Introduction
At present, there is a lack of effective treatment
for diabetes mellitus and the current antidiabetic therapies,
including insulin injection and administering of hypoglycemic
agents usually have various side effects (1). In addition, these agents are
relatively ineffective against certain long-term diabetic
complications and are expensive (2). Therefore, the development of
antidiabetic agents derived from natural sources may offer a
promising solution to prevent the adverse effects of current
antidiabetic therapies (3).
The pharmacological effects of various medicinal
plants have been previously examined, including effects on the
regulation of blood glucose levels and cell death in high
glucose-induced oxidative stress, which is caused by the excessive
generation of intracellular reactive oxygen species (ROS) (4–6).
Anthocyanins are a group of naturally derived plant pigments, which
are used as orally administered therapeutic agents due to their
nontoxic antioxidative, anti-inflammatory and anticancer properties
(7,8). Previous studies have suggested that
the different types of anthocyanins, including cyanidin,
delphinidin and peonidin isolated from natural products may be
useful in the prevention and/or treatment of aging, cancer,
arteriosclerosis, inflammation and diabetes (9,10).
However, the potential antidiabetic effects of cyanidin-3-glucoside
(C3G), which is isolated from mulberry fruits, on high
glucose-induced apoptosis remain to be elucidated.
Therefore, the present study investigated whether
C3G, isolated from mulberry fruits, protected MIN6N pancreatic
β-cells against high glucose-induced oxidative stress from high
glucose conditions in the pancreas via modulation of cell signaling
pathways.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS), penicillin-streptomycin (PS) and trypsin-EDTA
were purchased from Gibco-BRL (Grand Island, NY, USA). The
dichlorodihydrofluorescein-diacetate (H2DCF-DA)
apoptotic assay kit and nuclear extraction kit were obtained from
Molecular Probes (Carlsbad, CA, USA). The rat/mouse insulin
enzyme-linked immunosorbent assay kit was obtained from Linco
Research, Inc. (St. Charles, MO, USA). Kuromanin chloride (C3G
standard), Hoechst 33342 and the mitochondria isolation kit were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies
against c-Jun NH2-terminal kinase (JNK; 1:1,000
dilution; rabbit polyclonal antibody; #9252), phosphorylated
(p-)JNK (1:1,000 dilution; Thr183/Tyr185 mouse monoclonal antibody;
#9255S), extracellular signal-related kinase (ERK; 1:1,000
dilution; rabbit monoclonal antibody; #4695), p-ERK (1:1,000
dilution; Thr202/Tyr204 rabbit polyclonal antibody; #9101S), p38
(1:1,000 dilution; rabbit polyclonal antibody; #9212), p-p38
(1:1,000 dilution; Thr180/Tyr182 rabbit monoclonal immunoglobulin
(Ig)G antibody; #4631S), nuclear factor-κB(NF-κB) p65 (1:1,000
dilution; rabbit polyclonal antibody; #3034) and horseradish
peroxidase (HRP)-linked anti-rabbit IgG (1:2,000 dilution; #7074)
were purchased from Cell Signaling Technology, Inc. (Beverly, MA,
USA). Antibodies against β-actin (1:1,000 dilution; mouse
monoclonal IgG1; sc-47778), Bcl-2 (1:1,000 dilution;
mouse monoclonal IgG1; sc-7382), Bax (1:1,000 dilution;
rabbit polyclonal IgG; sc-493), caspase-3 (1:1,000 dilution; mouse
monoclonal IgG2a; sc-7272) and HRP-linked goat
anti-mouse IgG (1:2,000 dilution; sc-2005) were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). All other
chemicals were obtained from Sigma-Aldrich.
Extraction and identification of
anthocyanin
Mulberry fruit was purchased from a local company
(Sujuchon, Yecheon, Korea). For anthocyanin extraction, the
mulberry fruits were placed in 70% ethanol for 24 h at room
temperature (RT). Following centrifugation of the extract (600 × g,
20 min), the supernatants were filtered with Whatman filter paper
(0.45 μm), concentrated using a vacuum rotary evaporator (Eyela,
Tokyo, Japan), redissolved in triple distilled water and
lyophilized using a freeze dryer (IlShin, Seoul, Korea). The powder
was soaked in n-hexane for 24 h to remove the fats and oils.
The anthocyanins in the powder were then extracted with acidified
methanol (methanol and 1.0 N HCl; 85:15, v/v), centrifuged at
12,000 × g for 15 min to remove the precipitate and then filtered
through a 0.45 μm filter. The purified anthocyanin extract was
evaporated at 46°C to dryness and stored at 4°C. The anthocyanin
powder was redissolved in the culture medium and filtered using a
0.22 μm filter prior to cell culture. The pure C3G standard and
purified anthocyanin, isolated from mulberry fruits, were
identified using high-performance liquid chromatography (HPLC)
retention time and their purity was >99% (data not shown).
Reversed-phase HPLC was performed using Waters 486 detector
(Waters, Milford, MA, USA) under the following conditions: Column,
μBondapakC18 (Waters; 3.9×300 mm); flow rate, 0.5 ml/min; injection
volume, 10 μl; solvent, methanol/H2O/formic acid
(75:20:5); and column temperature, 46°C. The absorbance was
recorded at 520 nm using a Thermo Spectronic Genesys
ultraviolet-visible spectrophotometer (Spectronic Instruments;
Thermo Fisher Scientific, Waltham, MA, USA).
Cell culture
The MIN6N pancreatic β-cells were derived from a
mouse pancreatic islet. The cells were provided by Professor H. Y.
Kwon (College of Medicine, Hallym University, Chuncheon, Korea).
The MIN6N β-cells were cultured in DMEM (5.5 mM glucose)
supplemented with 10% inactivated FBS and 1% PS and were maintained
at 37°C in a humidified 5% CO2 incubator. The cells were
cultured to ~85% confluence and were harvested using 0.25%
trypsin-EDTA. The cells were then subcultured in 6- or 12-well
plates for 12 h until they reached confluence. The cells were then
treated with various concentrations (0, 10, 20, 50, 70, 100 and 200
μg/ml) of C3G for 18 h and then cultured for an additional 18 h in
DMEM containing either 25 mM glucose or 25 mM mannitol (mannitol
with 5.5 mM glucose in culture medium). The cells were maintained
in these culture conditions for all experiments.
Cell viability assay
The viability of the treated cells was measured
using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay. Briefly, 500 μg/ml MTT solution was added to
each well and incubated for 2.5 h at 37°C. The formazan crystals in
each well were dissolved in isopropyl alcohol and the absorbance of
each well was measured at 595 nm using an ELISA microplate reader
(Bio-Rad model 550; Bio-Rad Laboratories Inc., Hercules, USA).
Measurement of intracellular ROS and
image analysis
Following treatment of the cells, 5 μM
H2DCF-DA in phosphate-buffered saline (PBS, pH 7.38;
Gibco-BRL) was added and the fluorescence was measured at
excitation and emission wavelengths of 485 nm and 535 nm,
respectively, using a microplate spectrofluorometer (Molecular
Devices Corp., Sunnyvale, CA, USA). The production of intracellular
ROS was determined by image analysis of the cells, which were
seeded into coverslip-loaded 6-well plates. Subsequently,
H2DCF-DA solution (500 μl per well) was added to each
well of the plate, which was incubated for 2 h at 37°C. Images of
the stained cells were acquired using a fluorescence microscope
(Nikon Eclipse TE 300; Nikon, Tokyo, Japan).
Measurement of DNA fragmentation
The cells were treated with Hoechst 33342, a dye
used to detect DNA condensation and/or fragmentation, followed by
incubation at RT for 15 min. Images of the stained cells were
acquired using a fluorescence microscope to examine the degree of
DNA fragmentation.
Flow cytometric analysis
The apoptotic cells were examined using a
fluorescein isothiocyanate (FITC)-labeled Annexin V/propidium
iodide (PI) apoptosis detection kit (Moleclular Probes, Carlsbad,
CA, USA) according to the manufacturer’s instructions. The treated
cells were harvested and washed with PBS and then centrifuged at
600 × g for 5 min to collect the cell pellet. Subsequently, the
cells were resuspended in binding buffer (10 mM HEPES, 140 mM NaCl,
2.5 mM CaCl2; pH 7.4) and stained with FITC-labeled Annexin V/PI at
RT for 15 min in light-protected conditions. Flow cytometric
analysis was performed using a FACSCalibur flow cytometer (Becton
Dickinson, Mountain View, CA, USA) within 1 h of the supravital
staining. The apoptotic cell rate was calculated as the sum of
cells in the early and late phases of apoptosis divided by the
total number of events.
Western blot analysis
The treated cells were washed in 1X PBS and lysed in
lysis buffer [10 mM Tris-HCl (pH 7.5), 10 mM
NaH2PO4/NaHPO4 (pH 7.5), 130 mM
NaCl, 1% Triton X-100, 10 mM NaPPi, 1 mM phenylmethylsulphonyl
fluoride, 2 μg/ml pepstatin A] for 40 min on ice. The lysates were
centrifuged at 12,000 × g for 30 min at 4°C. The supernatant was
then collected and the protein content of the supernatant was
measured using a Bio-Rad protein assay kit (Bio-Rad Laboratories,
Inc.) prior to analysis. The total or fractionated protein samples
were loaded and separated using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto
nitrocellulose membranes (Bio-Rad Laboratories, Inc.). The
membranes were inhibited with 5% non-fat powdered milk in 1X
Tris-buffered saline containing 0.1% Tween-20 (TBS-T) for 1 h and
were incubated with primary antibodies at 4°C overnight. Finally,
the membranes were treated with HRP-linked secondary antibodies for
1 h at RT. The membranes were washed with TBS-T following the
binding reaction with each antibody. The detection of each protein
was performed using an enhanced chemiluminescence kit (Millipore,
Billerica, MA, USA).
Measurement of caspase-3 activity
The treated MIN6N β-cells were lysed in 500 μl lysis
buffer consisting of 10 mM Tris-HCl (pH 7.5), 10 mM
NaH2PO4/NaHPO4 (pH 7.5), 130 mM
NaCl, 1% Triton X-100 and 10 mM NaPPi. The activity of caspase-3 in
the lysates was evaluated using a caspase assay kit (BD
Biosciences, San Diego, CA, USA) according to the manufacturer’s
instructions.
Measurement of insulin secretion
The culture medium was collected from the treated
cells and the level of insulin released in the medium was measured
using a rat/mouse insulin enzyme-linked immunosorbent (ELISA) assay
kit (Linco Research Inc., St. Charles, MO, USA) according to the
manufacturer’s instructions.
Statistical analysis
All the measurements were obtained from at least
three independent experiments and the values are expressed as the
mean ± standard error. Statistical analysis was performed using
Student’s t-test to evaluate significant differences and analysis
of variance and Duncan’s multiple range tests (SAS, version 9.1;
SAS-Institute, Cary, NC, USA) for comparing multiple groups when
appropriate. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of C3G on cell viability and
generation of ROS in the MIN6N β-cells
High glucose conditions increase the osmolarity in
cells, therefore, the MIN6N β-cells were also cultured with 25 mM
mannitol as an osmotic control agent to distinguish between the
effects of glucose and osmotic pressure. High glucose significantly
decreased the viability of the cells to 72.9% of the control,
whereas no significant decrease in viability was observed following
culture with mannitol. The pretreatment of the cells with C3G at a
concentration of 70 μg/ml restored the cell viability to 83.8% of
the control (Fig. 1A). The
intracellular ROS levels in the high glucose-treated cells were
determined using the ROS-sensitive fluorescent probe,
H2DCF-DA. High glucose markedly increased the levels of
intracellular ROS; however, no effects on the intracellular ROS
levels were observed following culture with mannitol. Compared with
the cells under high glucose conditions, the cells pretreated with
70 μg/ml C3G exhibited a marked decrease in the high
glucose-dependent increase in intracellular ROS levels and green
fluorescence intensity (Fig. 1B and
C).
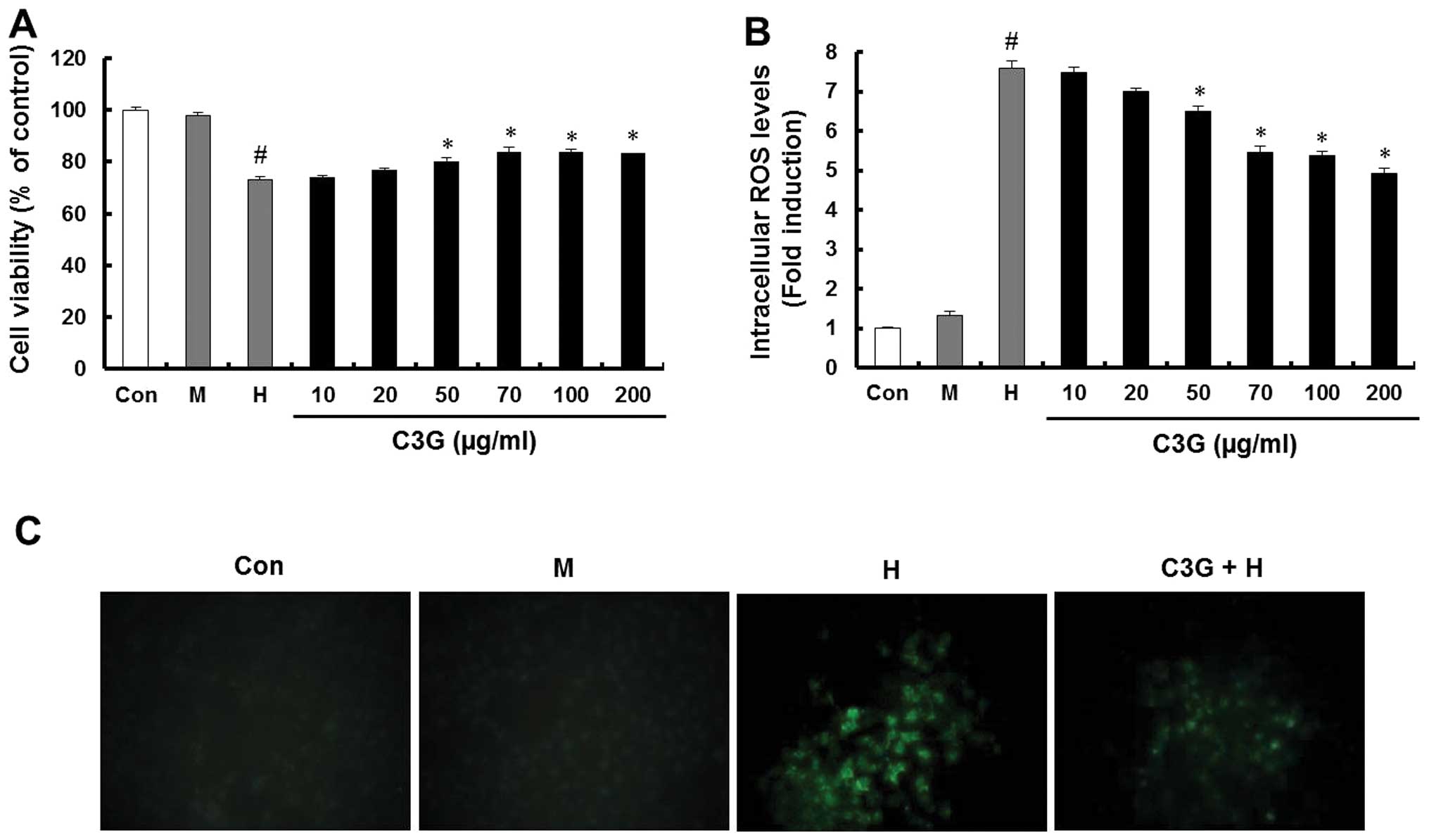 | Figure 1Effect of C3G on cell viability and
ROS generation in the MIN6N β-cells. The cells were cultured with
5.5 mM glucose (Con), 25 mM mannitol (M), 25 mM glucose (H) and
indicated doses of C3G with 25 mM glucose (C3G + H). (A) Cell
viability was determined using an
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
(B) Levels of intracellular ROS were measured using the
H2DCF-DA method. Data represent the mean ± standard
error of three independent experiments. #P<0.05
vs. Con; *P<0.05, vs. H group. (C)
Representative fluorescence images demonstrate the increase in
green fluorescence intensity of the DCF produced by ROS
(magnification, 400x). CG3, cyanidin-3-glucoside; ROS, reactive
oxygen species; Con, control; H2DCF-DA,
dichlorodihydrofluorescein-diacetate. |
Effect of C3G on MIN6N β-cell
apoptosis
To evaluate whether the growth-inhibitory effect of
high glucose was associated with apoptosis, double-staining was
performed using FITC-labeled Annexin V/PI. High glucose caused
apoptosis in 33.9% of the cells, which was significantly higher
compared with mannitol. However, pretreatment with 70 μg/ml C3G
significantly inhibited the high glucose-induced apoptotic cell
death (24.85%; Fig. 2A). The
fragmentation of DNA upon apoptosis induced by high glucose was
confirmed by staining the chromatin of the MIN6N β-cells using
Hoechst 33342. Only the high glucose-treated cells exhibited DNA
fragmentation, however, these chromatin changes were decreased
following pretreatment with 70 μg/ml C3G (Fig. 2B).
Effect of C3G on the altered expression
of apoptotic-associated proteins in the MIN6N β-cells
The present study performed western blot analysis to
determine the effects of C3G on the mitogen-activated protein
kinase (MAPK) signaling pathway in the MIN6N β-cells. The cells
were cultured under high glucose conditions, with or without C3G,
followed by examination of the phosphorylation levels of ERK, JNK
and p38. Treatment with C3G (50 and 70 μg/ml) led to dose-dependent
inhibition of the high glucose-dependent phosphorylation of all the
MAPK proteins (Fig. 3A). Apoptosis
is initiated via two pathways, the intrinsic pathway is
characterized by the release of cytochrome c and the
activation of Bcl-2 family proteins, whereas the extrinsic pathway
involves activation of apoptosis inducing factor (AIF), caspase-8
and caspase-10 (11). A previous
study demonstrated that the phosphorylation of MAPK proteins is
involved in the regulation of the mitochondrial
permeability-mediated activation of apoptotic proteins, including
Bcl-2 family proteins and cytochrome c (12). In the present study, the release of
cytochrome c and expression of Bcl-2 family proteins,
including Bcl-2 and Bax were confirmed (Fig. 3B). The protein expression of Bcl-2
decreased, whilst that of Bax increased in the high glucose-treated
cells. The expression of the Bcl-2 family proteins in the group
pretreated with 70 μg/ml C3G was regulated in a manner similar to
the control group. In addition, the present study investigated
whether high glucose induced the release of cytochrome c
from the mitochondria into the cytosol. Western blot analysis of
the cytosolic fraction revealed a significant release of cytochrome
c from mitochondria in the cells cultured with high glucose.
However, C3G (50 and 70 μg/ml) treatment inhibited the release of
cytochrome c (Fig. 3C). To
investigate whether high glucose induced the extrinsic apoptotic
pathway, the expression of AIF and caspase-8 was examined in the
MIN6N β-cells treated with high glucose. However, no significant
promotion of AIF or caspase-8 activation was observed in high
glucose-induced glucotoxicity (data not shown).
 | Figure 3Effect of C3G on the altered
expression of apoptotic-associated proteins in the MIN6N β-cells
treated with high glucose. The cells were cultured with 5.5 mM
glucose (Con), 25 mM mannitol (M), 25 mM glucose (H) and indicated
dose of C3G with 25 mM glucose (C3G + H). Subsequently, the cells
were harvested and the lysates were prepared. The expression levels
of (A) ERK, p-ERK, JNK, p-JNK, p38 MAPK and p-p38 MAPK, (B) Bcl-2
and Bax and (C) fractional cyt c were assessed using western blot
analysis. Data represent the mean ± standard error of three
independent experiments. The equal loading of total proteins in
each sample was confirmed by the expression of β-actin. CG3,
cyanidin-3-glucoside; Con, control; ERK, extracellular
signal-related kinase; p-, phosphorylated; JNK, c-Jun
NH2-terminal kinase; MAPK, mitogen-activated protein
kinase, Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein;
cyt c, cytochrome c. |
Effect of C3G on the translocation of
NF-κB, activation of caspase-3 and insulin secretion in the MIN6N
β-cells
NF-κB is involved in oxidative stress-induced cell
death in different cell types (12), therefore, the present study
examined the translocation of NF-κB from the cytosol into the
nucleus. High glucose induced the nuclear translocation of NF-κB
p65, however pretreatment with C3G (50 and 70 μg/ml) inhibited the
high glucose-induced nuclear translocation of NF-κB p65 (Fig. 4A). Caspase-3 is important in the
execution of apoptosis, therefore, the present study examined the
effect of C3G on the high glucose-induced activation of caspase-3.
The cleaved form of caspase-3 was detected in the high
glucose-treated group. However, compared with the high
glucose-treated group, the group pretreated with C3G (50 and 70
μg/ml) exhibited a decrease in the level of cleaved caspase-3
(Fig. 4B). To further investigate
the effects of C3G on caspase-3 activity, the activity of caspase-3
was analyzed using a caspase-3 assay kit. High glucose treatment
significantly increased the activity of caspase-3, however, C3G (70
μg/ml) significantly decreased the caspase-3 activity (Fig. 4C). The antidiabetic efficacy of C3G
was determined by measuring insulin release in the MIN6N β-cells
using a rat/mouse insulin ELISA kit. Treatment with C3G led to an
increase in insulin secretion compared with the high
glucose-treated control (74.8, vs. 84.2%; Fig. 4D).
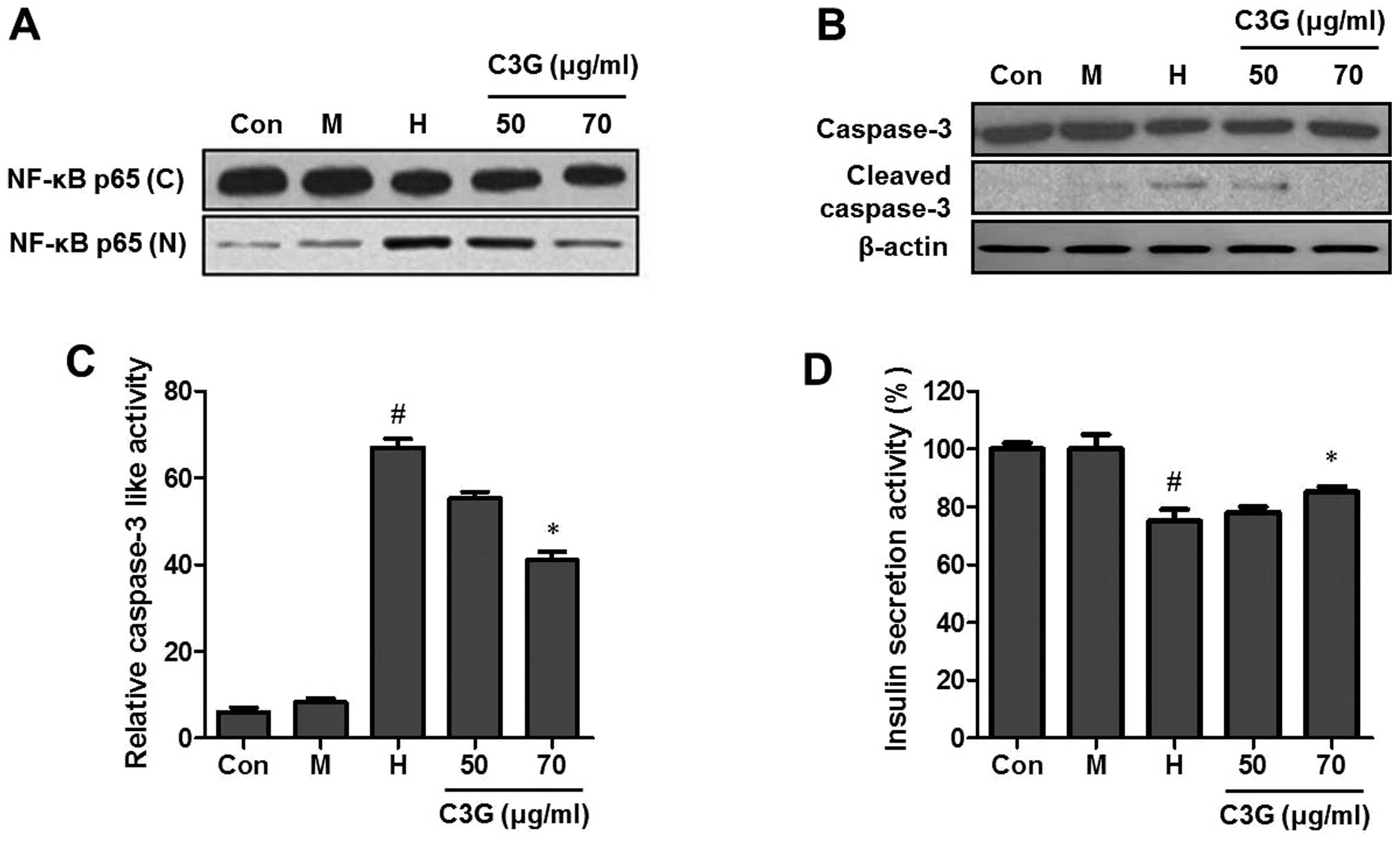 | Figure 4Effect of C3G on the translocation of
NF-κB, activation of caspase-3 and insulin secretion in the MIN6N
β-cells treated with high glucose. The cells were cultured with 5.5
mM glucose (Con), 25 mM mannitol (M), 25 mM glucose (H) and the
indicated doses of C3G with 25 mM glucose (C3G + H). Subsequently,
the cells were harvested and the lysates were prepared. The
expression levels of fractional (A) NF-κB and (B) caspase-3 and
cleaved caspase-3 were assessed using western blot analysis. The
equal loading of the total proteins in each sample was confirmed by
the expression of β-actin. (C) Effect of C3G on caspase-3 activity
in the MIN6N β-cells. (D) Effect of C3G on insulin secretion in the
MIN6N β-cells. Following treatment, the supernatants were collected
and insulin release was measured using a rat/mouse insulin ELISA
kit. All the experiments were representative of at least the
independent experiments. #P<0.05, vs. Con;
*P<0.05, vs. H group. CG3, cyanidin-3-glucoside;
NF-κB, nuclear factor-κB; Con, control. |
Discussion
Hyperglycemia causes glucotoxicity in vulnerable
cell types and contributes to the generation of intracellular ROS,
which results in apoptosis (13).
Consequently, ROS have been implicated in the aging process,
carcinogenesis, rheumatoid arthritis and inflammation(14). The excessive generation of
intracellular ROS by high glucose is particularly deleterious to
the pancreas and their levels are correlated with the loss of
β-cell mass, β-cell dysfunction and pancreas islet destruction
(15). In addition, insulin
deficiency, caused by the destruction of pancreatic β-cells,
induces long-term hyperglycemia, which leads to different types of
diabetic complications and serious pathological effects (16). The blood glucose concentration is
increased by various environmental stresses, eating, drinking and
smoking habits and thus leads to glucotoxicity in the tissues and
organs (17). Therefore,
inhibition of the glucotoxicity-induced excessive generation of ROS
has been considered to be an important therapeutic target for
protecting pancreatic β-cells in the prevention and/or treatment of
diabetes (18).
Previous studies have demonstrated that
anthocyanins, which are natural pigments obtained from various
plants, have inhibitory effects against ROS and
hyperglycemia-induced oxidative stress and, therefore, they are
used orally as phytotherapeutic agents (19,20).
Antioxidants from natural products have emerged as a novel class of
phytotherapeutic agents for diabetes (21). The findings of the present study
revealed that C3G exerted marked antioxidative effects by
inhibiting the generation of intracellular ROS and, thus, C3G may
be used as a novel therapeutic agent for the prevention and/or
treatment of various diseases.
The high glucose treatment significantly reduced
cell viability, however the cell viability was restored following
pretreatment with C3G (Fig. 1A).
High glucose conditions induce the generation of ROS, which are
harmful for pancreatic β-cells and cause an increase in the rate of
apoptosis and DNA fragmentation (22). The C3G treatment decreased the
intracellular ROS generation (Fig.
1B) and the rate of apoptosis in the high glucose-treated MIN6N
β-cells (Fig. 2A and 2B). These
results suggested that high glucose increased oxidative stress,
defined as cellular damage caused by ROS, and that C3G may
attenuate high glucose-induced oxidative stress in the MIN6N
pancreatic β-cells. Additional experiments were performed to
evaluate the signaling mechanism underlying the cytoprotection of
C3G against high glucose-induced apoptosis. The phosphorylation of
MAPK proteins activated the pro-apoptotic protein Bax. The
activated Bax induced the release of cytochrome c from the
mitochondria into the cytosol and the cytosolic cytochrome c
then activated caspase-3, which was important in the apoptotic
pathway and lead to apoptosis. However, the present study also
confirmed that pretreatment with C3G exerted protective effects
against high glucose-induced apoptosis by regulating the activation
of the intrinsic mitochondrial pathway-mediated proteins. In
addition, C3G markedly inhibited the nuclear translocation of NF-κB
in high glucose-treated cells. The phosphorylation of MAPK proteins
is important in the translocation of NF-κB into the nucleus
(23). The major mechanism
underlying the inhibition of NF-κB activation by C3G may be through
suppressing the phosphorylation and activation of the MAPK and
Bcl-2 proteins. Insulin secretion was significantly inhibited in
the MIN6N β-cells exposed to high glucose conditions. In
particular, pretreatment with 70 μg/ml C3G led to increased insulin
secretion.
In conclusion, the present study demonstrated that
C3G isolated from mulberry fruits protected the pancreatic β-cells
by inhibiting apoptosis via regulation of the intrinsic apoptotic
pathway-mediated proteins. These results suggested that C3G offers
potential as a novel chemopreventative agent for diabetes.
Acknowledgements
This study was supported by the High Value-added
Food Technology Development Program, Ministry of Agriculture, Food
and Rural Affairs.
References
|
1
|
Shvarts LS and Shub AI: Side effect of
insulin. Klin Med. 47:85–89. 1969.
|
|
2
|
Kim BY, Jung CH, Mok JO and Kim CH:
Factors associated with long-term oral hypoglycemic agent
responsiveness in korean patients with type 2 diabetes mellitus.
Diabetes Metab J. 35:282–289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee NJ, Norris SL and Thakurta S: Efficacy
and harms of the hypoglycemic agent pramlintide in diabetes
mellitus. Ann Fam Med. 8:542–549. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salimifar M, Fatehi-Hassanabad Z and
Fatehi M: A review on natural products for controlling type 2
diabetes with an emphasis on their mechanisms of actions. Curr
Diabetes Rev. 9:402–411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peng CH, Chyau CC, Chan KC, Chan TH, Wang
CJ and Huang CN: Hibiscus sabdariffa polyphenolic extract inhibits
hyperglycemia, hyperlipidemia, and glycation-oxidative stress while
improving insulin resistance. J Agric Food Chem. 59:9901–9909.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gupta S, Sharma SB, Prabhu KM and Bansal
SK: Protective role of Cassia auriculata leaf extract on
hyperglycemia-induced oxidative stress and its safety evaluation.
Indian J Biochem Biophys. 46:371–377. 2009.PubMed/NCBI
|
|
7
|
Zhao C, Giusti MM, Malik M, Moyer MP and
Magnuson BA: Effects of commercial anthocyanin-rich extracts on
colonic cancer and nontumorigenic colonic cell growth. J Agric Food
Chem. 52:6122–6128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Edirisinghe I, Banaszewski K, Cappozzo J,
Sandhya K, Ellis CL, Tadapaneni R, et al: Strawberry anthocyanin
and its association with postprandial inflammation and insulin. Br
J Nutr. 106:913–922. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noda Y, Kaneyuki T, Mori A and Packer L:
Antioxidant activities of pomegranate fruit extract and its
anthocyanidins: delphinidin, cyanidin, and pelargonidin. J Agric
Food Chem. 50:166–171. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oak MH, Bedoui JE, Madeira SV, Chalupsky K
and Schini-Kerth VB: Delphinidin and cyanidin inhibit PDGF
(AB)-induced VEGF release in vascular smooth muscle cells by
preventing activation of p38 MAPK and JNK. Br J Pharmacol.
149:283–290. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pan MH, Chiou YS, Cheng AC, Bai N, Lo CY,
Tan D, et al: Involvement of MAPK, Bcl-2 family, cytochrome c, and
caspases in induction of apoptosis by
1,6-O,O-diacetylbritannilactone in human leukemia cells. Mol Nutr
Food Res. 51:229–238. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saldeen J and Welsh N: p38 MAPK inhibits
JNK2 and mediates cytokine-activated iNOS induction and apoptosis
independently of NF-κB translocation in insulin-producing cells.
Eur Cytokine Netw. 15:47–52. 2004.PubMed/NCBI
|
|
13
|
Yu T, Jhun BS and Yoon Y: High-glucose
stimulation increases reactive oxygen species production through
the calcium and mitogenactivated protein kinase-mediated activation
of mitochondrial fission. Antioxid Redox Signal. 14:425–437. 2011.
View Article : Google Scholar :
|
|
14
|
Palsamy P and Subramanian S: Ameliorative
potential of resveratrol on proinflammatory cytokines,
hyperglycemia mediated oxidative stress, and pancreatic beta-cell
dysfunction in streptozotocin-nicotinamide-induced diabetic rats. J
Cell Physiol. 224:423–432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Noh H and Ha H: Reactive oxygen species
and oxidative stress. Contrib Nephrol. 170:102–112. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim WH, Lee JW, Suh YH, Lee HJ, Lee SH, Oh
YK, et al: AICAR potentiates ROS production induced by chronic high
glucose: roles of AMPK in pancreatic beta-cell apoptosis. Cell
Signal. 19:791–805. 2007. View Article : Google Scholar
|
|
17
|
Zou R, Yang L, Xue J, Ke M, Huang Q, Huang
Q, et al: RIP140 mediates hyperglycemia-induced glucotoxicity in
beta-cells via the activation of JNK and ERK1/2 signaling pathways.
Diabetes Res Clin Pract. View Article : Google Scholar
|
|
18
|
Lee SH, Park MH, Park SJ, Kim J, Kim YT,
Oh MC, Jeong Y, Kim M, Han JS and Jeon YJ: Bioactive compounds
extracted from Ecklonia cava by using enzymatic hydrolysis protects
high glucose-induced damage in INS-1 pancreatic β-cells. Appl
Biochem Biotechnol. 167:1973–1985. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harris CS, Asim M, Saleem A, Haddad PS,
Arnason JT and Bennett SA: Characterizing the cytoprotective
activity of Sarracenia purpurea L., a medicinal plant that inhibits
glucotoxicity in PC12 cells. BMC Complement Altern Med. 12:2452012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu W, Jia Q, Wang Y, Zhang Y and Xia M:
The anthocyanin cyanidin-3-O-beta-glucoside, a flavonoid, increases
hepatic glutathione synthesis and protects hepatocytes against
reactive oxygen species during hyperglycemia: Involvement of a
cAMP-PKA-dependent signaling pathway. Free Radic Biol Med.
52:314–327. 2012. View Article : Google Scholar
|
|
21
|
Hays NP, Galassetti PR and Coker RH:
Prevention and treatment of type 2 diabetes: current role of
lifestyle, natural product, and pharmacological interventions.
Pharmacol Ther. 118:181–191. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee SH, Park MH, Kang SM, Ko SC, Kang MC,
Cho S, Park PJ, Jeon BT, Kim SK, Han JS and Jeon YJ: Dieckol
isolated from Ecklonia cava protects against high-glucose induced
damage to rat insulinoma cells by reducing oxidative stress and
apoptosis. Biosci Biotechnol Biochem. 76:1445–1451. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Becatti M, Prignano F, Fiorillo C,
Pescitelli L, Nassi P, Lotti T, et al: The involvement of
Smac/DIABLO, p53, NF-κB, and MAPK pathways in apoptosis of
keratinocytes from perilesional vitiligo skin: Protective effects
of curcumin and capsaicin. Antioxid Redox Signal. 13:1309–1321.
2010. View Article : Google Scholar : PubMed/NCBI
|


















