparison
of expression of inflammatory cytokines in the spinal cord between
young adult and aged beagle dogs. Cell Mol Neurobiol. 33:615–624.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dinapoli VA, Benkovic SA, Li X, et al: Age
exaggerates proinflammatory cytokine signaling and truncates signal
transducers and activators of transcription 3 signaling following
ischemic stroke in the rat. Neuroscience. 170:633–644. 2010.
View Article : Google Scholar : PubMed/NCBI
|