Introduction
Stroke, with ischemia of the brain and hemorrhagic
injury as the predominant clinical manifestations, is the main
cause of mortality worldwide (1–3). The
annual number of stroke cases is increasing as the world population
is ageing. According to the World Health Organization, 15,000,000
individuals suffer a stroke worldwide each year, which undoubtedly
creates an increased financial and social burden to the surviving
population (4). There are
currently few preventative therapies available for stroke and the
only Food and Drug Administration approved ‘pharmacological’
intervention to reduce brain damage is tissue plasminogen activator
(5). However, only ~3–5% of
patients benefit from this, due to its narrow time window.
The blood-brain barrier (BBB) is a highly selective
permeability barrier, which separates the circulating blood from
the brain extracellular fluid in the central nervous system
(6). The BBB allows the passage of
water, certain gases and lipid soluble molecules by passive
diffusion, and the selective transport of molecules, including
glucose and amino acids, which are crucial to neurological function
(6). By contrast, the BBB may
prevent the entry of lipophilic, potential neurotoxins using an
active transport mechanism, which is important for protecting the
brain from fluctuations in the plasma composition and from
circulating agents (7).
Changes in the permeability of the BBB, which result
in the increase of vascular-derived substances into the brain,
leading to pathophysiologic processes and affecting the passage of
drugs and various metabolites into the brain parenchyma, have been
investigated (8,9).
Gualou Guizhi granules (GLGZG; approval no.
S20130001) are a standard prescribed drug at Fujian University of
Traditional Chinese Medicine (TCM) Affiliated Second People’s
Hospital (Fuzhou, China). It is a well-known traditional Chinese
formula, first recorded in ‘Essentials from the Golden Cabinet’
(10). GLGZG is comprised of six
herbs, including Trichosanthes kirilowii Maxim., Paeonia
lactiflora Pall., Cinnamomum cassia Presl.,
Glycyrrhiza uralensis Fisch., Zingiber officinale
Rosc. and Ziziphus jujuba Mill., according to the Yin-Yang
and Wu Hsing ‘Five Elements’ theory of TCM, in a weight ratio of
10:3:3:3:2:3 (10). It has been
used clinically to treat muscular spasticity following stroke,
epilepsy and spinal cord injury in China (11–13).
Our previous clinical study demonstrated beneficial effects of
GLGZG in stroke patients (unpublished data) and our previous
studies evaluated the effect of GLGZG on lipopolysaccharide
(LPS)-induced BV-2 murine microglial cells and demonstrated that
GLGZG had an effect on the Toll-like receptor (TLR)4/nuclear factor
(NF)-κB pathway and mitogen-activated protein kinase (MAPK)
signaling pathway (14,15). The neuroprotective effects of GLGZG
on glutamate-induced apoptosis in cultured BV-2 cells (16) and the neuroprotective effects of
GLGZG in vivo were investigated, which demonstrated that
GLGZG exerts its neuroprotective effects via the modulation of
excitatory amino acid levels and the expression of
N-methyl-D-aspartate (NMDA) and
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)
receptors (17,18).
Although previous studies have demonstrated the
neuroprotective effect of GLGZG in vivo and in vitro,
the BBB permeability of GLGZG under normal or ischemia/reperfusion
(I/R) injury remains to be elucidated. The permeability of the BBB
is an important factor for compounds used in the treatment of
neurodegenerative disorders. The present study investigated the BBB
permeability of GLGZG in normal and I/R injury models in
vivo and also further examined the neuroprotective effects of
GLGZG in vivo.
Materials and methods
Materials
GLGZG was provided by Fujian University of TCM
Affiliated Second People’s Hospital (Fuzhou, China).
High-performance liquid chromatography (HPLC) grade methanol and
acetonitrile were purchased from Merck Co. (Darmstadt, Germany).
Standard substances, including citrulline, gallic acid, albiflorin,
peoniflorin, liquiritin apioside, liquiritin, isoliquiritin
apioside, isoliquiritin, liquiritigenin, isoliquiritigenin and
glycyrrhizinic acid were purchased from the National Institute for
the Control of Pharmaceutical and Biological Products (Beijing,
China). All other chemical reagents used were of analysis
grade.
A total of 98 male Sprague-Dawley rats (4 weeks old;
weighing 280±20 g) were obtained from the Laboratory Animal Center
of Fujian University of TCM. The animals were housed under
controlled temperature conditions (21–23°C) with a relative
humidity of 55±5%, a 12-h light/dark cycle and free access to
standard rat diet and tap water. The study was approved by the
ethics committee of the Animal Experimental Center, Fujian
University of TCM (Fuzhou, China).
Model of focal cerebral I/R
An intraluminal suture was used for the induction of
focal cerebral ischemia, as described in Longa et al
(17–19), with modification. Briefly, the rats
were anesthetized using 10% chloral hydrate solution (0.3 ml/100 g,
intraperitoneally administered; Sinopharm Chemical Reagent Co.,
Ltd., Shanghai, China) and the left common carotid artery (CCA),
external carotid artery (ECA) and internal carotid artery (ICA)
were exposed by a short incision, and separated from the adjacent
nerves and tissue. A 3-0 silicon rubber-coated nylon monofilament
(Guangzhou Jialing Biotechnology Co., Ltd., Guangzhou, China) was
carefully inserted into the ICA and was advanced to occlude the
origin of the left middle cerebral artery (MCAO) until light
resistance was detected at 18–20 mm from the CCA bifurcation.
Following ischemia for 2 h, the filament was withdrawn allowing
reperfusion. Neurological defects were scored using a five-point
scale: 0, no neurological symptoms; 1, unable to completely extend
the front jaw on the other side; 2, rotating while crawling and
falling to the contralateral side; 3, unable to walk without
assistance; and 4, unconsciousness (16). Rats with a score of 1–3 points were
considered successful models of I/R and were used in the subsequent
experiments.
Analysis of the compounds in the plasma
and brain by HPLC-quadrupole-time of flight-mass spectrometry
(Q-TOF-MS)
A total of 15 rats were separated into a normal
group, a normal group with GLGZG treatment and an I/R model group
with GLGZG treatment. The rats in the normal group were subjected
to the surgical procedure with exposure of the ICA and ECA,
however, no MCAO was induced, and the rats received normal saline
treatment. The rats in the normal group with GLGZG treatment
received GLGZG (7.2 g/kg/day intragastrically), however, were not
exposed to MCAO surgery. The model group with GLGZG treatment
received GLGZG (7.2 g/kg/day intragastrically) and underwent MCAO
surgery. Following treatment with GLGZG for 15, 30 or 60 min (5
rats at each time point), the rats were deeply anesthetized with
10% chloral hydrate solution (0.3 ml/100 g, intraperitoneally
administered) and decapitated, then brains were resected
immediately, prior to plasma and brain tissue samples being
obtained. The brain tissue was homogenized in deionized water at a
ratio of 1:1 (w/v) using a glass pestle, and the homogenate was
centrifuged at 628 × g for 15 min at 4°C to obtain the supernatant.
Methanol (4-fold) was then added to the brain tissue homogenate or
plasma samples. The samples were then vortex mixed for 2 min,
followed by ultrasound-associated extraction using a KQ-500DE
single-frequency ultrasonic cleaner (Kunshan Ultrasonic Instruments
Co., Ltd., Kunshan, China) for 10 min. Following centrifugation at
628 × g for 10 min at 4°C, 2 μl supernatant was injected
into the HPLC system.
Chromatographic analysis was performed on a Shimadzu
HPLC system (Shimadzu Corporation, Kyoto, Japan) coupled with a
Bruker micrOTOF-Q II mass spectrometer (Bruker Daltonik, Bremen,
Germany). The HPLC system consisted of an LC-20A pump,
DGU-20A5 degasser, SIL-20A auto-injector and CTO-20A
column thermostat. The mobile phases consisted of 0.1% formic acid
(Aladdin Industrial Corporation, Shanghai, China) in (A) water and
(B) acetonitrile high performance liquid chromatography grade
(Merck Millipore, Darmstadt, Germany). The gradient elution program
was as follows: 15% B for 0–18 min; 15–48% B for 18–20 min; 48-15%
B for 20–25 min. Another chromatographic separation was performed
at a flow rate of 0.3 ml/min using a sample volume of 2 μl
and a Shimadzu Inertsil SP C18 5 μm (250×4.6 mm) column
(Shimadzu, Kyoto, Japan).
The microTOF-Q II mass spectrometer was equipped
with an electrospray ionization source and run in negative mode.
The acquisition parameters of Q-TOF-MS were as follows: Capillary,
−4.5 kV (negative mode); nebulizer pressure, 2.0 bar; dry gas
(N2) flow rate, 4.0 l/min; dry gas temperature, 180°C.
Funnel 1 and 2 were 200.0 Vpp; hexapole Rf, 100.0 Vpp; quadrupole
ion energy, 2.5 eV; collision Rf, 150.0 Vpp. The ion transfer time
was 80 μsec and the prepulse storage time was 5 μsec.
Argon (Fuzhou Huaxin Industrial Gases Co., Ltd., Fuzhou, China) was
applied as the collision gas and the collision energy were set
between 20 and 50 eV to obtain the fragment ions data. To obtain
highly accurate mass values, external calibration of the Q-TOF/MS
was performed daily prior to sample injection, and the enhanced
quadratic calibration mode was used for the calibration curve.
Neuroprotection of GLGZG in I/R
injury
Experimental grouping and
treatment
A total of 48 rats were divided into the three
experimental groups, described above and treatment was performed,
as described below. The experimental animals were grouped as:
Sham-operated group (n=16), in which the rats were subjected to the
surgical procedure to expose the ICA and ECA, however, no MCAO was
induced and the rats received normal saline; an MCAO model group
(n=16), in which the rats received normal saline and underwent MCAO
surgery; and a GLGZG group (n=16), in which the rats received GLGZG
treatment (7.2 g/kg) and underwent MCAO surgery. The rats were
treated daily for 1 week.
Scoring neurological defects
The neurobehavioral defects of the rats were
quantified using the five-point scale, as described above (16), and this evaluation was performed in
a blinded-manner by Miss. Yuqin Zhang.
2,3,5-triphenyltetrazolium chloride
(TTC) staining
A total of eight rats from each group were
sacrificed using 10% chloral hydrate and perfused transcardiacally
with 0.9% NaCl prior to decapitation and rapid removal of the
brain. The brains were stored at 20°C for 5 min and then dissected
into six coronal slices (2 mm) continuously from front to back. The
brain tissues were immersed in 2% TTC staining solution (cat no.
T8877; Sigma-Aldrich, St. Louis, MO, USA) in phosphate-buffered
saline (pH 7.4; Beyotime Institute of Biotechnology, Haimen,
China), and incubated at 37°C for 1 h and turned over six times. A
high-resolution digital camera (IXUS130; Canon, Inc., Tokyo, Japan)
was used to capture images and a Motic Med 6.0 Digital Medical
Image Analysis system (Motic Instruments Inc., Richmond, Canada)
was used to calculate the infarct volume as a percentage of the
viable cerebral tissue of the entire brain (20).
Cerebral histopathology
A total of eight rats from each group were
sacrificed using 10% chloral hydrate and perfused transcardiacally
with 4% paraformaldehyde solution and 0.9% NaCl (Beyotime Institute
of Biotechnology), following which the brains were rapidly removed.
The brains were paraffin-embedded (Beyotime Institute of
Biotechnology), prior to being dissected into a series of adjacent
5 μm thick sections and stained with hematoxylin and eosin
(Beyotime Institute of Biotechnology). Histopathological changes
were observed under a DM4000B light microscope (Leica Microsystems
GmbH, Wetzlar, China).
Statistical analysis
The data are expressed as the mean ± standard
deviation. Analysis of variance was performed to determine
significant differences between groups using SPSS 16.0 software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Analysis of the compounds in the plasma
and brain using HPLC-Q-TOF-MS
The standard components of GLGZG, including
citrulline, gallic acid, albiflorin, peoniflorin, liquiritin
apioside, liquiritin, isoliquiritin apioside, isoliquiritin,
liquiritigenin, isoliquiritigenin and glycyrrhizinic acid were
detected by HPLC-Q-TOF-MS (Fig.
1). The result demonstrated no peaks in the chromatograms of
the blank plasma and blank brain tissue homogenate. Citrulline,
albiflorin, peoniflorin, liquiritin apioside, liquiritin,
isoliquiritin apioside, isoliquiritin, liquiritigenin,
isoliquiritigenin and glycyrrhizinic acid were present in the
plasma from the rat model of MCAO and the normal rats. Only
citrulline, gallic acid, albiflorin, peoniflorin, liquiritin
apioside, liquiritin, liquiritigenin, isoliquiritigenin and
glycyrrhizinic acid were detected in the brain tissue homogenates
from the rat models of MCAO by HPLC-Q-TOF-MS. However, gallic acid,
isoliquiritigenin and glycyrrhizinic acid were not detected in the
brain tissue homogenate from the normal rat group compared with the
rat model of MCAO. Representative chromatograms of plasma and brain
tissue homogenate are shown in Figs.
2 and 3, respectively.
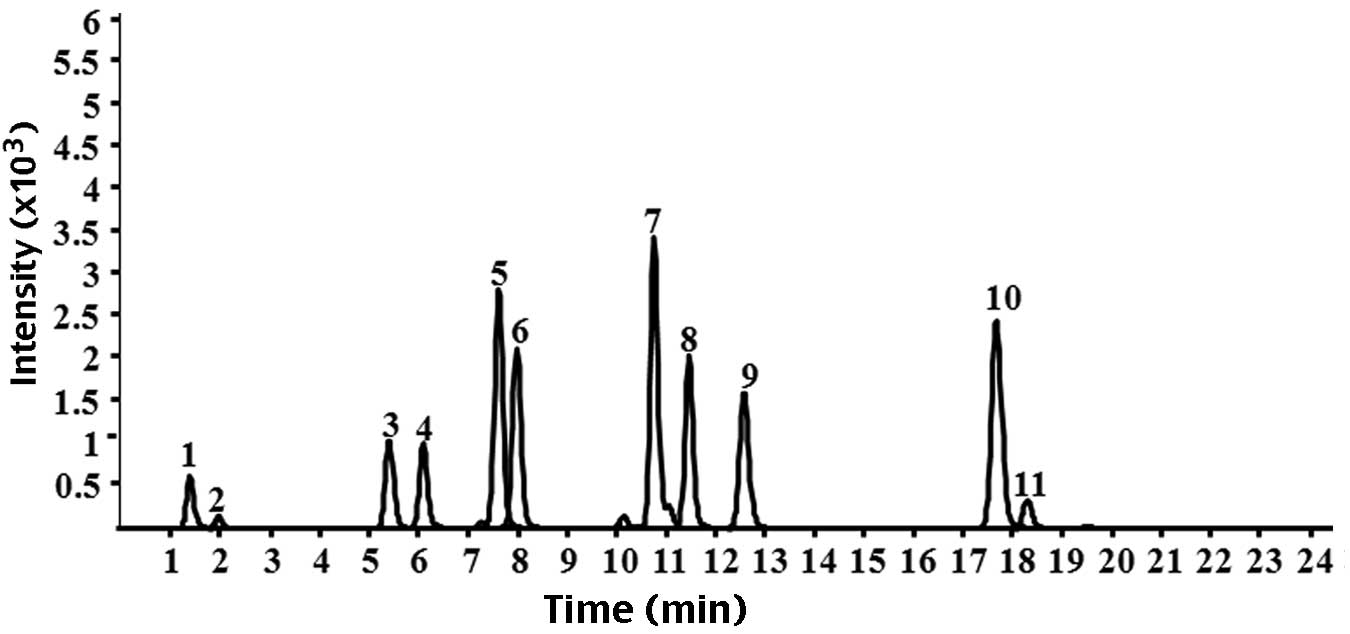 | Figure 1Chromatograms of the nine standard
reference compounds in high-performance liquid
chromatography-quadrupole-time of flight-mass spectrometry. 1,
citrulline; 2, gallic acid; 3, albiflorin; 4, peoniflorin; 5,
liquiritin apioside; 6, liquiritin; 7, isoliquiritin apioside; 8,
isoliquiritin; 9, liquiritigenin; 10, isoliquiritigenin; 11,
glycyrrhizinic acid. |
 | Figure 2Chromatograms of plasma following
treatment with Gualou Guizhi granule. (A) Blank plasma, (B) plasma
from normal rats and (C) plasma from rat models of middle cerebral
artery occlusion. 1, citrulline: 3, albiflorin; 4, peoniflorin; 5,
liquiritin apioside; 6, liquiritin; 7, isoliquiritin apioside; 8,
isoliquiritin; 9, liquiritigenin; 10, isoliquiritigenin; 11,
glycyrrhizinic acid. |
 | Figure 3Chromatograms of brain tissue
homogenate following treatment with Gualou Guizhi granule. (A)
Brain tissue homogenate, (B) brain tissue homogenate from normal
rats and (C) brain tissue homogenate from rat models of middle
cerebral artery occlusion. 1, citrulline; 2, gallic acid; 3,
albiflorin; 4, peoniflorin; 5, liquiritin apioside; 6, liquiritin;
9, liquiritigenin; 10, isoliquiritigenin; 11, glycyrrhizinic
acid. |
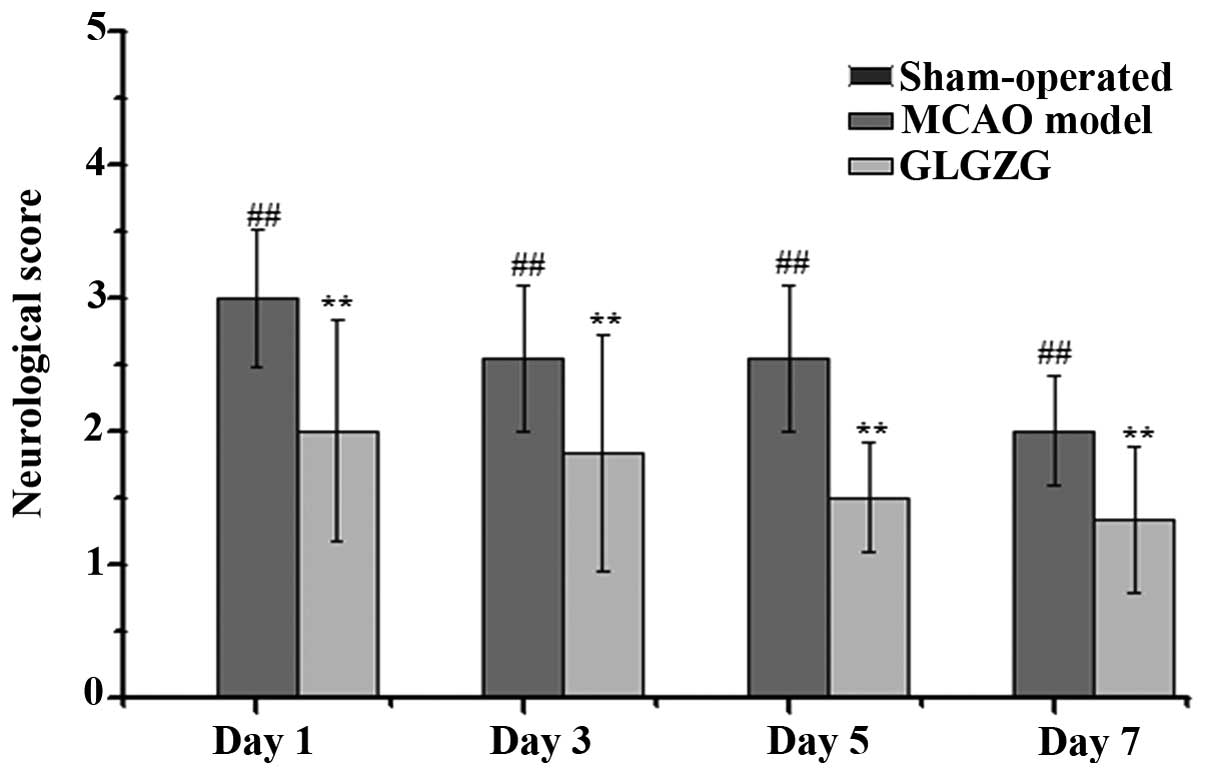
Treatment with GLGZG improves the
neurological deficits and attenuates cerebral infarct volume in
rats
The effect of GLGZG on neurological deficits was
evaluated by measuring the neurological performance using a
five-point scale, as described above. As shown in Fig. 4, the rats in the sham-operated
group (score: 0) exhibited no neurological deficits, while the rats
in the MCAO model group exhibited neurological deficit (score:
1–3), including circling towards the contralateral side with
reduced mobility, inability to completely extend the front jaw on
the other side, rotating while crawling and falling to the
contralateral side. The rats in the GLGZG treated group exhibited
significantly improved neurological symptoms between days 1 and 7,
particularly those in the high-dose group.
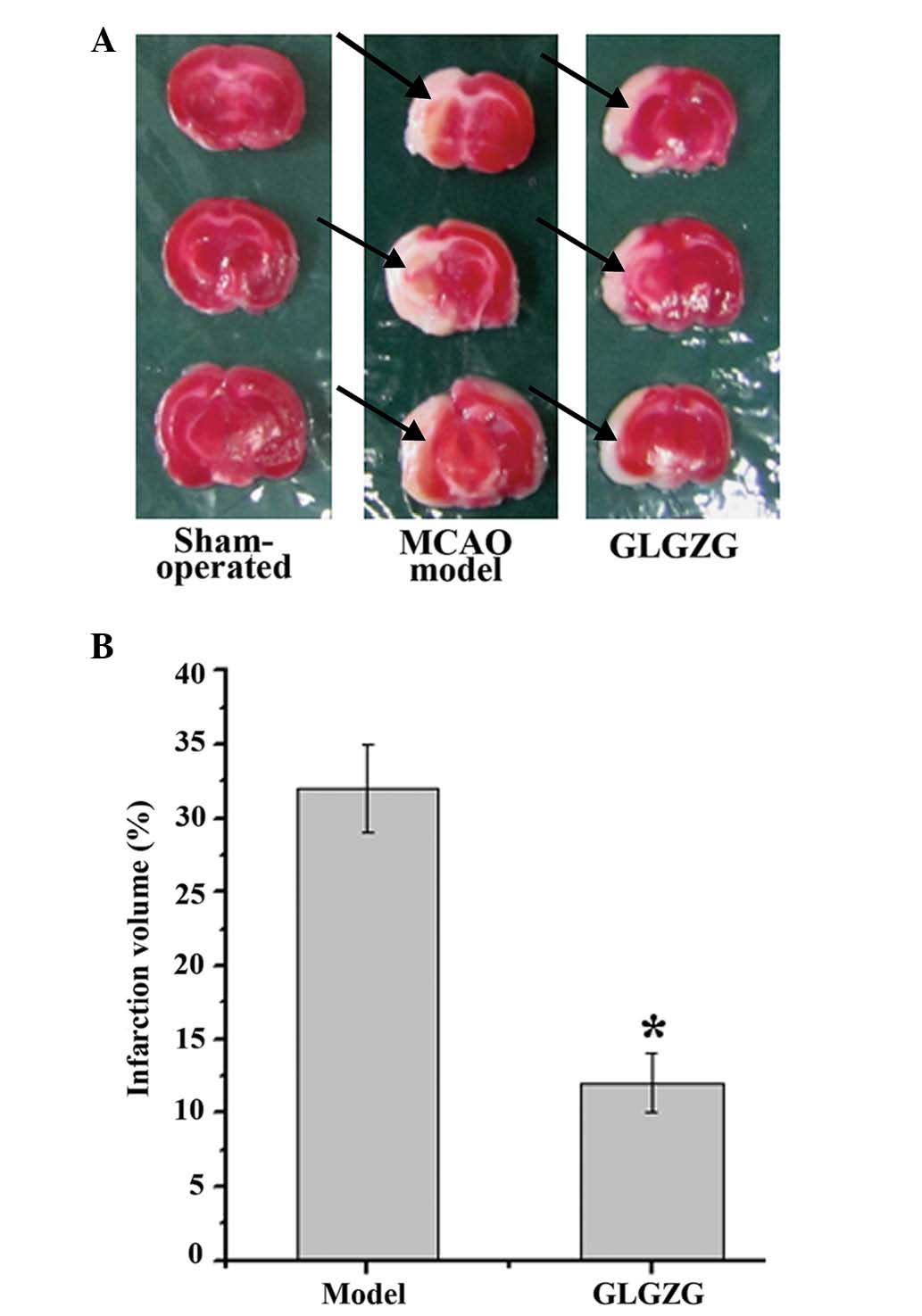
The infarct volumes of the rats were also determined
to evaluate the impact of GLGZG treatment for 7 days following
MCAO. As shown in Fig. 5A and B,
the mean infraction volume in the MCAO model group was
significantly higher compared with the sham-operated group.
Treatment with GLGZG reduced the infarct volume to 12±1.2% compared
with that of the MCAO model group.
Treatment with GLGZG improves cerebral
histopathology
As shown in Fig. 6,
in the sham-operated group, the brain tissue remained intact,
neurons maintained eumorphism and uniform distribution, cytoplasms
were pale pink and abundant and no inflammatory cell infiltrate was
observed. By contrast, the brain tissues had a larger infarct area,
neurons were disordered and reduced, with increased volume and
staining in the cytoplasms was shallower with vacuolization in the
MCAO model group. However, following treatment with GLGZG, the
infarct area was markedly reduced, the extent of damage was
decreased significantly and cytoplasmic hypervacuolization was
reduced.
Discussion
GLGZG is a well-known traditional Chinese formula,
which was first recorded in ‘Essentials from the Golden Cabinet’ in
~210 AD. It is now developed into granules as an easy-to-use form
(10). In order to investigate the
efficacy and mechanism underlying GLGZG, our previous studies
evaluated the effect of GLGZG on LPS-induced BV-2 murine microglial
cells and demonstrated that GLGZG has an inhibitory effect on the
TLR4/NF-κB (14) and the MAPK
signaling pathways (15). The
neuroprotective effects of GLGZG on glutamate-induced apoptosis in
cultured BV-2 cells was also investigated (16). In addition, to confirm the results
observed in vitro, a reversible model of focal ischemia
(MCAO model) was used to induce a model of focal brain ischemia
in vivo. It was revealed that GLGZG exerts neuroprotective
effects via the modulation of excitatory amino acid levels and the
expression levels of the NMDA and AMPA receptors (17,18).
Although possible mechanisms have been identified, it is known that
the BBB, a diffusion barrier, is responsible for strictly
controlling the exchanges between the blood and brain compartments
(6), whether the BBB changes
following cerebral I/R remains to be elucidated. Whether the BBB
alters following cerebral I/R injury, and which compounds of GLGZG
penetrate the BBB, remain to be fully elucidated.
During I/R, the brain environment may be altered.
Loss of blood supply to the brain results in a cascade of events
throughout the infarcted region, including depletion of adenosine
triphosphate, excitotoxic glutamate efflux, a neuronal component,
ionic imbalance, including increased intracellular calcium, loss of
metabolic function with increased acidosis, oxidative stress and
activation of the inflammatory processes (8). Ultimately, this causes the
destruction and/or dysfunction of brain cells, which leads to
clinically definable neurological deficits (6).
The present study confirmed the neuroprotective
effects of GLGZG in I/R injury, including decreased neurological
defects and infarct volume, and ameliorated cerebral
histopathology. Together with previous findings, these results
suggested that GLGZG is a promising alternative therapeutic
approach.
Under normal physiological conditions, the BBB
prevents the paracellular diffusion of hydrophilic solutes and the
efflux of hydrophobic molecules and drugs from the brain to the
blood by active transport (6).
However, the BBB is disrupted following ischemic stroke and the
subsequent onset of reperfusion. This affects the passage of drugs
and various metabolites into the brain (21). Therefore, penetration of the BBB by
therapeutic agents is a prerequisite for treatment of central
nervous diseases. The present study aimed to investigate the BBB
permeability of GLGZG using HPLC-Q-TOF-MS analysis to detect
compounds in the brain tissues of normal rats and rat models of I/R
injury in vivo.
The present study synchronously measured the
compounds of GLGZG in the plasma and brain tissue from normal and
model rats. Citrulline, albiflorin, peoniflorin, liquiritin
apioside, liquiritin, isoliquiritin apioside, isoliquiritin,
liquiritigenin, isoliquiritigenin and glycyrrhizinic acid were
detected in the blood of normal and model rats. Citrulline, gallic
acid, albiflorin, peoniflorin, liquiritin apioside, liquiritin,
isoliquiritin apioside, isoliquiritin, liquiritigenin,
isoliquiritigenin and glycyrrhizinic acid were detected in the
brain tissue from model rats, however, only citrulline, albiflorin,
peoniflorin, liquiritin apioside, liquiritin and liquiritigenin
were detected in the brain tissue from normal rats. Compounds with
high lipid solubility and low relative molecular mass penetrate the
BBB more easily than others (22),
and it was demonstrated that the detected compounds in the brain
tissue from normal rats conformed to these characteristics.
However, this was not observed in the brain tissues from the model
rats. Glycyrrhizinic acid, with a relative molecular mass of
822.92, was detected in brain tissue from the model rats, but not
the normal rats and gallic acid, isoliquiritigenin and
glycyrrhizinic acid were increased in the brain tissues from the
model rats compared with the normal rats, suggesting that these
compounds were able to penetrate the BBB rapidly and distribute
into the brain tissue as a result of BBB damage.
No previous investigations, to the best of our
knowledge, have demonstrated the penetration of glycyrrhizinic acid
into the brain. A previous study demonstrated that diammonium
glycyrrhizinate ameliorates inflammation in focal cerebral I/R
injury (21). Isoliquiritigenin, a
licorice chalconoid, is known to have vasorelaxant, antioxidant,
antiplatelet, anti-tumor, anti-allergenic, antiviral and estrogenic
properties, and Zhan and Yang demonstrated that isoliquiritigenin
exhibited protective effects in transient MCAO-induced focal
cerebral ischemia in rats (23,24).
Paeoniflorin, a monoterpene gluco-side, suggested to exhibit
several pharmacological effects attenuates ischemia-induced
pathological and behavioral changes (25–27).
Glycyrrhizin has been associated with numerous pharmacological
effects and has been observed to have a protective effect on focal
cerebral I/R-induced inflammation, oxidative stress and apoptosis
in rats (28,29). Additionally, the Institute of
Cancer Research reported that liquiritin exerts a neuroprotective
effect against focal cerebral I/R in male mice (30).
GLGZG contains several herbs and is chemically
complex, with hundreds of components. As mentioned above, certain
bioactive chemicals have been suggested to penetrate the BBB, enter
the brain and collectively exert therapeutic and modulatory
effects, providing a beneficial property with possible clinical use
in the treatment of ischemic stroke.
In conclusion, the present study demonstrated that
the BBB permeability of GLGZG increased significantly following I/R
injury in vivo, with citrulline, gallic acid, albiflorin,
peoniflorin, liquiritin apioside, liquiritin, liquiritigenin,
isoliquiritigenin and glycyrrhizinic acid being detected in the
brain following MCAO. In addition, GLGZG exhibited a protective
effect in ischemic injury in rats.
Acknowledgments
This study was performed at the State Ley Laboratory
of Chinese Pharmacies of Fujian Provincial Department of Science
and Technology, Collaborative Innovation Center for Rehabilitation
Technology and TCM Rehabilitation Research Center of the State
Administration of Traditional Chinese Medicine. This study was
funded by grants from the Important Subject of Fujian Province
Science and Technology Hall of China (no. 2012Y0041) and the Fujian
province Education Hall of China (no. JA12176).
References
|
1
|
Lloyd-Jones D, Adams RJ, Brown TM, et al:
Executive summary: heart disease and stroke statistics - 2010
update: a report from the American Heart Association. Circulation.
121:948–954. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu M, Wu BO, Wang WZ, Lee LM, Zhang SH
and Kong LZ: Stroke in China: epidemiology, prevention, and
management strategies. Lancet Neurolo. 6:456–464. 2007. View Article : Google Scholar
|
|
3
|
Centers for Disease Control and Prevention
(CDC): Prevalence of disabilities and associated health conditions
among adults - United States, 1999. MMWR Morb Mortals Wkly Rep.
50:120–125. 2001.
|
|
4
|
The World Health Report 2002. World Health
Organization; Geneva, Switzerland: 2002
|
|
5
|
Del Zoppo GJ, Saver JL, Jauch EC and Adams
HP Jr; American Heart Association Stroke Council: Expansion of the
time window for treatment of acute ischemic stroke with intravenous
tissue plasminogen activator: a science advisory from the American
Heart Association/American Stroke Association. Stroke.
40:2945–2948. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ballabh P, Braun A and Nedergaard M: The
blood-brain barrier: an overview: structure, regulation, and
clinical implications. Neurobiol Dis. 16:1–13. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Begley DJ and Brightman MW: Structural and
functional aspects of the blood-brain barrier. Prog Drug Res.
61:39–78. 2003.PubMed/NCBI
|
|
8
|
Sandoval KE and Witt KA: Blood-brain
barrier tight junction permeability and ischemic stroke. Neurobiol
Dis. 32:200–219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Strbian D, Durukan A, Pitkonen M,
Marinkovic I, Tatlisumak E, Pedrono E, et al: The blood-brain
barrier is continuously open for several weeks following transient
focal cerebral ischemia. Neuroscience. 153:175–181. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang ZJ, Jinkui YL, Lin Y, Yang PJ, Hou
XM and Yang YW: Synopsis of Golden Chamber. Macmillan Press;
Beijing, China: pp. 203–204. 2008, In Chinese.
|
|
11
|
Sun X: Research on formula treating
paralysis and spasticity from ‘treatise on febrile and
miscellaneous diseases’. Zhongguo Zhong Yi Ji Chu Yi Xue Za Zhi.
8:644–645. 2010.In Chinese.
|
|
12
|
Yang C, Chen L and Tao J: New usage of a
classical formula-Gua Lou Gui Zhi decoction. Liaoning J Tradit Chin
Med. 8:166–167. 2010.
|
|
13
|
Zhang L and Ai H: Effects of Gua Lou Gui
Zhi decoction on c-fos and c-jun in epileptic rats. Sichuan Hua Xi
Zhong Yi Yao Yan Jiu Suo. 23:21–22. 2005.
|
|
14
|
Hu H, Li Z, Zhu X, Lin R, Lin J, Peng J,
Tao J and Chen L: Gua Lou Gui Zhi decoction suppresses LPS-induced
activation of the TLR4/NF-κB pathway in BV-2 murine microglial
cells. Int J Mol. 31:1327–1332. 2013.
|
|
15
|
Hu H, Li Z, Zhu X, Lin R, Lin J, Peng J,
Tao J and Chen L: GuaLou GuiZhi decoction inhibits LPS-induced
microglial cell motility through the MAPK signaling pathway. In J
Mol Med. 32:1281–1286. 2013.
|
|
16
|
Li ZF, Lin RH, Mao JJ, Hu HX, Zhu XQ, Chen
WL and Chen LD: Protective effect of Gualou Guizhi decoction on
BV-2 cells injured by glutamate. Journal of Fujian University of
Traditional Chinese Medicine. 23:14–17. 2013.
|
|
17
|
Huang J, Tao J, Xue XH, et al: Gua Lou Gui
Zhi decoction exerts neuroprotective effects on post-stroke
spasticity via the modulation of glutamate levels and AMPA receptor
expression. Int J Mol Med. 31:841–848. 2013.PubMed/NCBI
|
|
18
|
Chen X, Li H, Huang M, Huang M, et al:
Effect of Gua Lou Gui Zhi decoction on focal cerebral
ischemia-reperfusion injury through regulating the expression of
excitatory amino acids and their receptors. Mol Med Rep.
10:248–254. 2014.PubMed/NCBI
|
|
19
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bederson JB, Pitts LH, Germano SM,
Nishimura MC, Davis RL and Bartkowski HM: Evaluation of
2,3,5-triphenyltetrazolium-chloride as a stain for detection and
quantification of experimental cerebralin farction in rats. Stroke.
17:1304–1308. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hou SZ, Li Y, Zhu XL, Wang ZY, Wang X and
Xu Y: Ameliorative effects of diammonium glycyrrhizinate on
inflammation in focal cerebral ischemic-reperfusion injury. Brain
Res. 1447:20–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weiss N, Miller F, Cazaubon S and Couraud
PO: The blood-brain barrier in brain homeostasis and neurological
diseases. Biochim Biophys Acta. 1788:842–857. 2009. View Article : Google Scholar
|
|
23
|
Zhan C and Yang J: Protective effects of
isoliquiritigenin in transient middle cerebral artery
occlusion-induced focal cerebral ischemia in rats. Pharmacol Res.
53:303–309. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kape R, Parniske M, Brandt S and Werner D:
Isoliquiritigenin, a strong nod gene- and glyceollin
resistance-inducing flavonoid from soybean root exudate. Appl
Environ Microbiol. 58:1705–1710. 1992.PubMed/NCBI
|
|
25
|
Liu DZ, Xie KQ, Ji XQ, Ye Y, Jiang CL, et
al: Neuroprotective effect of paeoniflorin on cerebral ischemic rat
by activating adenosine A1 receptor in a manner different from its
classical agonists. Br J Pharmacol. 146:604–611. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mao QQ, Zhong XM, Feng CR, Pan AJ, Li ZY,
et al: Protective effects of paeoniflorin against glutamate-induced
neurotoxicity in PC12 cells via antioxidant mechanisms and
Ca(2+) antagonism. Cell Mol Neurobiol. 30:1059–1066.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo RB, Wang GF, Zhao AP, Gu J, Sun XL and
Hu G: Paeoniflorin protects against ischemia-induced brain damages
in rats via inhibiting MAPKs/NF-κB-mediated inflammatory responses.
PLoS ONE. 7:e497012012. View Article : Google Scholar
|
|
28
|
Kim SW, Lim CM, Lee HK and Lee JK: The use
of Stronger NeoMinophagen C, a glycyrrhizin-containing preparation,
in robust neuroprotection in the postischemic brain. Anat Cell
Biol. 44:304–313. 2012. View Article : Google Scholar
|
|
29
|
Gong G, Xiang L, Yuan L, et al: Protective
effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral
ischemia/reperfusion-induced inflammation, oxidative stress, and
apoptosis in rats. PLoS One. 9:e894502014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun YX, Tang Y, Wu AL, Liu T, Dai XL,
Zheng QS and Wang ZB: Neuroprotective effect of liquiritin against
focal cerebral ischemia/reperfusion in mice via its antioxidant and
antiapoptosis properties. J Asian Nat Prod Res. 12:1051–1060. 2012.
View Article : Google Scholar
|