Introduction
Intestinal ischemia-reperfusion (IIR) is a
challenging clinical syndrome occurring in patients undergoing
major surgery, including cardiac surgery and liver transplantation
(1–4). IIR leads to local injury, but also
induces severe remote organ injury, particularly acute lung injury
(ALI), which is associated with high rates of mortality (5).
Previous studies have demonstrated that oxidative
stress is important in IIR-mediated ALI (6,7).
Reactive oxygen species (ROS), predominantly from neutrophil
sequestration, contribute to ALI (8) and high concentrations of ROS in the
bronchoalveolar lavage fluid are correlated with the severity of
ALI in rabbits induced by hemorrhagic shock and resuscitation
(9). The administration of
antioxidants has been observed to attenuate ALI in several models,
including hemorrhagic shock, IIR and sepsis (10–12).
Mast cells are widely distributed in the lungs in
order to maintain homeostasis of respiratory function. However,
mast cell degranulation can exacerbate ALI, as observed in our
previous study, which demonstrated that inhibiting the activation
of mast cells alleviated IIR-induced lung injury and reduced the
inflammatory response in a rodent model (13). ROS have been observed to mediate
mast cell degranulation in vitro (14), and excessive activation of mast
cells contributes to allergic and inflammatory diseases of the
respiratory system (15,16). However, there remains no direct
evidence demonstrating the role of mast cell activation by
oxidative stress in IIR-induced ALI. The present study hypothesized
that mast cell activation exacerbates IIR-mediated ALI primarily
through oxidative stress.
Sevoflurane (SEV) is one of the most commonly used
inhaled anesthetics (17,18). Preconditioning with SEV has been
demonstrated to protect against ischemia-reperfusion injury in
various organs, particularly in the lungs and brain (19,20).
It has been suggested that the antioxidant and anti-inflammatory
properties of SEV contribute to protection against sepsis,
ventilation-induced lung injury and lipopolysaccharide-induced lung
injury (21–23). However, the role of SEV
preconditioning in IIR-mediated ALI remains to be elucidated. The
present study investigated whether SEV preconditioning prevents
against IIR-induced ALI through inhibition of the synergistic
actions between mast cell activation and oxidative stress.
Materials and methods
Animals and treatment
A total of 60 female adult Sprague-Dawley rats
(weighing 200–250 g) were obtained from the Animal Centre of Sun
Yat-sen University (Guangzhou, China). The use and care of animals,
in addition to the experimental and surgical procedures, were
reviewed and approved by the Institutional Animal Care and Use
Committee of Sun Yat-Sen University and the Ethics Committee of Sun
Yat-Sen University. The animals were housed under a 14 h:10 h
light-dark cycle at room temperature between 18 and 26°C and 60–70%
humidity. Food and water were available ad libitum. The rats
were randomly divided into 10 groups (6 rats/group): Normal saline
(NS), SEV, apocynin (AP), sham, IIR, IIR + compound 48/80 (CP), SEV
+ IIR, SEV + IIR + CP, AP + IIR and AP + IIR + CP). The treatments
administered to these groups was as follows: NS group, 1 ml NS
only; SEV group, 2.3% SEV inhalation only; iii) AP group, 2.5 mg/kg
AP only; sham, administration of 1 ml NS each day for 3 days prior
to surgical isolation of the superior mesenteric artery (SMA)
without occlusion; IIR, administration of 1 ml NS each day for 3
days prior to IIR, established by occluding the SMA for 75 min,
followed by 2 h reperfusion; IIR + CP, IIR with administration of
CP (0.75 mg/kg) via the caudal vein 5 min prior to reperfusion;
vii) SEV + IIR, IIR following exposure to 2.3% SEV each day for 3
days; SEV + IIR + CP, IIR + CP following exposure to 2.3% SEV each
day for 3 days; AP + IIR, IIR following pretreatment with AP each
day for 3 days; and AP + IIR + CP, IIR + CP following pretreatment
with AP each day for 3 days. In the NS, AP and SEV groups, the
animals did not undergo surgery, but received saline (1 ml; KeyGen
Biotech. Co., Ltd., Nanjing, China) or AP (2.5 mg/kg;
Sigma-Aldrich, St. Louis, MO, USA) via intraperitoneal injection,
or 2.3% SEV (Maruishi Pharmaceutical Co., Ltd., Osaka, Japan) by
inhalation. In the sham group, the SMA was isolated, but not
clamped; in the IIR group, all rats received SMA separation and
clipping for 75 min, followed by 2 h reperfusion. For the groups
treated with NS, AP or SEV, the rats were injected with NS (1 ml)
or AP (2.5 mg/kg), or administered with 2.3% SEV via inhalation for
three consecutive days prior to surgery. For the groups treated
with CP, 0.75 mg/kg CP (Sigma-Aldrich) was injected via the caudal
vein 5 min prior to reperfusion. The rats in the remaining IIR
groups received the same volume of normal saline (1 ml). The
experimental procedure used in the present study is presented in
Fig. 1. A heating lamp was used to
maintain the body temperatures of the rats. The optimal doses of
SEV (2.3%), AP (2.5 mg/kg) and CP (0.75 mg/kg) were adjusted, in
accordance with those previously described (24–26)
with modifications.
 | Figure 1Experimental procedures. The
blank/drug control group received no surgery and were administered
with NS, SEV or AP only. In the sham group, surgery, involvong
isolation of the SMA was performed, without occlusion. The IIR
animals received pretreatment with NS, SEV or AP prior to SMA
occlusion surgery, followed by treatment with CP or NS prior to 2 h
reperfusion. The NS (1 ml) and AP (2.5 mg/kg) were administered via
intraperitoneal injection and 2.3% SEV was provided by inhalation
for three consecutive days prior to surgery. CP (0.75 mg/kg) was
administrated via the caudal vein 5 min prior to reperfusion. NS,
normal saline; SEV, sevoflurane; AP, apocynin; SMA, superior
mesenteric artery; IIR, intestinal ischemia-reperfusion; CP,
compound 48/80. |
Preparation of tissue specimens
The rats were sacrificed through overdose of
pentobarbital (70 mg/kg; intraperitoneal injection; Sigma-Aldrich)
2 h after reperfusion. Blood samples (2 ml) were obtained from the
abdominal aorta and centrifuged (Centrifuge 5804; Eppendorf,
Hamburg, Germany) at 1,699 × g for 15 min at 4°C, and the resultant
plasma samples were stored at -80°C until analysis. A thoracotomy
was immediately performed and the right upper lung was removed,
fixed in 10% formaldehyde (Sigma-Aldrich) and embedded in paraffin
(Leica Biosystems, Nussloch, Germany) for sectioning. The middle
lobe of the right lung was removed and used to measure the wet/dry
(w/d) weight ratio. The inferior lobes of the right and left lungs
were removed and preserved in liquid nitrogen (Guangzhou Pearl
River Industrial Gases Co. Ltd., Guangzhou, China) for further
analysis of oxidative stress, mast cell activation and inflammatory
indicators.
Measurement of lung w/d ratio
The middle lobes of the right lungs were weighed
(weightwet) immediately using a precision balance
[Mettler-Toledo (Schweiz) GmbH, Greifensee, Switzerland], and
re-weighed (weightdry) following incubation at 95°C in
an oven (876–1 Vacuum Drying Oven; Nantong Science Instrument
Factory, Nantong, China) for 24 h. The w/d ratio was calculated as
follows: w/d = weightwet / weightdry.
Histopathological examination
The lung tissues embedded in paraffin were dissected
into 4-μm microsections (ZQP-86; Zhejiang Xiangshan
Scientific Precision Instrument Factory, Xiangshan, China), which
were stained and observed under a light microscope (Eclipse E200;
Nikon, Tokyo, Japan). Hematoxylin and eosin (Beyotime Institute
Biotechnology, Shanghai, China) staining was used to assess
pathological injury, while staining with toluidine blue (Beijing
Leagene Biotech Co., Ltd., Beijing, China) was used to count the
number of mast cells. The degree of lung injury was assessed using
a scoring system, described by Hofbauer et al (27). According to this scoring system,
edema of the alveoli and mesenchyme, intra-alveolar inflammatory
cell infiltrates, alveolar hemorrhage and atelectasis were graded
on a scale between 0 and 4. The grades were as follows: 0, normal,
<15% of space occupied by tissue and >85% occupied by
alveolar space; 1, 15%–25% of space occupied by tissue and 75%-85%
occupied by alveolar space; 2, 25%–50% occupied by tissue and
50%–75% occupied by alveolar space; 3, 50%–75% occupied by tissue
and 25%–50% occupied by alveolar space; and 4, 75%–100% occupied by
tissue and 0%–25% occupied by alveolar space. For the sections
stained with toluidine blue, cells containing blue-purple granules
in the cytoplasm were considered to be mast cells.
Detection of the levels of β -hexosa
minidase
β-hexosaminidase is one of the specific enzymes
synthesized by mast cells, and mast cell activation is accompanied
by the release of histamine and β-hexosaminidase. While histamine
is metabolized rapidly, β-hexosaminidase is metabolized less
readily and can be used as an index of mast cell activation
(28). In the present study, the
lung tissues were homogenized with cold normal saline and the
homogenates were centrifuged at 1,699 × g for 15 min at 4°C. The
supernatants were then transferred into fresh tubes for detection.
The activities of β-hexosaminidse in the lung tissue homogenates
and blood samples were detected using a β-hexosaminidase kit,
according to the manufacturer’s instructions (Nanjing KeyGen
Biotech. Co., Ltd.).
Detection of the levels of
malondialdehyde (MDA) and hydrogen peroxide
(H2O2)
The levels of MDA and H2O2 in
the homogenates of the lung tissues were measured using the MDA
Detection kit and the H2O2 Detection kit,
according to the manufacturer’s instructions (KeyGen Biotech. Co.,
Ltd.).
Detection of the activity levels of
interleukin (IL-6) and myeloperoxidase (MPO) activity
The concentration of IL-6 is an independent marker
for the inflammatory response. MPO is an enzyme induced by
activated neutrophils and is considered an indicator of neutrophil
infiltration (29,30). The activities of IL-6 and MPO in
the lung tissues were measured using the IL-6 Detection kit and the
MPO Detection kit (KeyGen Biotech Co., Ltd.), according to the
manufacturer’s instructions.
Western blotting
Prosurfactant protein C (proSP-C) is the precursor
of surfactant protein C, which is exclusively produced in alveolar
type II cells to prevent lung collapse (31). Trypatse is one of the
characteristic markers of mast cell activation (32). The expression of NADPH oxidase
reflects the level of oxidative stress, and p47phox and
gp91phox are subunits of NADPH oxidase (33). Intercellular adhesion molecule-1
(ICAM-1, CD54) is an important early marker of immune activation
and response (34). Western blot
analyses were performed, as previously described (24). The primary antibodies used in the
present study were as follows: Anti-ICAM-1 mouse monoclonal
antibody (1:500; sc-8439; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), antitryptase rabbit polyclonal antibody (1:500;
sc-32889; Santa Cruz Biotechnology, Inc.), anti-proSP-C polyclonal
rabbit antibody (1:1000; AB3786; EMD Millipore, Billerica, MA,
USA), anti-p47phox polyclonal rabbit antibody (1:1,000;
sc-14015; Santa Cruz Biotechnology, Inc.) and
anti-gp91phox polyclonal rabbit antibody (1:1,000;
sc-20782; Santa Cruz Biotechnology, Inc.). The images were analyzed
used ImageJ software, version 1.41 (National Institutes of Health,
Bethesda, MA, USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analyses were performed using SPSS software,
version 13.0 (SPSS Inc., Chicago, IL, USA). The statistical
significance of differences between the groups were evaluated by
one-way analysis of variance followed by Bonferroni’s post-hoc test
for unpaired values. P<0.05 was considered to indicate a
statistically significant difference.
Results
SEV and AP increase antioxidant capacity
and inactivate mast cells in normal rat lungs
As shown in Fig.
2A, the pulmonary structures were normal following treatments
with NS, SEV and AP for 3 days. Treatment with SEV or AP did not
significantly change the number of mast cells (Fig. 2B), expression of tryptase or the
levels of β-hexosaminidase in either the plasma or the lung tissues
(Fig. 2C). However, the expression
levels of p47phox and gp91phox were
downregulated in the lungs (P<0.05), inhibiting NAPDH enzyme
activity (Fig. 2D).
SEV and AP attenuate IIR-induced lung
injury
As shown in Fig.
3A, the lung structures in the sham group were normal. IIR
resulted in severe damage to the lungs, with collapse of the
alveoli, interstitial edema, haemorrhage in the alveoli and
mesenchyme, neutrophil infiltration and atelectasis. In addition,
treatment with CP aggravated IIR-induced lung injury. Pretreatment
with SEV and AP significantly prevented the lung damage induced by
IIR and IIR + CP. The pathological injury score and w/d ratio in
the lungs were in accordance with the pathological changes observed
under the light microscope (Fig. 3B
and C).
SEV and AP decrease the downregulation in
the expression of proSP-C induced by IIR and IIR + CP
The expression levels of proSP-C were significantly
reduced in the IIR group and were reduced further in the IIR + CP
group compared with the sham group (Fig. 4). SEV and AP inhibited the
downregulated expression of proSP-C induced by IIR and IIR + CP.
These results suggested that IIR caused damage to type II alveolar
epithelial cells, that mast cell degranulation exacerbated the
damage, and that SEV and AP protected the type II alveolar
epithelial cells from the injury induced by IIR and mast cell
degranulation.
SEV and AP inhibit the IIR-induced
activation of mast cells
As shown in Fig. 5,
IIR resulted in an increase in the number of mast cells and
expression levels of tryptase in the lung tissues, and the levels
of β-hexosaminidase in the lungs and plasma. CP aggravated these
changes. Pretreatment with SEV and AP effectively alleviated the
changes induced by IIR and IIR + CP.
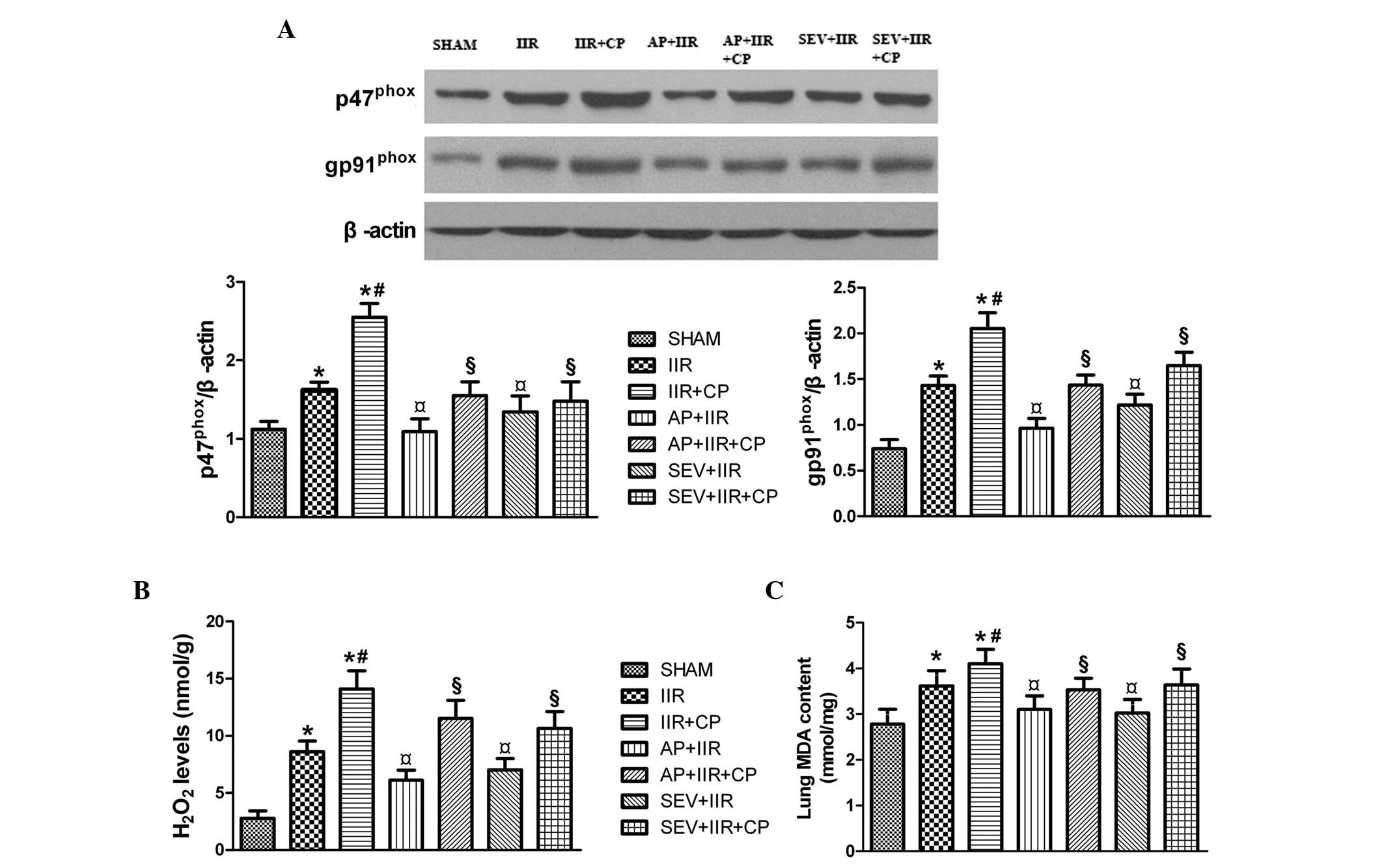
SEV and AP attenuate IIR-induced
oxidative stress
NADPH oxidase is crucial for ROS generation, and
p47phox and gp91phox are the predominant
subunits of NADPH oxidase (35).
As shown in Fig. 6, IIR increased
the expression levels of p47phox and gp91phox
in the lungs, which were further upregulated by CP. Pretreatment
with SEV and AP significantly reversed this upregulation in the
expression levels of p47phox and gp91phox.
The changes observed in the levels of H2O2
and MDA were consistent with the changes observed in the expression
of p47phox and gp91phox. These results
suggested that SEV and AP alleviated the oxidative injury in the
lungs, induced by IIR, by inhibiting the activity of NADPH
oxidase.
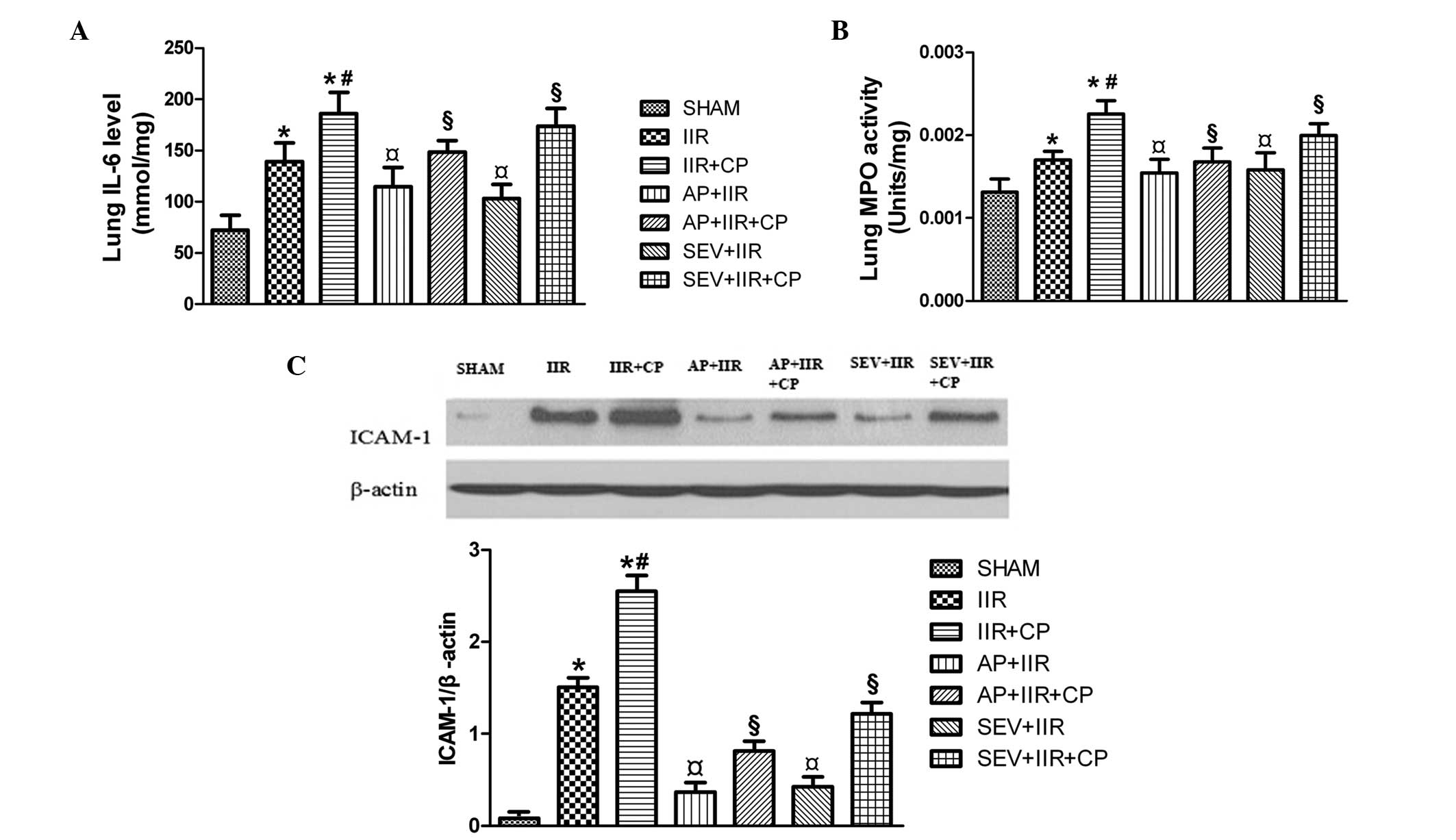
SEV and AP inhibit the IIR-induced
inflammatory response
As shown in Fig. 7,
IIR increased the levels of IL-6, activity of MPO and the protein
expression levels of ICAM-1 levels, and these were increased
further by CP. Pretreatment with SEV and AP effectively reduced the
levels of IL-6, activity of MPO and protein expression of ICAM-1,
which was induced by IIR and IIR + CP. These results suggested that
SEV and AP inhibited IIR-induced lung injury by inhibiting
inflammatory responses.
Discussion
The present study demonstrated that oxidative stress
is important in IIR-induced ALI, evidenced as significant
elevations in the expression levles of p47phox and
gp91phox in the lungs, in addition to increases in the
levels of H2O2 and MDA. Furthermore, mast
cells were found to exacerbate IIR-mediated ALI, revealed by
significant increases in the pathological injury score and w/d
weight ratio of the lungs and reductions in the expression of
proSP-C in the lungs. Notably, pretreatment with the antioxidant,
AP, or with SEV not only attenuated ALI, but also inhibited mast
cell degranulation-mediated exacerbation in the presence of CP. To
the best of our knowledge, the present study was the first to
demonstrate the ability of SEV to limit ALI by inhibiting the
synergistic action between oxidative stress and mast cell
activation. The results offer promising therapeutic benefits
against IIR-mediated ALI.
Several previous studies have reported that
oxidative stress and uncontrolled inflammation contribute to the
process of IIR-mediated ALI (36–38).
In the present study, significantly increased expression levels of
the p47phox and gp91phox NADPH enzymes and
increased levels of MDA and H2O2 were
observed in the IIR group. In addition, pretreatment with the NADPH
oxidase inhibitor, AP, attenuated ALI by significantly reducing the
protein expression levels of p47phox and
gp91phox and the levels of MDA and
H2O2, further suggesting that oxidative
stress is central in the process of ALI.
Several previous studies have demonstrated that mast
cells are important in the process of ALI in different models,
including sepsis, hemorrhagic shock and small IIR injury (39–41).
Mast cells, which contain large quantities of cytokines and
proteases, are widely distributed around the capillaries and lymph
vessels of the connective tissue in the respiratory system. When
activated, the released mediators are able to exacerbate
IIR-induced ALI. Several factors contribute to mast cell
degranulation (26,42,43).
A previous study demonstrated that phenyl N-tertbutylnitrone, a ROS
scavenger, reduced the enhancement of peritoneal mast cell activity
induced by supernatant from colonic biopsies, indicating that ROS
is involved in mast cell activation in vitro (44). However, the precise role of
oxidative stress in mast cell degranulation during the process of
IIR-mediated ALI remains to be fully elucidated. In the present
study, AP, an NADPH oxidase inhibitor, attenuated IIR-induced ALI
and oxidative stress compared with the IIR group. AP also inhibited
this exacerbation in the presence of the mast cell activator, CP.
These observations suggested that mast cell activation, induced by
oxidative stress, is pivotal in IIR-mediated ALI.
SEV is one of the most commonly used volatile
anesthetics and, in addition to its anesthetic effects, several
studies have demonstrated that SEV exhibits antioxidant and
anti-inflammatory properties (20,21).
Preconditioning with SEV has been demonstrated to protect the
heart, kidneys and lungs against ischemia-reperfusion injury in
vitro and in vivo (45–49).
In agreement with previous studies, the results of the present
study indicated that SEV preconditioning attenuated IIR-mediated
ALI by downregulating oxidative stress and the inflammatory
response, as did treatment with AP. These results further confirmed
SEV preconditioning as beneficial against ischemia-reperfusion
injury.
Uncontrolled inflammation also contributes to the
process of IIR-mediated ALI (50,51).
In line with the previous studies, the observations of the present
study also demonstrated that the activity of MPO, and the
expression levels of ICAM-1 and IL-6 were significantly increased
following IIR challenge, and these elevations were further enhanced
in the presence of CP. The findings indicated that oxidative stress
and mast cell activation, in addition to their synergistic action,
contributed to the pulmonary inflammatory response and were
important in the process of IIR-induced ALI. Similarly,
preconditioning with SEV and AP inhibited the exacerbations induced
by IIR combined with CP, therfotr, the results suggested that SEV
protected against IIR-mediated ALI by inhibiting the synergistic
effects of mast cells and oxidative stress (52–55).
In conclusion, SEV was observed to attenuate
IIR-induced lung injury by inhibiting mast cell activation,
minimizing oxidative damage and suppressing their synergistic
effects. These results may have an implication in the clinical
treatment of IIR-mediated ALI.
Acknowledgments
The current study was supported by the 985 Project
(grant no. 82000–3281901); the National Natural Science Foundation
of China (grant no. 30972858); and the Science and Technology
Planning Project of Guangdong Province, China (grant no.
2012B061700071).
References
|
1
|
Douzinas EE, Orfanos SE, Livaditi O, et
al: Hypoxemic resuscitation prevents pulmonary capillary
endothelial dysfunction induced by normoxemic resuscitation from
hemorrhagic shock. Crit Care Med. 37:869–875. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haglund U and Bergqvist D: Intestinal
ischemia-the basics. Langenbecks Arch Surg. 384:233–238. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mallick IH, Yang W, Winslet MC and
Seifalian AM: Ischemia-reperfusion injury of the intestine and
protective strategies against injury. Dig Dis Sci. 49:1359–1377.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kosieradzki M, Lisik W, Rowiński W and
Małkowski P: Progress in abdominal organ transplantation. Med Sci
Monit. 17:RA282–291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Frutos-Vivar F, Ferguson ND and Esteban A:
Epidemiology of acute lung injury and acute respiratory distress
syndrome. Semin Respir Crit Care Med. 27:327–336. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ben DF, Yu XY, Ji GY, et al: TLR4 mediates
lung injury and inflammation in intestinal ischemia-reperfusion. J
Surg Res. 174:326–333. 2012. View Article : Google Scholar
|
|
7
|
Guzel A, Kanter M, Pergel A and Erboga M:
Anti-inflammatory and antioxidant effects of infliximab on acute
lung injury in a rat model of intestinal ischemia/reperfusion. J
Mol Histol. 43:361–369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chabot F, Mitchell JA, Gutteridge JM and
Evans TW: Reactive oxygen species in acute lung injury. Eur Respir
J. 11:745–757. 1998.PubMed/NCBI
|
|
9
|
Tasoulis MK, Livaditi O, Stamatakos M, et
al: High concentrations of reactive oxygen species in the BAL fluid
are correlated with lung injury in rabbits after hemorrhagic shock
and resuscitation. Tohoku J Exp Med. 219:193–199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JH, Jo YH, Kim K, et al: Effect of
N-acetylcysteine (NAC) on acute lung injury and acute kidney injury
in hemorrhagic shock. Resuscitation. 84:121–127. 2013. View Article : Google Scholar
|
|
11
|
Gan X, Su G, Zhao W, Huang P, Luo G and
Hei Z: The mechanism of sevoflurane preconditioning-induced
protections against small intestinal ischemia reperfusion injury is
independent of mast cell in rats. Mediators Inflamm.
2013:3787032013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Campos R, Shimizu MH, Volpini RA, et al:
N-acetylcysteine prevents pulmonary edema and acute kidney injury
in rats with sepsis submitted to mechanical ventilation. Am J
Physiol Lung Cell Mol Physiol. 302:L640–L650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang P, Liu D, Gan X, et al: Mast cells
activation contribute to small intestinal ischemia reperfusion
induced acute lung injury in rats. Injury. 43:1250–1256. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van den Elsen LW, Nusse Y, Balvers M, et
al: n-3 Long-chain PUFA reduce allergy-related mediator release by
human mast cells in vitro via inhibition of reactive oxygen
species. Br J Nutr. 109:1821–1831. 2013. View Article : Google Scholar
|
|
15
|
Sawaguchi M, Tanaka S, Nakatani Y, et al:
Role of mast cells and basophils in IgE responses and in allergic
airway hyperrespon-siveness. J Immunol. 188:1809–1818. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xia YC, Harris T, Stewart AG and Mackay
GA: Secreted factors from human mast cells trigger inflammatory
cytokine production by human airway smooth muscle cells. Int Arch
Allergy Immunol. 160:75–85. 2013. View Article : Google Scholar
|
|
17
|
Chandler JR, Myers D, Mehta D, et al:
Emergence delirium in children: a randomized trial to compare total
intravenous anesthesia with propofol and remifentanil to
inhalational sevoflurane anesthesia. Paediatr Anaesth. 23:309–315.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bi SS, Deng CH, Zhou TY, et al:
Remifentanil-sevoflurane interaction models of circulatory response
to laryngoscopy and circulatory depression. Br J Anaesth.
110:729–740. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Casanova J, Garutti I, Simon C, et al: The
effects of anesthetic preconditioning with sevoflurane in an
experimental lung auto-transplant model in pigs. Anesth Analg.
113:742–748. 2011.PubMed/NCBI
|
|
20
|
Hu X, Zhang Y, Li W, Liu J and Li Y:
Preconditioning with sevoflurane ameliorates spatial learning and
memory deficit after focal cerebral ischemia-reperfusion in rats.
Int J Dev Neurosci. 31:328–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bedirli N, Demirtas CY, Akkaya T, et al:
Volatile anesthetic preconditioning attenuated sepsis induced lung
inflammation. J Surg Res. 178:e17–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiong XQ, Lin LN, Wang LR and Jin LD:
Sevoflurane attenuates pulmonary inflammation and
ventilator-induced lung injury by upregulation of HO-1 mRNA
expression in mice. Int J Nanomedicine. 6:1075–1081. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song SY, Zhou B, Yang SM, Liu GZ, Tian JM
and Yue XQ: Preventive effects of sevoflurane treatment on lung
inflammation in rats. Asian Pac J Trop Med. 6:53–56. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ye R, Yang Q, Kong X, et al: Sevoflurane
preconditioning improves mitochondrial function and long-term
neurologic sequelae after transient cerebral ischemia: role of
mitochondrial permeability transition. Crit Care Med. 40:2685–2693.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paterniti I, Galuppo M, Mazzon E, et al:
Protective effects of apocynin, an inhibitor of NADPH oxidase
activity, in splanchnic artery occlusion and reperfusion. J Leukoc
Biol. 88:993–1003. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gan X, Liu D, Huang P, Gao W, Chen X and
Hei Z: Mast-cell-releasing tryptase triggers acute lung injury
induced by small intestinal ischemia-reperfusion by activating
PAR-2 in rats. Inflammation. 35:1144–1153. 2012. View Article : Google Scholar
|
|
27
|
Hofbauer B, Saluja AK, Bhatia M, et al:
Effect of recombinant platelet-activating factor acetylhydrolase on
two models of experimental acute pancreatitis. Gastroenterology.
115:1238–1247. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mirzahosseini A, Dalmadi B and Csutora P:
Histamine receptor H4 regulates mast cell degranulation and IgE
induced FcεRI upregulation in murine bone marrow-derived mast
cells. Cell Immunol. 283:38–44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Neveu WA, Allard JL, Raymond DM, et al:
Elevation of IL-6 in the allergic asthmatic airway is independent
of inflammation but associates with loss of central airway
function. Respir Res. 11:282010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mullane KM, Kraemer R and Smith B:
Myeloperoxidase activity as a quantitative assessment of neutrophil
infiltration into ischemic myocardium. J Pharmacol Methods.
14:157–167. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grek CL, Newton DA, Spyropoulos DD and
Baatz JE: Hypoxia up-regulates expression of hemoglobin in alveolar
epithelial cells. Am J Respir Cell Mol Biol. 44:439–447. 2011.
View Article : Google Scholar :
|
|
32
|
Frieri M, Patel R and Celestin J: Mast
cell activation syndrome: a review. Curr Allergy Asthma Rep.
13:27–32. 2013. View Article : Google Scholar
|
|
33
|
Kleniewska P, Piechota A, Skibska B and
Gorąca A: The NADPH oxidase family and its inhibitors. Arch Immunol
Ther Exp (Warsz). 60:277–294. 2012. View Article : Google Scholar
|
|
34
|
Shen A, Yang J, Gu Y, et al:
Lipopolysaccharide-evoked activation of p38 and JNK leads to an
increase in ICAM-1 expression in Schwann cells of sciatic nerves.
FEBS J. 275:4343–4353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Elnakish MT, Hassanain HH, Janssen PM,
Angelos MG and Khan M: Emerging role of oxidative stress in
metabolic syndrome and cardiovascular diseases: important role of
Rac/NADPH oxidase. J Pathol. 231:290–300. 2013.PubMed/NCBI
|
|
36
|
Wang J, Qiao L, Li S and Yang G:
Protective effect of ginsenoside Rb1 against lung injury induced by
intestinal ischemia-reperfusion in rats. Molecules. 18:1214–1226.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mao YF, Zheng XF, Cai JM, et al:
Hydrogen-rich saline reduces lung injury induced by intestinal
ischemia/reperfusion in rats. Biochem Biophys Res Commun.
381:602–605. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rossman JE, Caty MG, Zheng S, et al:
Mucosal protection from intestinal ischemia-reperfusion reduces
oxidant injury to the lung. J Surg Res. 73:41–46. 1997. View Article : Google Scholar
|
|
39
|
Ramos L, Peña G, Cai B, Deitch EA and
Ulloa L: Mast cell stabilization improves survival by preventing
apoptosis in sepsis. J Immunol. 185:709–716. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fishman JE, Sheth SU, Levy G, et al:
Intraluminal nonbacterial intestinal components control gut and
lung injury after trauma hemorrhagic shock. Ann Surg.
260:1112–1120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao W, Gan X, Su G, et al: The
interaction between oxidative stress and mast cell activation plays
a role in acute lung injuries induced by intestinal
ischemia-reperfusion. J Surg Res. 187:542–552. 2014. View Article : Google Scholar
|
|
42
|
Wingard CJ, Walters DM, Cathey BL, et al:
Mast cells contribute to altered vascular reactivity and
ischemia-reper-fusion injury following cerium oxide nanoparticle
instillation. Nanotoxicology. 5:531–545. 2011. View Article : Google Scholar :
|
|
43
|
Mukundan C, Gurish MF, Austen KF, Hechtman
HB and Friend DS: Mast cell mediation of muscle and pulmonary
injury following hindlimb ischemia-reperfusion. J Histochem
Cytochem. 49:1055–1056. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Han W, Lu X, Jia X, Zhou T and Guo C:
Soluble mediators released from PI-IBS patients’ colon induced
alteration of mast cell: involvement of reactive oxygen species.
Dig Dis Sci. 57:311–319. 2012. View Article : Google Scholar
|
|
45
|
Zhao J, Wang F, Zhang Y, et al:
Sevoflurane preconditioning attenuates myocardial
ischemia/reperfusion injury via caveolin-3-dependent
cyclooxygenase-2 inhibition. Circulation. 128(11 Suppl 1): 121–129.
2013. View Article : Google Scholar
|
|
46
|
Zhou SP, Liao WT, Yang LK and Sun L:
Effects of sevoflurane pretreatment on renal Src and FAK expression
in diabetic rats after renal ischemia/reperfusion injury. Mol Cell
Biochem. 384:203–211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu R, Ishibe Y and Ueda M:
Isoflurane-sevoflurane admin-stration before ischemia attenuates
ischemia-reperfusion-induced injury in isolated rat lungs.
Anesthesiology. 92:833–840. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kong HY, Zhu SM, Wang LQ, He Y, Xie HY and
Zheng SS: Sevoflurane protects against acute kidney injury in a
small-size liver transplantation model. Am J Nephrol. 32:347–355.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang Q, Dong H, Deng J, et al: Sevoflurane
preconditioning induces neuroprotection through reactive oxygen
species-mediated up-regulation of antioxidant enzymes in rats.
Anesth Analg. 112:931–937. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Breithaupt-Faloppa AC, Fantozzi ET, de
Assis-Ramos MM, et al: Protective effect of estradiol on acute lung
inflammation induced by an intestinal ischemic insult is dependent
on nitric oxide. Shock. 40:203–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Guido BC, Zanatelli M, Tavares-de-Lima W,
Oliani SM and Damazo AS: Annexin-A1 peptide down-regulates the
leukocyte recruitment and up-regulates interleukin-10 release into
lung after intestinal ischemia-reperfusion in mice. J Inflamm
(Lond). 10:102013. View Article : Google Scholar
|
|
52
|
Neri M, Fineschi V, Di Paolo M, et al:
Cardiac Oxidative Stress and Inflammatory Cytokines Response After
Myocardial Infarction. Curr Vasc Pharmacol. 2013.
|
|
53
|
Thiyagarajan R, Subramanian SK, Sampath N,
et al: Association between cardiac autonomic function, oxidative
stress and inflammatory response in impaired fasting glucose
subjects: cross-sectional study. PLoS One. 7:e418892012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Könczöl M, Weiß A, Gminski R, Merfort I
and Mersch-Sundermann V: Oxidative stress and inflammatory response
to printer toner particles in human epithelial A549 lung cells.
Toxicol Lett. 216:171–180. 2013. View Article : Google Scholar
|
|
55
|
Lloberas N, Torras J, Herrero-Fresneda I,
et al: Postischemic renal oxidative stress induces inflammatory
response through PAF and oxidized phospholipids. Prevention by
antioxidant treatment FASEB J. 16:908–910. 2002.
|