Introduction
Colorectal carcinoma (CRC) comprises colon and
rectal cancer and is one of the most common types of malignant
digestive tract tumor in humans. It is the fourth most common type
of cancer in males and the third most common cancer in females
worldwide (1). CRC is one of the
major causes of cancer-associated mortality (2). In general, CRC incidence rates
continue to increase in developing countries, whereas incidence
rates are stabilizing or decreasing in developed countries. The
increase of CRC in developing countries may reflect the adoption of
western lifestyles and behaviors, including the consumption of
high-fat diets, physical inactivity and smoking (3,4). It
has been established that cancer stem cells (CSCs) have a phenotype
that can be defined by their cell of origin [stem cell (SC) or
early progenitor cells] and by oncogenic events, which contribute
to their transformation (5).
Certain studies have provided evidence to support this concept
(5,6). One approach for identifying shared SC
markers is to focus on conserved stem and progenitor cell
functions, which may be inherited by the malignant SC
compartment.
Aldehyde dehydrogenase 1 (ALDH1) is a detoxifying
enzyme responsible for the oxidation of intracellular aldehydes
(7–10). ALDH1 thereby confers resistance to
alkylating agents (11). Through
its role in oxidizing retinol to retinoic acid, ALDH1 is important
in the early differentiation of SCs (12). It has been demonstrated that murine
and human hematopoietic and neural stem and progenitor cells have a
high ALDH1 activity (13–16). Increased ALDH1 activity has also
been identified in SC populations in multiple myeloma and acute
myeloid leukemia (16,17). Thus, ALDH1 activity may provide a
common marker for normal and malignant stem and progenitor cells.
Previous observations have suggested that ALDH1 is a marker for
CSCs in various types of malignancy and that ALDH1 in CSCs is
associated with chemoresistance and an increased potential for
malignancy (18–24).
Previous immunohistochemistry results demonstrated
that ALDH1 expression was higher in breast and lung cancer compared
with normal tissue (25,26), while in malignant ovarian tumors
expression levels were lower than that in normal ovaries (27). However, to the best of our
knowledge, whether the expression of ALDH1 in human CRC is
associated with clinicopathological features and the
differentiation of tumor cells has not previously been
investigated. Thus, the present study aimed to elucidate whether
the expression of ALDH1 is a significant clinicopathological
prognostic factor in patients with CRC using immunofluorescence
staining and enzyme-linked immunosorbent assay (ELISA).
Materials and methods
Patients and tissue specimens
The fresh tissues of primary CRCs were obtained from
the Department of Pathology at the Red Flag Hospital Affiliated to
Mudanjiang Medical College (Mudanjiang, China) between January 1,
2010 and October 20, 2012. Prior to surgery, none of the patients
involved in the present study had received any type of therapy,
including radiation or chemotherapy. Of these tumor specimens, 20
were well differentiated, 20 were moderately differentiated, 20
were poorly differentiated and 10 were mucinous carcinomas. A total
of 20 fresh samples were collected for ELISA, including 10
colorectal tissues and 10 corresponding adjacent normal mucous
tissues (>5 cm from the margin of the tumor), which were stored
at −80°C until use. All tissue samples were fixed in formalin,
embedded in paraffin and deparaffinized for immunofluorescence
staining. Written informed consent was obtained from the patient’s
families and all protocols were reviewed and approved by the
Ethical Committee of Mudanjiang Medical College.
Indirect immunofluorescence
Indirect immunofluorescence was performed as
previously described (28). The
sections were rinsed with Tris-buffered saline (TBS; Sigma-Aldrich,
Shanghai, China) and blocked in buffer for 30 min in a humidified
chamber at 37°C. For ALDH1 staining, the sections were incubated at
4°C overnight with a rabbit monoclonal anti-ALDH1 antibody (1:400;
EarthOx; cat no. HZ348711). The slides were then incubated with a
fluorescent-conjugated monoclonal secondary antibody (1:150;
EarthOX; HZ369731; 30 min; 37°C; Fluor 491–520 nm). Following
incubation with the secondary antibody, the sections were rinsed
three times with TBS and Tween 20 and then coverslipped. Finally,
the samples were examined by fluorescence microscopy (Nikon Eclipse
80i; Nikon, Tokyo, Japan). The assessed tumor area, based on
immunofluorescence staining, was used to calculate the density of
the positive cell staining.
Evaluation of labeling
Evaluation of the immunofluorescence staining for
ALDH1 expression was performed independently by two pathologists.
Imaging analysis of the colorectal tumors for ALDH1 expression was
performed in one selected area (magnification, ×200) per case.
ELISA for ALDH1
The ALDH1 levels in tissue homogenate of the tumor
tissue were quantified using a commercially available ELISA kit
(Tianjin Haoyang Biological Manufacture Co., Ltd., Tianjin, China),
according to the manufacturer’s instructions. An ELISA standard
curve was generated and then according to the optical density data,
the ALDH1 content of the normal and malignant tumor tissues was
determined.
Statistical analysis
All the data were analyzed using SPSS software
version 11.0 for Windows (SPSS, Inc., Chicago, IL, USA). The
association between ALDH1 immunofluorescence expression and the
clinicopathological parameters was evaluated using the
χ2 test. P<0.05 was considered to indicate a
statistically significant difference. The association of ALDH1
content between primary colorectal tissues and normal colorectal
tissues was assessed using the Pearson χ2 test.
P<0.001 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
A total of 70 patients were enrolled in the present
study; 50% were male (35 of 70) and 50% were female (35 of 70). The
median age of the patients was 61.7 years (range 22–80 years). A
total of 45 (64.2%) patients were >60 years old and 25 (35.7%)
were <60 years old. The diagnosis in all patients was colorectal
adenocarcinoma and was classified by the degree of tumor cell
differentiation (well, moderately or poorly differentiated). Of the
70 patients, 20 patients (28.5%) had well-differentiated tumors, 20
(28.5%) had moderately differentiated tumors, 20 (28.5%) had poorly
differentiated tumors and 10 (14.3%) had mucinous carcinomas. A
total of 29 patients were at Dukes’ stage A+B (41.4%) and 41
patients were at Dukes’ stage C+D (58.5%). According to the
tumor-node metastasis (TNM) staging system, 10 patients (14.3%)
were at stage I, 19 patients (27.1%) were at stage II, 32 patients
(45.7%) were at stage III and 9 patients (12.9%) were at stage IV.
Lymph node metastases were present in 36 patients (51.4%) and
absent in 34 patients (48.6%; Table
I).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Clinical data | n | (%) |
|---|
| Total cases | 70 | |
| Male | 35 | (50) |
| Female | 35 | (50) |
| Age (years) |
| ≥60 | 45 | (64.2) |
| <60 | 25 | (35.7) |
| Median (years) | 61.7 | (22–80) |
| Differentiation |
| Well | 20 | (28.5) |
| Moderate | 20 | (28.5) |
| Poor | 20 | (28.5) |
| Mucinous
carcinomas | 10 | (14.3) |
| Dukes’ stage |
| A+B | 29 | (41.4) |
| C+D | 41 | (58.5) |
| TNM stage |
| I | 10 | (14.3) |
| II | 19 | (27.1) |
| III | 32 | (45.7) |
| IV | 9 | (12.9) |
| Lymph node
metastasis |
| Positive | 36 | (51.4) |
| Negative | 34 | (48.6) |
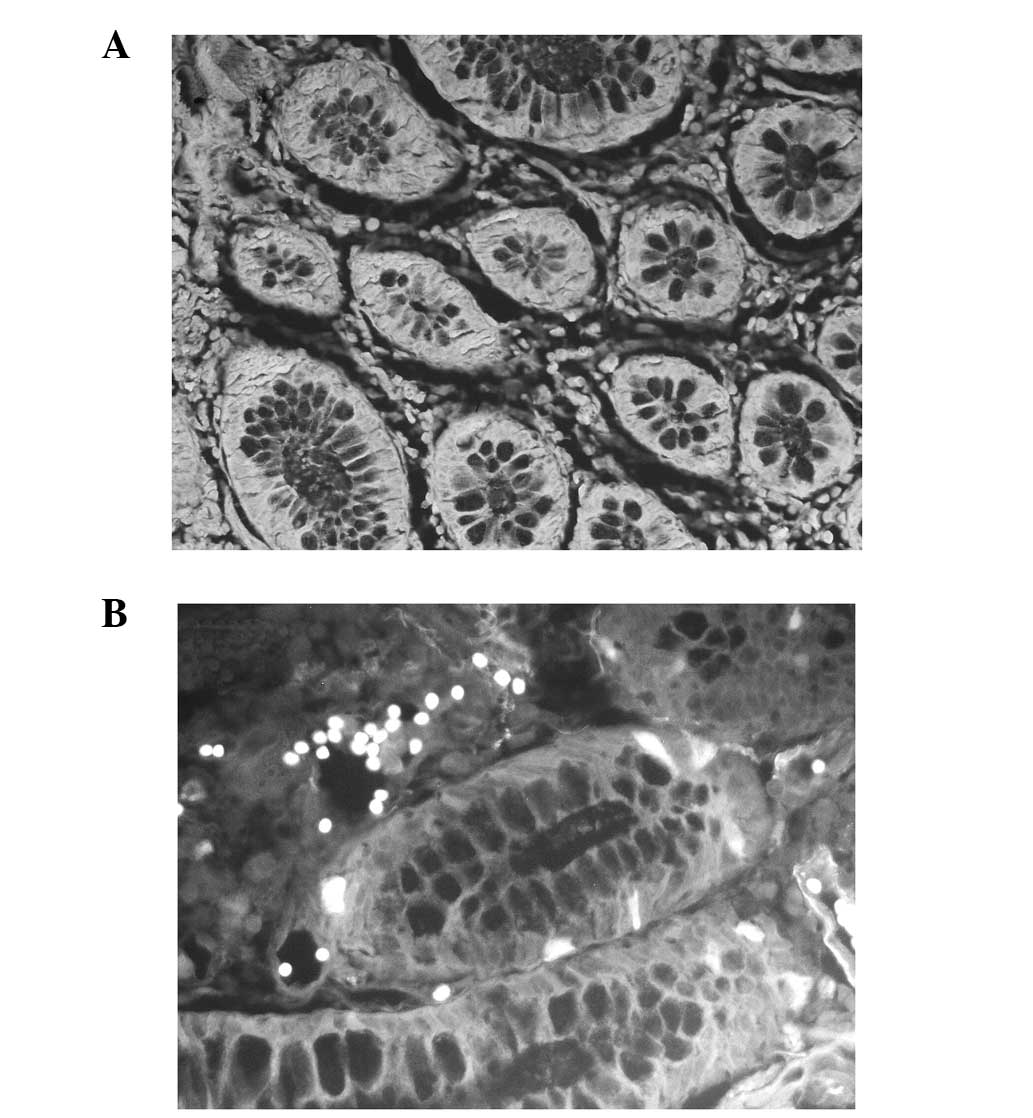
ALDH1 expression in normal colorectal
tissue and CRC tissue
Compared with normal colorectal tissue, CRC tissue
exhibited markedly higher levels of ALDH1 protein expression.
Normal mucosal specimens at least 5 cm distant from the primary
colorectal carcinomas were obtained from 10 patients with CRC.
ALDH1 was detected in the cytoplasm of the CRC tissue and certain
normal colorectal tissue. Staining was observed in the cytoplasm of
the epithelial cells. In the 10 normal controls, ALDH1 reactivity
was demonstrated in ~10% of the epithelial cells. Of the 70
colorectal cancer specimens, a high rate of positive staining was
observed (≥20%). Patients exhibiting ≥20% positive cells were
classified as ALDH1 positive and the remainder as ALDH1 negative.
The results of the immunofluorescence staining for ALDH1 in the
normal colorectal tissue and the CRC tissue are shown in Fig. 1.
Correlation between ALDH1 positive
expression and various clinicopathological features
The association between ALDH1 protein and the
clinicopathological features of the CRC patients are summarized in
Table II. No significant
correlation was identified between ALDH1 expression and age,
gender, tumor size or lymph node metastasis. ALDH1 expression,
however, was closely associated with the differentiation of tumor
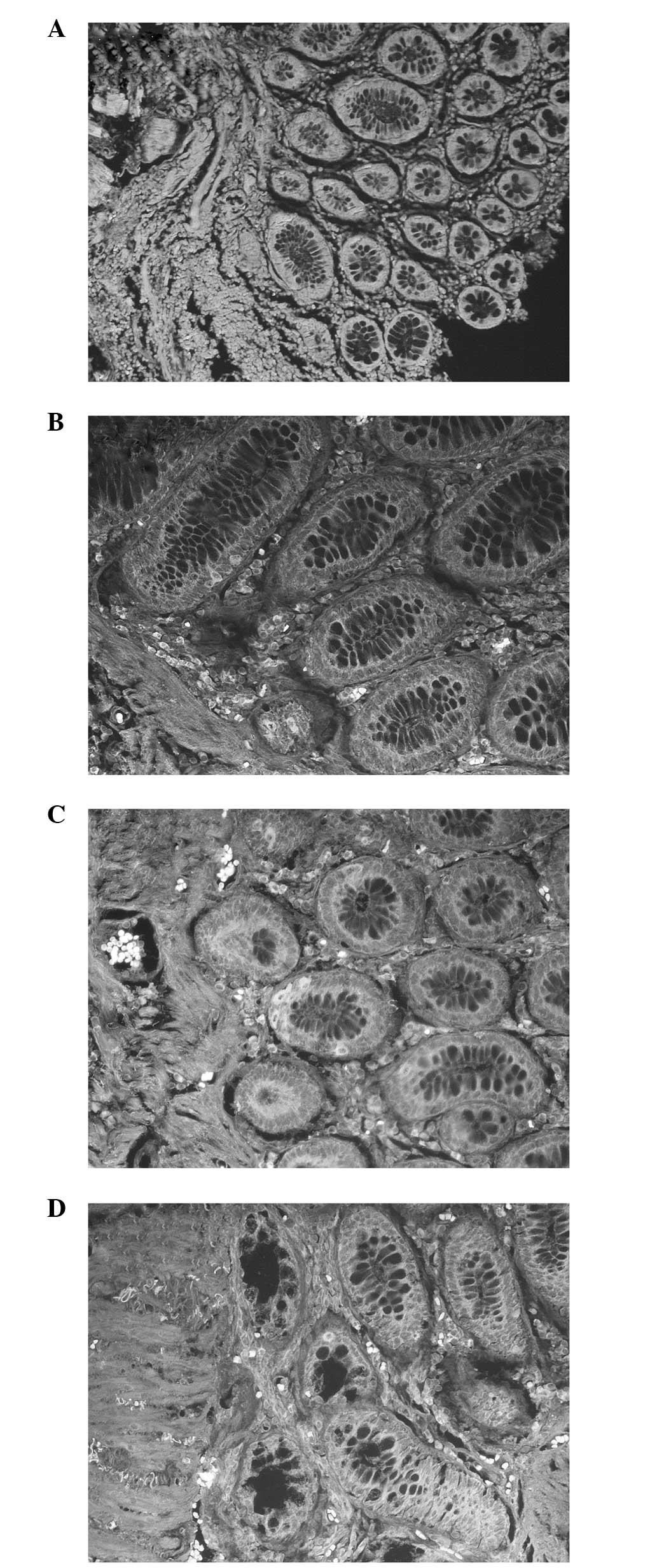
cells. The results revealed that the greater the degree of
differentiation of the tumor cell, the lower the rate of ALDH1
staining. A higher ALDH1 band intensity was detected in the poorly
differentiated malignant tumors (Fig.
2A) compared with the mucinous carcinomas and well or
moderately differentiated tumors (Fig.
2B–D). A positive correlation was observed between the
differentiation of tumor cells and the proportion of ALDH1
immunostained cells (χ2=16.43; P<0.05) among the
examined samples. Of the 10 mucinous carcinoma cases, three ALDH1
positive cases were noted. Of the 20 well-differentiated cases,
ALDH1 expression was noted in 7 cases (35%). Of the 20 moderately
differentiated cases, ALDH1 expression was noted in 13 cases (65%)
and of the 20 poorly differentiated cased, ALDH1 expression was
noted in 18 cases (90%). Dukes’ C+D stages demonstrated a higher
expression rate of ALDH1 (70.7%) compared with Dukes’ A+B stages
(41.4%; P<0.05). Using the TNM staging system, positive staining
of the ALDH1 protein was identified in 3 out of the 9 cases of TNM
stage I (33.3%); 9 cases were identified in 20 of the TNM stage II
samples (45%); 21 cases were identified in 32 of the TNM stage III
samples (65.6%) and 8 cases were identified in 9 of the TNM stage
IV samples (88.9%; χ2=7.94; P<0.05). The
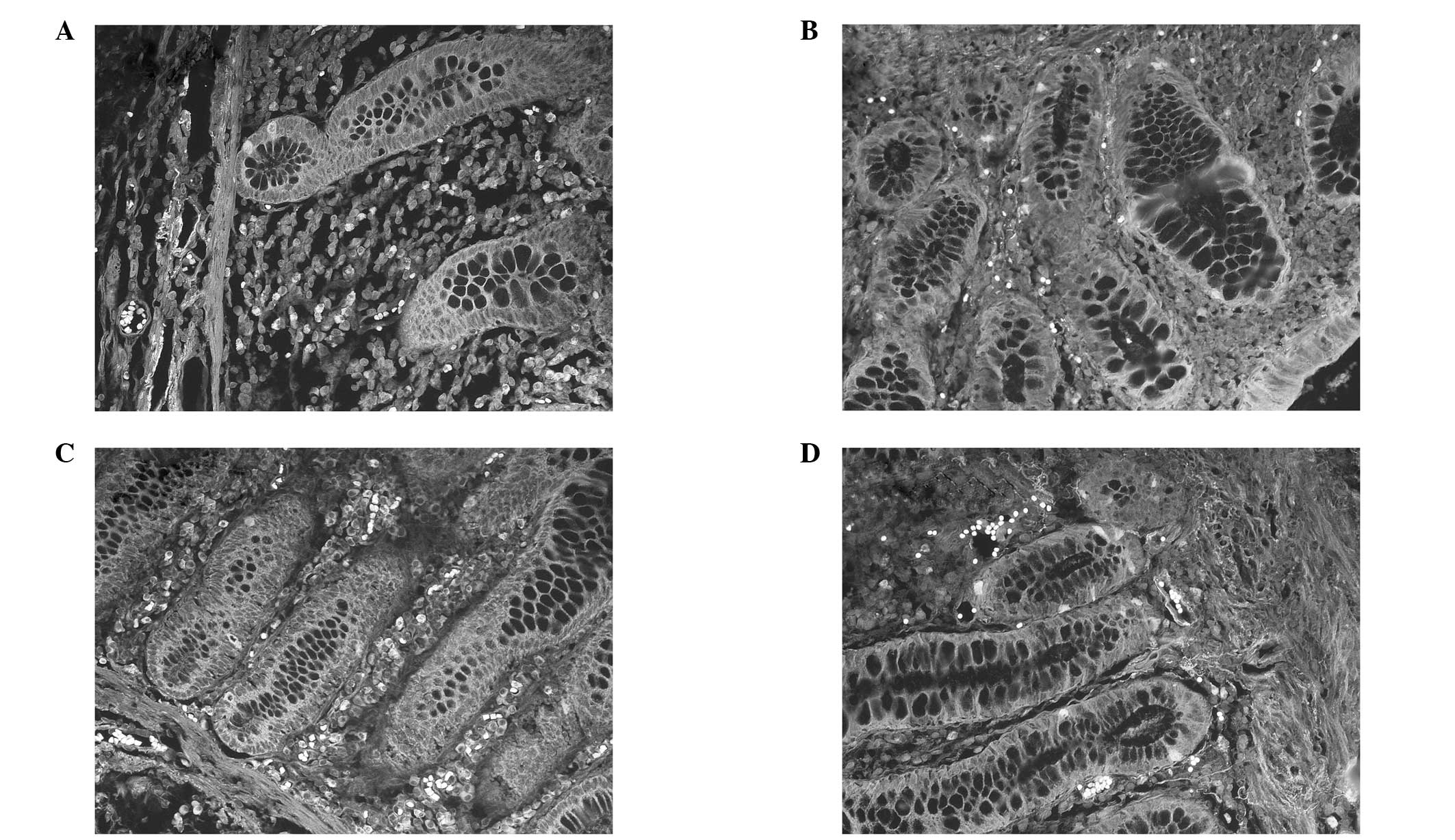
immunofluorescence staining for ALDH1 expression in various TNM
stages is shown in Fig. 3.
 | Table IICorrelation between ALDH1 positive
expression and various clinicopathological features in colorectal
carcinoma patients. |
Table II
Correlation between ALDH1 positive
expression and various clinicopathological features in colorectal
carcinoma patients.
| Clinicopathological
feature | n | Positive | Negative | Positive rate
(%) | χ2 | P-value |
|---|
| Gender |
| Male | 35 | 22 | 13 | 62.8 | 0.530 | P>0.05 |
| Female | 35 | 19 | 16 | 54.3 | | |
| Age (years) |
| ≥60 | 45 | 26 | 19 | 57.8 | 0.033 | P>0.05 |
| <60 | 25 | 15 | 10 | 60.0 | | |
| Tumor size
(cm) |
| ≤3 | 15 | 8 | 7 | 53.3 | | |
| >3 | 55 | 33 | 22 | 60.0 | 0.216 | P>0.05 |
|
Differentiation |
| Mucinous
carcinomas | 10 | 3 | 7 | 30.0 | | |
| Well | 20 | 7 | 13 | 35.0 | 16.431 | P<0.05 |
| Moderate | 20 | 13 | 7 | 65.0 | | |
| Poor | 20 | 18 | 2 | 90.0 | | |
| Duke’s stage |
| A+B | 29 | 12 | 17 | 41.4 | 6.031 | P<0.05 |
| C+D | 41 | 29 | 12 | 70.7 | | |
| TNM stage |
| I | 9 | 3 | 6 | 33.3 | | |
| II | 20 | 9 | 11 | 45.0 | 7.940 | P<0.05 |
| III | 32 | 21 | 11 | 65.6 | | |
| IV | 9 | 8 | 1 | 88.9 | | |
| Lymph node
metastasis |
| Positive | 36 | 24 | 12 | 66.7 | 2.000 | P>0.05 |
| Negative | 34 | 17 | 17 | 50.0 | | |
Quantitative detection of ALDH1 in CRC
tissue and in normal colorectal tissue
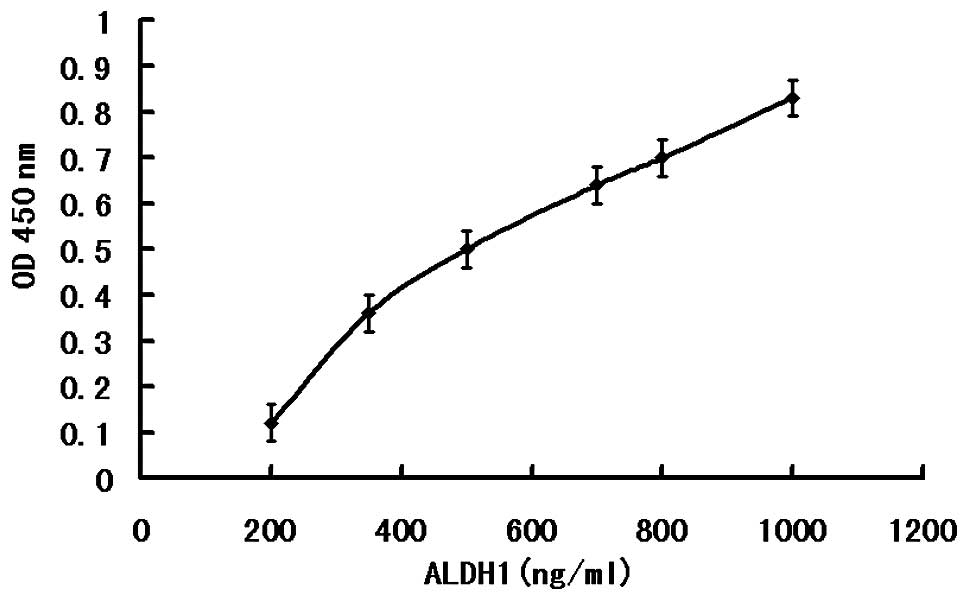
According to the ELISA kit instructions, an ELISA
standard curve was drawn (Fig. 4),
and then according to the standard curve, the levels of ALDH1 in
the normal and malignant tumor tissues were calculated. In the CRC
tissue, the content of ALDH1 was 559.0±151.76 ng/ml, whereas, in
normal colorectal tissue, the content of ALDH1 was 298.6±58.43
ng/ml. Using a Pearson χ2 test (t=5.064; P<0.001;
Table III), the ALDH1 content
was found to be significantly increased in the human colorectal
cancer tissue compared with the normal colorectal tissue.
 | Table IIIQuantitative results of ALDH1 in
colorectal carcinoma tissue and in normal colorectal tissue. |
Table III
Quantitative results of ALDH1 in
colorectal carcinoma tissue and in normal colorectal tissue.
| Sample | n | ALDH1 mean ± SD,
(ng/ml) |
|---|
| Colorectal
carcinoma tissue | 10 | 559.00±151.76 |
| Normal colorectal
tissue | 10 | 298.60±58.43 |
Discussion
The cancer stem cell theory was initially proposed
by Hamburger and Salmon (29), who
demonstrated that only a small percentage of tumor cells was able
to form colonies in soft agar. According to the CSC hypothesis,
cancer originates from SCs that exhibit pluripotency and
self-renewal (30). CSCs are
hypothesized to lead to the initiation, progression and recurrence
of cancer. Certain tumor-initiating cells share common cell surface
markers, leading to their designation as SCs (18).
CSCs can be defined by the cell of origin (SCs or
early progenitor cells). Identifying the normal and malignant
stem/progenitor cells by the same marker, supports the hypothesis
that stem and progenitor cells are primary targets of
transformation, and also adds further evidence to the cancer stem
cell hypothesis. The candidate marker ALDH1 is a good choice, which
is in accordance with the above theory that CSCs can be defined by
the cell of origin (SCs) (21).
One key finding of the present study was that,
compared with the normal colorectal tissues, the tumor tissues
expressed a high rate of ALDH1 staining (≥20%). A second key
finding of the present study was the correlation between the
differentiation degree of the tumor cell and the expression of
ALDH1. Through indirect immunofluorescence staining, it was
demonstrated that with decreased differentiation (from well to
poorly differentiated), the ALDH1-positive staining rates increased
(χ2=16.43; P<0.05). The reason for mucinous carcinoma
exhibiting the lowest staining rate may be attributed to the mucago
of mucinous carcinomas affecting the staining of ALDH1 in cancer
cells. The results also suggested that poorly differentiated cancer
cells expressed increased colorectal SCs compared with
well-differentiated cells. These findings supported those of Huang
et al, which revealed that the overall proliferative cell
population increases during colorectal tumorigenesis (19). A third important finding was that
low-grade tumors exhibited higher expression of ALDH1 compared with
high grade tumors (P<0.05). Additionally, Dukes’ C+D stages
demonstrated a higher expression of ALDH1 compared with Dukes’ A+B
stages (P<0.05). Western blotting was used to determine the
protein content of ALDH1, however, no significant findings were
obtained. Considering that the protein content was small, ELISA was
used to analyze the content of ALDH1 in fresh CRC tissues. Through
an ELISA assay, the present study also found an increased level of
ALDH1 in CRC tissues compared with normal colorectal tissues.
In conclusion, the present study supported the CSC
hypothesis by demonstrating that normal and malignant SCs share a
common functional marker. As a CSC marker shared by normal
colorectal tissues and CRC tissues, ALDH1 may be important in CRC
carcinogenesis and may be used as an important marker for CSC. It
can be regarded as a valuable marker in CRC patients and, through
detecting the content of ALDH1, may assist in the differentiation
of colorectal carcinogenesis from normal colorectal tissues. At
present, ALDH1 is attracting increased attention, however, whether
ALDH1 negative cells also possess the same ability as SCs has yet
to be elucidated. As the sample size of the present study was
limited, further studies with larger samples are warranted to
assess the possibility that ALDH1 expression may be used in the
pathological evaluation of tissue histology to predict disease
prognosis and the response to chemotherapy in patients with
CRC.
Acknowledgements
The authors would like to thank Dr Ying-Wu of the
Red Flag Hospital Affiliated to Mudanjiang Medical College
(Mudanjiang, China) for her contribution to experiments and data
and Ms. Jia-Ning Qiu of Jilin University (Changchun, China) for her
linguistic contributions. This study was supported by project
grants from the Science and Technology Department of Heilongjiang
Province in China (grant no. D201259).
Abbreviations:
|
ALDH1
|
aldehyde dehydrogenase 1
|
|
CRC
|
colorectal carcinoma
|
|
CSC
|
cancer stem cell
|
|
IFAS
|
indirect florescence antibody
staining
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
SC
|
stem cell
|
|
AML
|
acute myeloid leukemia
|
References
|
1
|
Center MM, Jemal A and Ward E:
International trends in colorectal cancer incidence rates. Cancer
Epidemiol Biomarkers Prev. 18:1688–1694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Long N, Moore MA, Chen W, Gao CM, Lai MS
and Mizoue T: Cancer epidemiology and control in north-east Asia -
past, present and future. Asian Pac J Cancer Prev. 11:107–148.
2010.PubMed/NCBI
|
|
4
|
Peppone LJ, Mahoney MC, Cummings KM, et
al: Colorectal cancer occurs earlier in those exposed to tobacco
smoke: implications for screening. J Cancer Res Clin Oncol.
134:743–751. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jamieson CH, Ailles LE, Dylla SJ, et al:
Granulocyte-macrophage progenitors as candidate leukemic stem cells
in blast-crisis CML. N Engl J Med. 351:657–667. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kelly LM and Gilliland DG: Genetics of
myeloid leukemias. Annu Rev Genomics Hum Genet. 3:179–198. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duester G: Families of retinoid
dehydrogenases regulating vitamin A function: production of visual
pigment and retinoic acid. Eur J Biochem. 267:4315–4324. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Magni M, Shammah S, Schiró R, et al:
Induction of cyclophosphamide-resistance by aldehyde-dehydrogenase
gene transfer. Blood. 87:1097–1103. 1996.PubMed/NCBI
|
|
9
|
Sophos NA and Vasiliou V: Aldehyde
dehydrogenase gene superfamily: the 2002 update. Chem Biol
Interact. 143–144:5–22. 2003. View Article : Google Scholar
|
|
10
|
Yoshida A, Rzhetsky A, Hsu LC and Chang C:
Human aldehyde dehydrogenase gene family. Eur J Biochem.
251:549–557. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dylla SJ, Beviglia L, Park IK, et al:
Colorectal cancer stem cells are enriched in xenogeneic tumors
following chemotherapy. PLoS One. 3:e24282008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chute JP, Muramoto GG, Whitesides J, et
al: Inhibition of aldehyde dehydrogenase and retinoid signaling
induces the expansion of human hematopoietic stem cells. Proc Natl
Acad Sci USA. 103:11707–11712. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Armstrong L, Stojkovic M, Dimmick I, et
al: Phenotypic characterization of murine primitive hematopoietic
progenitor cells isolated on basis of aldehyde dehydrogenase
activity. Stem Cells. 22:1142–1151. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hess DA, Meyerrose TE, Wirthlin L, et al:
Functional characterization of highly purified human hematopoietic
repopulating cells isolated according to aldehyde dehydrogenase
activity. Blood. 104:1648–1655. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hess DA, Wirthlin L, Craft TP, et al:
Selection based on CD133 and high aldehyde dehydrogenase activity
isolates long-term reconstituting human hematopoietic stem cells.
Blood. 107:2162–2169. 2006. View Article : Google Scholar
|
|
16
|
Matsui W, Huff CA, Wang Q, et al:
Characterization of clonogenic multiple myeloma cells. Blood.
103:2332–2336. 2004. View Article : Google Scholar
|
|
17
|
Pearce DJ, Taussig D, Simpson C, et al:
Characterization of cells with a high aldehyde dehydrogenase
activity from cord blood and acute myeloid leukemia samples. Stem
Cells. 23:752–760. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deng S, Yang X, Lassus H, et al: Distinct
expression levels and patterns of stem cell marker, aldehyde
dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS
One. 5:e102772010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang EH, Hynes MJ, Zhang T, et al:
Aldehyde dehydrogenase 1 is a marker for normal and malignant human
colonic stem cells (SC) and tracks SC overpopulation during colon
tumorigenesis. Cancer Res. 69:3382–3389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li T, Su Y, Mei Y, et al: ALDH1A1 is a
marker for malignant prostate stem cells and predictor of prostate
cancer patients’ outcome. Lab Invest. 90:234–244. 2010. View Article : Google Scholar
|
|
21
|
Ginestier C, Hur MH, Charafe-Jauffret E,
et al: ALDH1 is a marker of normal and malignant human mammary stem
cells and a predictor of poor clinical outcome. Cell Stem Cell.
1:555–567. 2007. View Article : Google Scholar
|
|
22
|
Ma S, Chan KW, Lee TK, et al: Aldehyde
dehydrogenase discriminates the CD133 liver cancer stem cell
populations. Mol Cancer Res. 6:1146–1153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Croker AK, Goodale D, Chu J, et al: High
aldehyde dehydrogenase and expression of cancer stem cell markers
selects for breast cancer cells with enhanced malignant and
metastatic ability. J Cell Mol Med. 13:2236–2252. 2009. View Article : Google Scholar
|
|
24
|
Jiang F, Qiu Q, Khanna A, et al: Aldehyde
dehydrogenase 1 is a tumor stem cell-associated marker in lung
cancer. Mol Cancer Res. 7:330–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nogami T, Shien T, Tanaka T, et al:
Expression of ALDH1 in axillary lymph node metastases is a
prognostic factor of poor clinical outcome in breast cancer
patients with 1–3 lymph node metastases. Breast Cancer. 21:58–65.
2014. View Article : Google Scholar
|
|
26
|
Patel M, Lu L, Zander DS, et al: ALDH1A1
and ALDH3A1 expression in lung cancers: correlation with histologic
type and potential precursors. Lung Cancer. 59:340–349. 2008.
View Article : Google Scholar
|
|
27
|
Penumatsa K, Edassery SL, Barua A, et al:
Differential expression of aldehyde dehydrogenase 1a1 (ALDH1) in
normal ovary and serous ovarian tumors. J Ovarian Res. 3:282010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Vlierberghe RL, Sandel MH, Prins FA,
et al: Four-color staining combining fluorescence and brightfield
microscopy for simultaneous immune cell phenotyping and
localization in tumor tissue sections. Microsc Res Tech. 67:15–21.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hamburger AW and Salmon SE: Primary
bioassay of human tumor stem cells. Science. 197:461–463. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Boman BM and Wicha MS: Cancer stem cells:
a step toward the cure. J Clin Oncol. 26:2795–2799. 2008.
View Article : Google Scholar : PubMed/NCBI
|