The Ki67 antigen, which encodes two protein isoforms
with molecular weights of 345 and 395 kDa, was originally
identified by Scholzer and Gerdes in the early 1980s (1). The Ki67 protein has a half-life of
only ~1–1.5 h. It is present during all active phases of the cell
cycle (G1, S, G2 and M), but is absent in resting cells (G0)
(2,3). In later phases of mitosis (during
anaphase and telophase), a sharp decrease in Ki67 levels occurs
(4). Expression of the Ki67
protein (pKi67) is associated with the proliferative activity of
intrinsic cell populations in malignant tumors, allowing it to be
used as a marker of tumor aggressiveness (5,6). The
prognostic value of pKi67 has been investigated in a number of
studies with its potential as a reliable marker having been shown
in cancers of the breast, soft tissue, lung, prostate, cervix and
central nervous system (7–11).
Current classification schemes may require revision
where biological behavior and prognostic significance of these
tumors is concerned, as an increasing number of studies have
suggested that Ki67 may be an important factor in cancer grading
and prognostic evaluation. It has been shown that Ki67
immunohistochemical (IHC) staining is an effective method of
assessing the prognosis in a number of tumor types (12,13).
Although pKi67 is a key marker associated with
proliferating cancer cells and a poor prognosis, its full potential
in increasing proliferation has not been evaluated. In syngeneic
animal models with subcutaneous or orthotopic bladder cancer,
prostate cancer or renal cell carcinoma, antisense oligonucleotides
induced tumor growth inhibition (14,15),
potentially through the inhibition of Ki-67, indicating the
involvement of Ki67 in tumor cell proliferation.
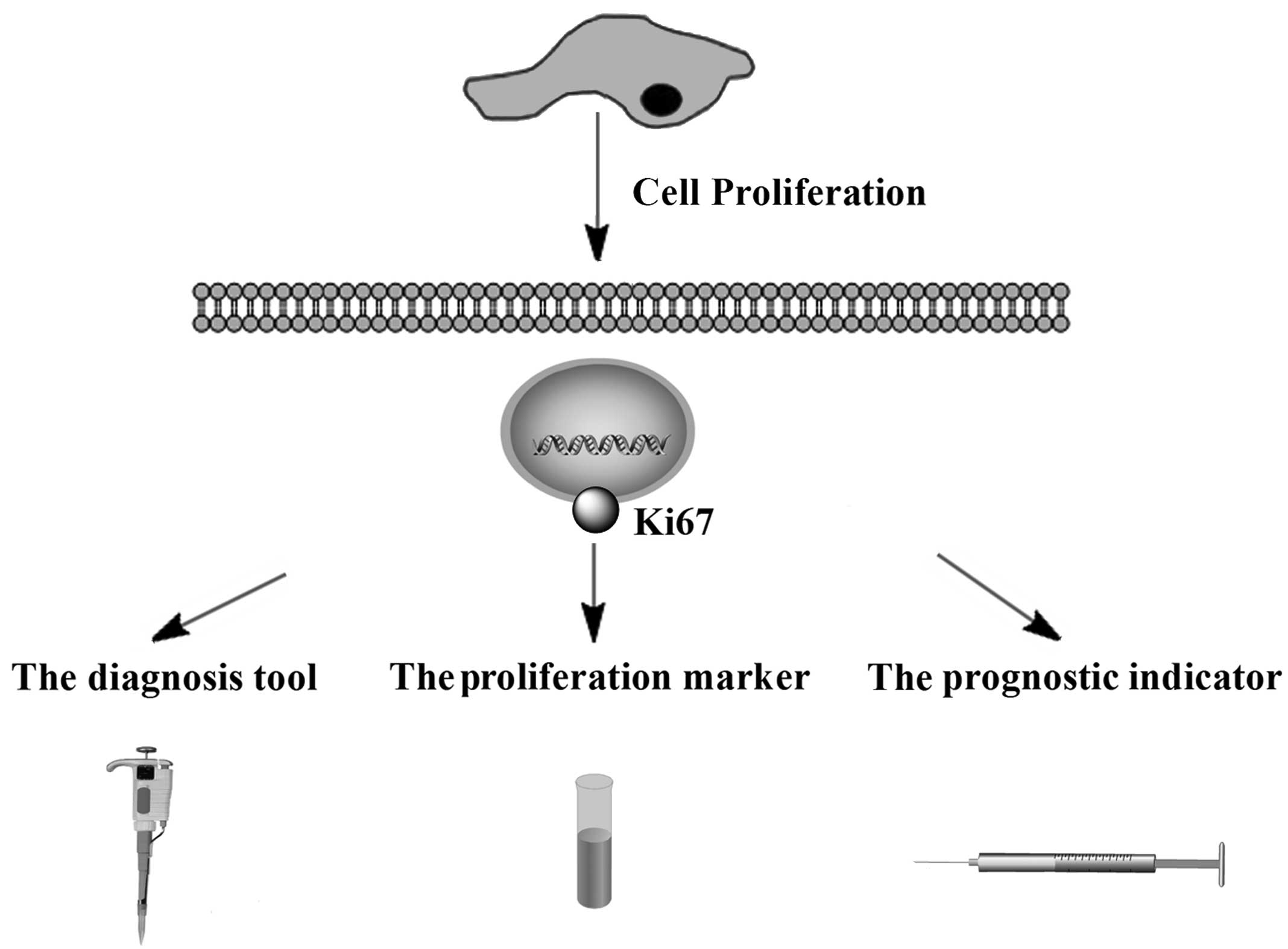
This review provides an update on the current
knowledge of Ki67 and the evidence regarding the prognostic and
predictive role of this marker (Fig.
1). It also discusses the laboratory techniques used to
determine Ki67 levels.
The Ki67 antigen was originally identified in the
1980s, as a proliferation-associated nuclear antigen, which is only
detected in dividing cells (G1-, S-, G2- and M-phase) and not in
quiescent cells (G0 phase) (16).
Ki67 levels are low in the G1 and S phases and peak early in
mitosis. In later phases of mitosis, a sharp decrease in Ki67
levels occurs (17). The gene
encoding Ki67 is a continuous sequence of 29,965-bp length located
on chromosome 10q25-ter and is comprised of 15 exons with sizes
ranging from 67 to 6845 bp and 14 introns with sizes ranging from
87 to 3569 bp. Exon 13 contains 16 homologous segments of 366 bp
(Ki67 repeats) located at the center of this gene. The complete
gene is comprised of a 74 bp 5′ region and a 264 bp 3′ region in
the Ki67 protein (18–20).
The quantity of pKi67 present at any time during the
cell cycle is regulated by a precise balance between synthesis and
degradation, as indicated by its short half life of 1–1.5 h
(20,21). Ki67 protein expression coincides
with the transit of cells through mitosis and undergoes
phosphorylation and dephosphorylation during mitosis in
vivo, rendering it susceptible to protease degradation.
Furthermore, its structure indicates that its expression is
regulated by proteolytic pathways, such as those controlled by the
key regulatory complex cyclin B/cyclin-dependent kinase 2 (1,22).
pKi67 is known to share structural similarities (including a
so-called fork head-associated domain) with other proteins, such as
DUN1 and RAD, which are involved in cell cycle regulation (23).
The characterization of the Ki67 promoter region is
essential for understanding gene transcription, and it is therefore
important to investigate this in order to develop targeted
interventions aimed at modulating gene expression (24). In a previous study, deletion
analysis and a dual luciferase reporter assay were used to locate
the Ki-67 core promoter from −223 to +12 nt relative to the
transcriptional initiation site, which is a TATA less, GC rich
region comprised of several putative Sp1 binding sites. It was
demonstrated that the region from −223 to +12 nt could drive the
transcription of the Ki-67 gene, and that the Sp1 binding site is
essential for the transcriptional regulation of the Ki-67 gene.
(25). An electrophoretic mobility
shift assay revealed three Sp1-binding sites in the Ki67 promoter
that are essential for its basal transcriptional activity.
It was found that expression of p53 is correlated
with that of Ki67 in several types of cancer, including oral
squamous cell cancer and breast cancer (26). How p53 may affect Ki67 gene
expression is not yet clear. As there are three Sp1-binding sites
in the Ki67 promoter and as p53 represses the transcription of
genes at Sp1-binding sites of promoters (20,26,27),
it is likely that p53 inhibits Ki67 promoter activity via p53- and
Sp1-dependent pathways. It is hypothesized that there are at least
two transcriptional regulatory mechanisms. One is that the
p53-binding motifs affect the transcriptional repression of the
Ki67 promoter. The other is a possible interaction between p53 and
Sp1 at Sp1-binding sites on the Ki67 promoter.
Scoring systems are based on the percentage of tumor
cells stained by an antibody. In order to assess the methodology of
a study, a publication is scored using The World Health
Organization’s classification system (28). The score is determined by various
aspects of methodology, which are grouped into three main
categories: The scientific design, the description of the
laboratory methods used to identify the presence of pKi67, DNA/RNA
or antibodies against Ki67 (29,30),
and some clinical reports that have used the techniques for Ki67
detection (31–34). Briefly, an automated bright-field
microscope and software are used to detect and classify. After the
Ki67-stained slide has been scanned at ×5 magnification, a trained
clinical laboratory scientist, who is blinded to the histological
diagnoses and patient survival data, randomly selects at least
eight fields representative of the range of Ki67 immunostaining in
the previously encircled tumor for evaluation with an automated
bright-field microscope at ×20 (35). The percentage of Ki-67-positive
tumor nuclei (Ki-67 index) was quantitated for each case using the
Ariol® SL-50 Image Analyzer and imaging software (Genetix Corp,
Boston, MA, USA)
Multiple clinical laboratories have reported the
successful use of Ki67 as a diagnostic tool (36–39).
Expression of Ki67, as evaluated by immunostaining has become the
gold standard, with a cutoff level of between 10 and 14%
positively-stained cells defined as high risk in terms of prognosis
(40–42). The St Gallen Consensus in 2009
considered the Ki-67 labelling index important for selecting the
addition of chemotherapy to endocrine therapy in hormone
receptor-positive breast cancers. In addition, tumors may be
classified as low, intermediate, and highly proliferating,
according to the Ki-67 labelling index of ≤15%, 16%–30%, and
>30%, respectively (43).
Tissue microarray technology has become increasingly important over
the past decade. There are concerns with the scoring reliability of
tissue microarrays due to tumor heterogeneity. To overcome this
issue, certain experts have used multiple cores when conducting
this analysis (44,45). However, a number of pathologists
have expressed the view that using a manual counting procedure will
obtain a more reliable score, which may lead to differences in
interpretation between examiners with consequent variability in
diagnoses (46). By contrast,
reverse transcription-quantitative polymerase chain reaction
assays, with convenient quantification of target transcripts,
result in objective and continuous data variables.
Nowadays, formalin-fixed paraffin-embedded tissue
samples are routinely used for gene expression analysis, which can
aid in overcoming technical difficulties (47–50).
The use of formalin-fixed, paraffin-embedded (FFPE) tissue
overcomes the most prominent issues related to research on
relatively rare diseases: limited sample size, availability of
control tissue, and time frame.
FFPE samples are easy to handle and store and are
suitable for diagnostic histology, immunohistochemistry, and in
situ hybridization. Therefore, this sample type is almost
always available for diseases in which treatment involves surgery
to remove parts of or almost the entire affected organ.
Furthermore, invaluable clinical information and follow-up data are
often accessible in conjunction with FFPE samples (3). Lastly, the tissue is usually
available in amounts adequate for use in GEM studies and obtained
biologically relevant and disease-specific, significant genes;
cancer-related genes.
The data on Ki67 as a diagnostic marker is scarce
and based on varying laboratory and statistical methods. Cancer has
a complex pathogenesis and reliable early diagnosis is difficult.
Symptoms usually do not appear until the disease has progressed to
an advanced stage. Therefore, further research into diagnostic and
prognostic markers may aid early diagnosis. Notably, the expression
of Ki67 reflects the tumor proliferation rate and correlates with
initiation, progression, metastasis and prognosis of a number of
types of tumors (18,67–72).
Certain regulators of these processes, such as Smac (73–76),
minichromosome maintenance 7 (77), p53 (10,78–80),
Bcl-2 (81), proliferating cell
nuclear antigen (PCNA) (75) and
CD105 (82) have been
investigated. In a number of studies, Ki67 appeared to be closely
correlated with pancreatic tumor severity as well as with
expression of Smac and thus may be useful as a diagnostic and
prognostic marker or, in conjunction with Smac, as an indicator of
treatment efficacy (73–75). In a further study, Chen et
al (83) reported that
utilizing Ki67 LI and vascular endothelial growth factor scoring is
useful to effectively and accurately predict outcomes and optimize
personal therapy in judging the outcomes of non-muscle invasive
bladder cancer This novel molecular grading system could enhance
the efficiency of the conventional system.
Colorectal carcinoma is the fourth leading cause of
cancer-related mortality worldwide (84). It has been demonstrated that the
Ki67 LI was higher in Dukes’ stage B than in Dukes’ stage C
carcinoma. They concluded that the positive rate of Ki-67 antibody
in poorly differentiated adenocarcinoma and mucinous carcinomas was
significantly lower than in well differentiated and moderately
differentiated adenocarcinomas, suggesting that proliferative
activity is low in cancers with poor differentiation. On the other
hand, it has been demonstrated that the Ki-67 LI is high in well to
moderately differentiated, non-mucinous adenocarcinomas in an early
Dukes’ stage (A or B) as compared with that in poorly
differentiated, mucinous adenocarcinomas or signet-ring cell
colorectal carcinomas in an advanced Dukes’ stage (C). MIB-1 is a
monoclonal antibody that recognizes a fixation resistant epitope of
the Ki67 antigen and it is used to estimate the proliferative
fraction of neoplasia (85). Using
MIB-1, it was observed that Ki67 LI was high in Grade I and Grade
II as compared with the Grade III carcinomas.
The Ki67 index is a diagnostic and prognostic aid in
several fields of pathology and an established predictive tool in
others (37,86–90).
However, existing Ki67 index estimations are time-consuming and
cumbersome, and may be subject to inter-observer variability
(90–93). To improve the accuracy of the Ki67
index, current research recommends the use of an IHC cocktail,
which detects Ki67 and the melanocytic marker, melanoma antigen
recognized by T cells (MART1) (94). In melanocytic pathology, current
research favors using Ki67/MART1 double stains to accurately
distinguish Ki67-positive melanocytic cells from other
proliferating Ki67-positive cells, including lymphocytes, stromal
cells and epithelial cells (95,96).
The usability and cost benefit of automated MART1-verified Ki67
indices in routine settings require investigation in a prospective
study with a consecutive inclusion of specimens. When predicting a
clinical outcome for the individual patient, automated
MART1-verified Ki67 indices may be more reliable as a result of a
reduction in false positive results with this assay.
It is known that Ki67 is expressed in all cell-cycle
phases outside of the resting phase G0. Academics recommend its use
as a prognostic marker over mitotic rate (97–100). A number of studies have shown a
correlation between proliferative markers and tumor grade (13,82,101). Studies have also suggested a
predictive role for pKi67, in that an individual patient may be
treated with a specific regimen based on the degree of pKi67
expression (78). While a
prognostic biomarker indicates the likely course of the disease in
an untreated individual, a predictive biomarker identifies
subpopulations of patients who are most likely to respond to a
given therapy. The results of one study indicated that the Ki67
labeling index is an independent prognostic factor for survival
rate, including all stage and grade categories (102). Studies demonstrated a correlation
between the ratio of Ki67-positive malignant cells and patient
survival (103,104). Vogt and Klapper (105) showed that the Ki67 index was
associated with prognosis in specimens from patients with primary
and relapsed mantle cell lymphoma in the large cohort, which was
included in the first of a number of studies on MCLs. The
pretherapeutic assessment of Ki67 expression is becoming more
important in the evaluation of tumor aggressiveness and the
selection of adequate treatment.
In a number of tumors there appears to be a degree
of correlation between pKi67 expression and patient survival, e.g.
cervical and uterine cancer, non-Hodgkin’s lymphoma and large bowel
cancer (106,107). Indeed, Kimura et al
(6) found that cases of colorectal
carcinoma showing a high pKi67 expression at the site of deepest
invasion had a worse prognosis. These findings may be the result of
the marked heterogeneity of pKi67 expression in carcinomas.
Numerous studies have similarly confirmed the utility of the Ki67
proliferation index as a prognostic indicator in cancer, as it
shows a correlation with primary tumor size, lymphatic invasion,
metastases, tumor proliferation activity measured by DNA flow
cytometry and shorter patient survival times.
Mucosal malignant melanoma (MMM) of the sinonasal
tract accounts for 1% of all mucosal melanomas and 3–4% of
malignant neoplasms of the sinonasal tract (108). Intense immunohistochemical
staining for Ki67 is correlated with a poor prognosis in various
malignancies (95,109,110). One study reported that scores of
≤40% for Ki67 and ≤80% for PCNA were correlated with good prognosis
in anorectal malignant melanoma (74,111). Another study showed that scores
of ≤20% for Ki67 and ≤35% for PCNA were correlated with a good
prognosis in cutaneous malignant melanoma (84,112). These studies showed that Ki67 is
negatively correlated with a more favorable prognosis, which
indicates that the Ki67 antigen may be useful as a diagnostic and
prognostic factor for MMM.
Ki-67 is a sensitive protein associated with cell
proliferation. Owing to high cell proliferation, frequently
associated with the Ki-67 protein labeling index, Ki67 may be a
promising factor for targeted molecular therapies.
Antisense oligodeoxynucleotides (ASOs) have been
studied as specific tools for treating tumors (14,113). In syngeneic animal models,
Ki-67-ASOs markedly inhibit tumour growth (114,115). However, there are several factors
that effect their application, including nuclease degradation
(116). In order to improve the
characteristics of ASOs, the development of their derivatives
requires promotion, particularly that of peptide nucleic acids
(PNAs) (117). PNAs are a DNA
mimic that can target DNA and RNA with high specifically and
affinity (105,118,119). In vitro, anti-K-67 PNAs
yielded a stronger inhibitive effect on Ki-67 expression than ASOs,
and had greater effects on the proliferation and apoptosis of human
renal carcinoma cells (115).
In recent years a new technique, known as RNA
interference (RNAi), has been developed, which suppresses gene
expression mediated by siRNAs (120). siRNA-inhibition of Ki-67
expression has been shown to lead to significant inhibition of
proliferation (14,121). While synthetic siRNAs can rapidly
knock down target genes and achieve similar effects, the result is
transient (73). The most
significant obstacles in the use of siRNAs are efficient uptake and
long-term stability (122). To
resolve these problems, certain studies have investigated the
transfection of plasmid vectors to stably synthesize so-called
“short hairpin RNAs” (shRNAs) in host cells, which makes it
possible alter native cell processes (123–125).
Concurrently, another study constructed a novel
oncolytic adenovirus-based shRNA expression system named ZD55, an
E1B 55kDa-deficient oncolytic adenovirus that is similar to
ONYX-015 (126), which has the
ability to deliver Ki-67- shRNA and the lytic ability of oncolytic
adenoviruses. ZD55-Ki67 induces silencing of the Ki-67 gene,
allowing for efficient tumor-specific viral replication and
inducing the apoptosis of tumor cells in vitro and in nude
mice (127).
Furthermore, the microinjection of antibodies
directed against the Ki-67 was shown to result in a decreased rate
of cell division (128,129). In this technique, the nuclear
localization presents a major hurdle, due to the need for
intracellular and intranuclear delivery of targeting and
therapeutic moieties. Zhang et al (129) used a liposomally encapsulated
construct to design photo immunoconjugate-encapsulating liposomes
(PICELs). Non-cationic PICELs are particularly useful for the
subcellular delivery of mAbs and provide multi-functional
constructs for imaging and therapy.
In summary, due to its ubiquitous expression in all
proliferating cells and the prognostic value of the Ki-67 index in
many cancers, pKi-67 is an potentially attractive therapeutic
target in cancer, and strategies that inactivate pKi-67 are a
promising anti-proliferative approach, with potentially broad
applicability in cancer treatment (14). Hence, targeting pathways and
molecular markers implicated in cancer cell growth is a promising
avenue for the development of effective therapies.
As a proliferation marker to measure the growth
fraction of cells in human tumors, the expression of Ki67 is
strongly associated with cell proliferation and is widely used in
routine pathology. pKi67 is well characterized at the molecular
level and extensively used as a prognostic and predictive marker in
cancer. Based on the studies presented here, Ki67 may be a
promising molecular candidate for the diagnosis and treatment of a
wide range of malignancies.
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81372916, 81372460
and 81101702), the Science and Technology Department of Xuzhou city
(grant no. XM13B084), the ‘Six Talent Peaks’ Project of Jiangsu
Province (grant no. 2013-WSN-014), the Key University Science
Research Project of Jiangsu Province (grant nos. 11KJA320002,
12KJA320001 and 11KJB320017) and the Science and Technology
Department of Jiangsu province (grant nos. BK20141142, BK2013348
and BK2011207).
|
1
|
Scholzen T and Gerdes J: The Ki-67
protein: from the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shirendeb U, Hishikawa Y, Moriyama S, et
al: Human papillomavirus infection and its possible correlation
with p63 expression in cervical cancer in Japan, Mongolia, and
Myanmar. Acta Histochem Cytochem. 42:181–190. 2009. View Article : Google Scholar
|
|
3
|
Hooghe B, Hulpiau P, Van Roy F and De
Bleser PD: ConTra: a promoter alignment analysis tool for
identification of transcription factor binding sites across
species. Nucleic Acids Res. 36:W128–W132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Modlin IM, Moss SF, Chung DC, et al:
Priorities for improving the management of gastroenteropancreatic
neuroendocrine tumors. J Natl Cancer Inst. 100:1282–1289. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klöppel G, Perren A and Heitz PU: The
gastroenteropancreatic neuroendocrine cell system and its tumors:
the WHO classification. Ann N Y Acad Sci. 1014:13–27. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown DC and Gatter KC: Ki67 protein: the
immaculate deception? Histopathology. 40:2–11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishihara M, Mukai H, Nagai S, et al:
Retrospective analysis of risk factors for central nervous system
metastases in operable breast cancer: effects of biologic subtype
and Ki67 overexpression on survival. Oncology. 84:135–140. 2013.
View Article : Google Scholar
|
|
8
|
Sorbye SW, Kilvaer TK, Valkov A, et al:
Prognostic impact of Jab1, p16, p21, p62, Ki67 and Skp2 in soft
tissue sarcomas. PLoS One. 7:e470682012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sorbye SW, Kilvaer TK, Valkov A, et al:
Prognostic impact of CD57, CD68, M-CSF, CSF-1R, Ki67 and TGF-beta
in soft tissue sarcomas. BMC Clin Pathol. 12:72012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ciancio N, Galasso MG, Campisi R, et al:
Prognostic value of p53 and Ki67 expression in fiberoptic bronchial
biopsies of patients with non small cell lung cancer. Multidiscip
Respir Med. 7:292012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Josefsson A, Wikström P, Egevad L, et al:
Low endoglin vascular density and Ki67 index in Gleason score 6
tumours may identify prostate cancer patients suitable for
surveillance. Scand J Urol Nephrol. 46:247–257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iatropoulos MJ and Williams GM:
Proliferation markers. Exp Toxicol Pathol. 48:175–181. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jacquemier JD, Penault-Llorca FM, Bertucci
F, et al: Angiogenesis as a prognostic marker in breast carcinoma
with conventional adjuvant chemotherapy: a multiparametric and
immunohistochemical analysis. J Pathol. 184:130–135. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kausch I, Lingnau A, Endl E, et al:
Antisense treatment against Ki-67 mRNA inhibits proliferation and
tumor growth in vitro and in vivo. Int J Cancer. 105:710–716. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Fang L, Cheng Q, et al: Effects of
G250 promoter controlled conditionally replicative adenovirus
expressing Ki67-siRNA on renal cancer cell. Cancer Sci.
103:1880–1888. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gerlach C, Sakkab DY, Scholzen T, et al:
Ki-67 expression during rat liver regeneration after partial
hepatectomy. Hepatology. 26:573–578. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Le Guellec S, Perallon R, Alunni JP, et
al: Neoadjuvant treatment of breast cancer: implications for the
pathologist. Ann Pathol. 31:442–454. 2011.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yerushalmi R, Woods R, Ravdin PM, et al:
Ki67 in breast cancer: prognostic and predictive potential. Lancet
Oncol. 11:174–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Duchrow M, Schlüter C, Wohlenberg C, et
al: Molecular characterization of the gene locus of the human cell
proliferation-associated nuclear protein defined by monoclonal
antibody Ki-67. Cell Prolif. 29:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Halm U, Tannapfel A, Breitung B, et al:
Apoptosis and cell proliferation in the
metaplasia-dysplasia-carcinoma-sequence of Barrett’s esophagus.
Hepatogastroenterology. 47:962–966. 2000.PubMed/NCBI
|
|
21
|
Rahmanzadeh R, Hüttmann G, Gerdes J and
Sholzen T: Chromophore-assisted light inactivation of pKi67 leads
to inhibition of ribosomal RNA synthesis. Cell Prolif. 40:422–430.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Castro LA, Elias LS, Oton-Leite AF, et al:
Long-term effects of nifedipine on human gingival epithelium: a
histopathological and immunohistochemical study. J Oral Sci.
52:55–62. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Panteva MT, Salari R, Bhattacharjee M and
Chong LT: Direct observations of shifts in the β-sheet register of
a protein-peptide complex using explicit solvent simulations.
Biophys J. 100:L50–L52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tian H, Qian GW, Li W, et al: A critical
role of Sp1 transcription factor in regulating the human Ki-67 gene
expression. Tumour Biol. 32:273–283. 2011. View Article : Google Scholar
|
|
25
|
Chen F, Song J, Di J, et al: IRF1
suppresses Ki-67 promoter activity through interfering with Sp1
activation. Tumour Biol. 33:2217–2225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakano T, Ohno T, Ishikawa H, et al:
Current advancement in radiation therapy for uterine cervical
cancer. J Radiat Res. 51:1–8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim BH, Bae YS, Kim SH, et al: Usefulness
of Ki-67 (MIB-1) immunostaining in the diagnosis of pulmonary
sclerosing hemangiomas. APMIS. 121:105–110. 2013. View Article : Google Scholar
|
|
28
|
Steels E, Paesmans M, Berghmans T, et al:
Role of p53 as a prognostic factor for survival in lung cancer: a
systematic review of the literature with a meta-analysis. Eur
Respir J. 18:705–719. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martin B, Paesmans M, Mascaux C, et al:
Ki-67 expression and patients survival in lung cancer: systematic
review of the literature with meta-analysis. Br J Cancer.
91:2018–2025. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Faes T, Pecceu A, Van Calenbergh S and
Moerman P: Chorangiocarcinoma of the placenta: a case report and
clinical review. Placenta. 33:658–661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Almeida JC, Menezes RP, Kuckelhaus SA,
Bocca AL and Figueiredo F: Prognostic value of morphologic and
clinical parameters in pT2 – pT3 prostate cancer. Int Braz J Urol.
33:662–672. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vaira V, Fedele G, Pyne S, et al:
Preclinical model of organotypic culture for pharmacodynamic
profiling of human tumors. Proc Natl Acad Sci USA. 107:8352–8356.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hofman MS and Hicks RJ: Changing paradigms
with molecular imaging of neuroendocrine tumors. Discov Med.
14:71–81. 2012.PubMed/NCBI
|
|
34
|
Laurinavicius A, Plancoulaine B,
Laurinaviciene A, et al: A methodology to ensure and improve
accuracy of Ki67 labelling index estimation by automated digital
image analysis in breast cancer tissue. Breast Cancer Res.
16:R352014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hayashi Y, Takei H and Kurosumi M: Ki67
immunohistochemical staining: the present situation of diagnostic
criteria. Nihon Rinsho. 7:428–432. 2012.(In Japanese).
|
|
36
|
Zizi-Sermpetzoglou A, Moustou E,
Petrakopoulou N, et al: Atypical polypoid adenomyoma of the uterus.
A case report and a review of the literature. Eur J Gynaecol Oncol.
33:118–121. 2012.PubMed/NCBI
|
|
37
|
Zini L, Porpiglia F and Fassnacht M:
Contemporary management of adrenocortical carcinoma. Eur Urol.
60:1055–1065. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Viale G: Pathological work up of the
primary tumor: getting the proper information out of it. Breast.
20(Suppl 3): S82–S86. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Leong AS and Zhuang Z: The changing role
of pathology in breast cancer diagnosis and treatment.
Pathobiology. 78:99–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ibrahim T, Farolfi A, Scarpi E, et al:
Hormonal receptor, human epidermal growth factor receptor-2, and
Ki67 discordance between primary breast cancer and paired
metastases: Clinical impact. Oncology. 84:150–157. 2013. View Article : Google Scholar
|
|
41
|
Chlebowski RT, Col N, Winer EP, et al;
American Society of Clinical Oncology Breast Cancer Technology
Assessment Working Group. American Society of Clinical Oncology
technology assessment of pharmacologic interventions for breast
cancer risk reduction including tamoxifen, raloxifene, and
aromatase inhibition. J Clin Oncol. 20:3328–3343. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Blancato J, Singh B, Liu A, Liao DJ and
Dickson RB: Correlation of amplification and overexpression of the
c-myc oncogene in high grade breast cancer: FISH, in situ
hybridization and immunohistochemical analysis. Br J Cancer.
90:1612–1619. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jonat W and Arnold N: Is the Ki-67
labelling index ready for clinical use? Ann Oncol. 22:500–502.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sapino A, Marchiò C, Senetta R, et al:
Routine assessment of prognostic factors in breast cancer using a
multicore tissue microarray procedure. Virchows Arch. 449:288–296.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Camp RL, Neumeister V and Rimm DL: A
decade of tissue microarrays: progress in the discovery and
validation of cancer biomarkers. J Clin Oncol. 26:5630–5637. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Konsti J, Lundin M, Joensuu H, et al:
Development and evaluation of a virtual microscopy application for
automated assessment of Ki-67 expression in breast cancer. BMC Clin
Pathol. 11:32011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Klimowicz AC, Bose P, Nakoneshny SC, et
al: Basal Ki67 expression measured by digital image analysis is
optimal for prognostication in oral squamous cell carcinoma. Eur J
Cancer. 48:2166–2174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sánchez-Navarro I, Gámez-Pozo A,
González-Barón M, et al: Comparison of gene expression profiling by
reverse transcription quantitative PCR between fresh frozen and
formalin-fixed, paraffin-embedded breast cancer tissues.
Biotechniques. 48:389–397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Farragher SM, Tanney A, Kennedy RD and
Paul Harkin D: RNA expression analysis from formalin fixed paraffin
embedded tissues. Histochem Cell Biol. 130:435–445. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mittempergher L, de Ronde JJ, Nieuwland M,
et al: Gene expression profiles from formalin fixed paraffin
embedded breast cancer tissue are largely comparable to fresh
frozen matched tissue. PLoS One. 6:e171632011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Karamitopoulou E, Perentes E, Tolnay M and
Probst A: Prognostic significance of MIB-1, p53, and bcl-2
immunoreactivity in meningiomas. Hum Pathol. 29:140–145. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Geyer FC, Rodrigues DN, Weigelt B and
Reis-Filho JS: Molecular classification of estrogen
receptor-positive/luminal breast cancers. Adv Anat Pathol.
19:39–53. 2012. View Article : Google Scholar
|
|
53
|
Claudio PP, Zamparelli A, Garcia FU, et
al: Expression of Cell-Cycle-regulated Proteins pRb2/p130, p107,
p27kip1, p53, mdm-2, and Ki-67 (MIB-1) in Prostatic Gland
Adenocarcinoma1. Clin Cancer. 8:1808–15. 2002.
|
|
54
|
Hu HY, Liu H, Zhang JW, et al: Clinical
significance of Smac and Ki-67 expression in pancreatic cancer.
Hepato-gastroenterology. 59:2640–2643. 2012.PubMed/NCBI
|
|
55
|
McCormick D, Chong H, Hobbs C, et al:
Detection of the Ki-67 antigen in fixed and wax-embedded sections
with the monoclonal antibody MIB-1. Histopathology. 22:355–360.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Merkel D, Dresseler L and McGuire WL: Flow
cytometry, cellular DNAcontent, and prognosis in human malignancy.
J Clin Oncol. 5:1690–1703. 1987.PubMed/NCBI
|
|
57
|
Clark GM, Mathieu M-C, Owens MA, et al:
Prognostic significance of S-phase fraction in good-risk,
node-negative breast cancer patients. J Clin Oncol. 10:428–432.
1992.PubMed/NCBI
|
|
58
|
Fernandez EB, Sesterhenn IA, McCarthy WF,
et al: Proliferating cellnuclear antigen expression to predict
occult disease in clinical stage Inonseminomatous testicular germ
cell tumors. J Urol. 152:1133–1138. 1994.PubMed/NCBI
|
|
59
|
de Aguiar PH, Aires R, Laws ER, et al:
Labeling index in pituitary adenomas evaluated by means of MIB-1:
is there a prognostic role?. A critical review. Neurol Res.
32:1060–1071. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Prayson RA: The utility of MIB-1/Ki-67
immunostaining in the evaluation of central nervous system
neoplasms. Adv Anat Pathol. 12:144–148. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lind-Landström T, Habberstad AH, Sundstrøm
S and Torp SH: Prognostic value of histological features in diffuse
astrocytomas WHO grade II. Int J Clin Exp Pathol. 5:152–158.
2012.PubMed/NCBI
|
|
62
|
Nabi U, Nagi AH and Sami W: Ki-67
proliferating index and histological grade, type and stage of
colorectal carcinoma. J Ayub Med Coll Abbottabad. 20(4): 44–8.
2008.PubMed/NCBI
|
|
63
|
Hegazy A, Daoud SA and Ibrahim WS: Role of
Ki-67, P53 and Bcl-2 in Advanced Colorectal Carcinoma. Academic
Journal of Cancer Research. 7(3): 168–172. 2014.
|
|
64
|
Inwald EC, Klinkhammer-Schalke M and
Hofstädter F: Ki-67 is a prognostic parameter in breast cancer
patients: results of a large population-based cohort of a cancer
registry. Breast Cancer Res Treat. 139(2): 539–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Klöppel G, Perren A and Heiitz PU: The
gastroenteropancreatic neuroendocrine cell system and its tumors:
The WHO classification. Ann NY Acad Sci. 1014:13–27. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Morimoto R, Satoh F, Murakami O, et al:
Immunohistochemistry of a proliferation marker Ki67/MIB1 in
adrenocortical carcinomas: Ki67/MIB1 labeling index is a predictor
for recurrence of adrenocortical carcinomas. Endocr J. 55:49–55.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gupta N, Srinivasan R and Rajwanshi A:
Functional biomarkers in cervical precancer: an overview. Diagn
Cytopathol. 38:618–623. 2010.
|
|
68
|
Jalava P, Kuopio T, Juntti-Patinen L, et
al: Ki67 immunohistochemistry: a valuable marker in prognostication
but with a risk of misclassification: proliferation subgroups
formed based on Ki67 immunoreactivity and standardized mitotic
index. Histopathology. 48:674–682. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang B: Neoadjuvant endocrine therapy for
postmenopausal estrogen receptor-positive patients with breast
cancer. Zhonghua Zhong Liu Za Zhi. 33:241–244. 2011.(In Chinese).
PubMed/NCBI
|
|
70
|
Gentile V, Vicini P, Giacomelli L, et al:
Detection of human papillomavirus DNA, p53 and ki-67 expression in
penile carcinomas. Int J Immunopathol Pharmacol. 19:209–215.
2006.PubMed/NCBI
|
|
71
|
Kroeze SG, Bijenhof AM, Bosch JL and Jans
JJ: Diagnostic and prognostic tissuemarkers in clear cell and
papillary renal cell carcinoma. Cancer Biomark. 7:261–268.
2010.PubMed/NCBI
|
|
72
|
D’Angelo E and Prat J: Uterine sarcomas: a
review. Gynecol Oncol. 116:131–139. 2010. View Article : Google Scholar
|
|
73
|
Yu JY, DeRuiter SL and Turner DL: RNA
interference by expression of short-interfering RNAs and hairpin
RNAs in mammalian cells. Proc Natl Acad Sci USA. 99:6047–6052.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li L, Thomas RM, Suzuki H, et al: A small
molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell
death. Science. 305:1471–1474. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ben-Izhak O, Bar-Chana M, Sussman L, et
al: Ki67 antigen and PCNA proliferation markers predict survival in
anorectal malignant melanoma. Histopathology. 41:519–525. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hu HY, Liu H, Zhang JW, et al: Clinical
significance of Smac and Ki-67 expression in pancreatic cancer.
Hepatogastroenterology. 59:2640–2643. 2012.PubMed/NCBI
|
|
77
|
Liu YZ, Jiang YY, Hao JJ, et al:
Prognostic significance of MCM7 expression in the bronchial
brushings of patients with non-small cell lung cancer (NSCLC). Lung
Cancer. 77:176–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Golmohammadi R and Pejhan A: The
prognostic value of the P53 protein and the Ki67 marker in breast
cancer patients. J Pak Med Assoc. 62:871–875. 2012.PubMed/NCBI
|
|
79
|
Tihan T, Davis R, Elowitz E, et al:
Practical value of Ki-67 and p53 labeling indexes in stereotactic
biopsies of diffuse and pilocytic astrocytomas. Arch Pathol Lab
Med. 124:108–113. 2000.PubMed/NCBI
|
|
80
|
Iamaroon A, Khemaleelakul U, Pongsiriwet S
and Pintong J: Co-expression of p53 and Ki67 and lack of EBV
expression in oral squamous cell carcinoma. J Oral Pathol Med.
33:30–36. 2004. View Article : Google Scholar
|
|
81
|
Boonyaphiphat P, Pruegsanusak K and
Thongsuksai P: The prognostic value of p53, Bcl-2 and Bax
expression in laryngeal cancer. J Med Assoc Thai. 95:1317–1320.
2012.PubMed/NCBI
|
|
82
|
Tadbir AA, Pardis S, Ashkavandi ZJ, et al:
Expression of Ki67 and CD105 as proliferation and angiogenesis
markers in salivary gland tumors. Asian Pac J Cancer Prev.
13:5155–5159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chen JX, Deng N, Chen X, et al: A novel
molecular grading model: combination of Ki67 and VEGF in predicting
tumor recurrence and progression in non-invasive urothelial bladder
cancer. Asian Pac J Cancer Prev. 13:2229–2234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hoogerbrugge N, Hermens RP, Nagengast F,
et al: Tumour examination to detect hereditary colorectal cancer.
Ned Tijdschr Geneeskd. 156:A49822012.(In Dutch).
|
|
85
|
Nabi U, Nagi AH and Sami W: Ki-67
proliferating index and histological grade, type and stage of
colorectal carcinoma. J Ayub Med Coll Abbottabad. 20:44–48.
2008.PubMed/NCBI
|
|
86
|
Tvedskov TF: Staging of women with breast
cancer after introduction of sentinel node guided axillary
dissection. Dan Med J. 59:B44752012.PubMed/NCBI
|
|
87
|
Wang MJ, Pei DS, Qian GW, et al: p53
regulates Ki-67 promoter activity through p53-and Sp1-dependent
manner in HeLa cells. Tumour Biol. 32:905–912. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Grala B, Markiewicz T, Kozowski W, et al:
New automated image analysis method for the assessment of Ki-67
labeling index in meningiomas. Folia Histochem Cytobiol.
47:587–592. 2009.PubMed/NCBI
|
|
89
|
Tuominen VJ, Ruotoistenmäki S, Viitanen A,
et al: ImmunoRatio: a publicly available web application for
quantitative image analysis of estrogen receptor (ER), progesterone
receptor (PR), and Ki-67. Breast Cancer Res. 12:R562010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Gilles FH, Tavaré CJ, Becker LE, et al:
Pathologist interobserver variability of histologic features in
childhood brain tumors: results from the CCG-945 study. Pediatr Dev
Pathol. 11:108–117. 2008. View Article : Google Scholar
|
|
91
|
Grzybicki DM, Liu Y, Moore SA, et al:
Interobserver variability associated with the MIB-1 labeling index:
high levels suggest limited prognostic usefulness for patients with
primary brain tumors. Cancer. 92:2720–2726. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Hsu CY, Ho DM, Yang CF and Chiang H:
Interobserver reproducibility of MIB-1 labeling index in astrocytic
tumors using different counting methods. Mod Pathol. 16:951–957.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Walker RA: Quantification of
immunohistochemistry - issues concerning methods, utility and
semiquantitative assessment I. Histopathology. 49:406–410. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Bertucci F, Finetti P, Roche H, et al:
Comparison of the prognostic value of genomic grade index, Ki67
expression and mitotic activity index in early node-positive breast
cancer patients. Ann Oncol. 24:625–632. 2013. View Article : Google Scholar
|
|
95
|
Nielsen PS, Riber-Hansen R, Raundahl J and
Steiniche T: Automated quantification of MART1-verified Ki67
indices by digital image analysis in melanocytic lesions. Arch
Pathol Lab Med. 136:627–634. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Nielsen PS, Riber-Hansen R and Steiniche
T: Immunohistochemical double stains against Ki67/MART1 and
HMB45/MITF: promising diagnostic tools in melanocytic lesions. Am J
Dermatopathol. 33:361–370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Petit T, Wilt M, Velten M, et al:
Comparative value of tumour grade, hormonal receptors, Ki-67, HER-2
and topoisomerase II alpha status as predictive markers in breast
cancer patients treated with neoadjuvant anthracycline-based
chemotherapy. Eur J Cancer. 40:205–211. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chang J, Ormerod M, Powles TJ, et al:
Apoptosis and proliferation as predictors of chemotherapy response
in patients with breast carcinoma. Cancer. 89:2145–2152. 2000.
View Article : Google Scholar
|
|
99
|
Viale G, Giobbie-Hurder A, Regan MM, et
al: Prognostic and predictive value of centrally reviewed Ki-67
labeling index in postmenopausal women with endocrine-responsive
breast cancer: results from Breast International Group Trial 1–98
comparing adjuvant tamoxifen with letrozole. J Clin Oncol.
26:5569–75. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Yerushalmi R, Woods R, Ravdin PM, et al:
Ki67 in breast cancer: prognostic and predictive potential. Lancet
Oncol. 11:174–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Park JY, Kim KR and Nam JH:
Immunohistochemical analysis for therapeutic targets and prognostic
markers in low-grade endometrial stromal sarcoma. Int J Gynecol
Cancer. 23:81–89. 2013. View Article : Google Scholar
|
|
102
|
Nagao K, Yamamoto Y, Hara T, et al: Ki67
and BUBR1 may discriminate clinically insignificant prostate cancer
in the PSA range <4 ng/ml. Jpn J Clin Oncol. 41:555–564. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Machowska M, Wachowicz K, Sopel M, et al:
Nuclear location of tumor suppressor protein maspin inhibits
proliferation of breast cancer cells without affecting
proliferation of normal epithelial cells. BMC Cancer. 14:1422014.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Nielsen PS, Riber-Hansen R, Jensen TO, et
al: Proliferation indices of phosphor-histone H3 and Ki67: strong
prognostic markers in a consecutive cohort with stage I/II
melanoma. Mod Pathol. 26(3): 404–13. 2013. View Article : Google Scholar
|
|
105
|
Vogt N and Klapper W: Variability in
morphology and cell proliferation in sequential biopsies of mantle
cell lymphoma at diagnosis and relapse: clinical correlation and
insights into disease progression. Histopathology. 62:334–342.
2013. View Article : Google Scholar
|
|
106
|
Palmqvist R, Sellberg P, Oberg A, et al:
Low tumour cell proliferation at the invasive marginis associated
with a poor prognosis in Dukes’ stage B colorectal cancers. Br J
Cancer. 79:577–581. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kimura T, Tanaka S, Haruma K, et al:
Clinical significance of MUC1 and E-cadherin expression, cellular
proliferation, and angiogenesis at the deepest invasive portion of
colorectal cancer. Int J Oncol. 16:55–64. 2000.
|
|
108
|
Holdcraft J and Gallagher JC: Malignant
melanomas of the nasal and paranasal sinus mucosa. Ann Otol Rhinol
Laryngol. 78:5–20. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Rinaldo A, Shaha AR, Patel SG and Ferlito
A: Primary mucosal melanoma of the nasal cavity and paranasal
sinuses. Acta Otolaryngol. 121:979–982. 2001. View Article : Google Scholar
|
|
110
|
Bisgaard ML: Young age colorectal cancer
and identification of hereditary non-polyposis colorectal cancer
cohorts. Br J Surg. 94:1055–1056. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Niezabitowski A, Czajecki K, Ryś J, et al:
Prognostic evaluation of cutaneous malignant melanoma: a
clinicopathologic and immunohistochemical study. J Surg Oncol.
70:150–160. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Tuleta I, Bauriedel G, Steinmetz M, et al:
Apoptosis-regulated survival of primarily extravascular cells in
proliferative active poststent neointima. Cardiovasc Pathol.
19:353–360. 2010. View Article : Google Scholar
|
|
113
|
Wajed SA, Laird PW and DeMeester TR: DNA
methylation: an alternative pathway to cancer. Ann Surg. 234:10–20.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zheng JN, Ma TX, Cao JY, et al: Knockdown
of Ki-67 by small interfering RNA leads to inhibition of
proliferation and induction of apoptosis in human renal carcinoma
cells. Life Sci. 78:724–729. 2006. View Article : Google Scholar
|
|
115
|
Zheng JN, Sun YF, Pei DS, et al:
Anti-Ki-67 peptide nucleic acid affects the proliferation and
apoptosis of human renal carcinoma cells in vitro. Life Sci.
76:1873–1881. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Chan JH, Lim S and Wong WS: Antisense
oligonucleotides: from design to therapeutic application. Clin Exp
Pharmacol Physiol. 33:533–540. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Paulasova P and Pellestor F: The peptide
nucleic acids (PNAs): a new generation of probes for genetic and
cytogenetic analyses. Ann Genet. 47:349–358. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Smolina IV and Demidov VV:
Sequence-universal recognition of duplex DNA by oligonucleotides
via pseudocomplementarity and helix invasion. Chem Biol.
10:591–595. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Demidov VV and Frank-Kamenetskii MD: Two
sides of the coin: affinity and specificity of nucleic acid
interactions. Trends Biochem Sci. 29:62–71. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Demidov VV, Potaman VN, Frank-Kamenetskii
MD, et al: Stability of peptide nucleic acids in human serum and
cellular extracts. Biochem Pharmacol. 48:1310–1313. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Tuschl T: RNA interference and small
interfering RNAs. Chembiochem. 2:239–245. 2001. View Article : Google Scholar
|
|
122
|
Zheng JN, Pei DS, Mao LJ, et al:
Inhibition of renal cancer cell growth in vitro and in vivo with
oncolytic adenovirus armed short hairpin RNA targeting Ki-67
encoding mRNA. Cancer Gene Ther. 16:20–32. 2009. View Article : Google Scholar
|
|
123
|
Pai SI, Lin YY, Macaes B, Meneshian A,
Hung CF and Wu TC: Prospects of RNA interference therapy for
cancer. Gene Ther. 13:464–477. 2006. View Article : Google Scholar
|
|
124
|
Brummelkamp TR, Bernards R and Agami R: A
system for stable expression of short interfering RNAs in mammalian
cells. Science. 296:550–553. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Lieberman J, Song E, Lee SK and Shankar P:
Interfering with disease: opportunities and roadblocks to
harnessing RNA interference. Trends Mol Med. 9:397–403. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Moore CB, Guthrie EH, Huang MT and Taxman
DJ: Short hairpin RNA (shRNA): design, delivery, and assessment of
gene knockdown. Methods Mol Biol. 629:141–158. 2010.PubMed/NCBI
|
|
127
|
Zhu H, Guo W, Zhang L, et al: Bcl-XL small
interfering RNA suppresses the proliferation of
5-fluorouracil-resistant human colon cancer cells. Mol Cancer Ther.
4:451–456. 2005.PubMed/NCBI
|
|
128
|
Starborg M, Gell K, Brundell E and Höög C:
The murine Ki-67 cell proliferation antigen accumulates in the
nucleolar and heterochromatic regions of interphase cells and at
the periphery of the mitotic chromosomes in a process essential for
cell cycle progression. J Cell Sci. 109:143–153. 1996.PubMed/NCBI
|
|
129
|
Zhang P, Steelant W, Kumar M and
Scholfield M: Versatile photosensitizers for photodynamic therapy
at infrared excitation. J Am Chem Soc. 129:4526–4527. 2007.
View Article : Google Scholar : PubMed/NCBI
|