Introduction
Macroangiopathy is a major cause of mortality and
morbidity in diabetes mellitus. In addition, atherosclerotic and/or
arteriosclerotic changes in the cardiovascular system may result in
the development and progression of cardiovascular diseases
associated with diabetes (1).
Endothelial dysfunction is recognized as one of the early and
prominent stages in the formation and development of
atherosclerotic lesions (2), in
which endothelial proliferation and apoptosis have been observed
(3). Impairment of endothelial
function has been reported to occur in the early stage of diabetes,
prior to clinically detectable angiopathy and hypertension
(4). Hyperglycemia has been
demonstrated to disrupt the cell cycle, increase DNA damage, delay
cell replication and induce apoptosis in endothelial cells
(5–8). The Akt (protein kinase
B)/phosphatidylinositol 3-kinase (PI3K) pathway has a critical role
in insulin signaling as well as cell apoptosis (9,10).
Molecular events linking high glucose with endothelial cell
apoptosis were reported to be involved in the nuclear
factor-κB-associated upregulation of cyclooxygenase-2 as well as
the increased production of reactive oxygen species via the
Akt/PI3K pathway in human umbilical vein endothelial cells (HUVECs)
(9,11).
A previous study reported that tribbles homolog
(TRIB) 3, a mammalian homolog of Drosophila tribbles, may be
associated with modulated glucose metabolism through directly
binding to Akt in the livers of diabetic mice (10). In addition, TRIB3 protein
overexpression was reported to result in hyperglycemia and inhibit
the activity of Akt (10).
Subsequent clinical studies have proposed that functional TRIB3
missense polymorphisms may be associated with hyperinsulinemia,
dyslipidemia and cardiovascular disease (12,13).
Another previous study demonstrated that the TRIB3 Q84R
polymorphism increased the risk of metabolic syndrome and insulin
resistance (14). In addition, the
R84 allele was found to be associated with a predisposition to
carotid atherosclerosis. These previous studies demonstrated a link
between TRIB3 and atherosclerosis in diabetes; therefore, the
present study aimed to investigate the involvement of TRIB3 protein
in endothelial cell dysfunction. In order to investigate the effect
of TRIB3 on endothelial cell dysfunction, the present study
stimulated HUVECs with high glucose concentrations and transfected
with an anti-TRIB3 inhibitor. The expression levels of miR-21 and
HUVECs dysfunction were subsequently measured.
Materials and methods
Cell culture
HUVECs were purchased from the China Center for Type
Culture Collection (Wuhan University, Wuhan, China). Cells were
grown in endothelial cell basal medium containing M199 (Gibco-BRL,
Carlsbad, CA, USA), 10% fetal calf serum (Tianjin Lisheng
Pharmaceutical Co., Ltd., Tianjin, China), 40 ng/ml growth factors
(rHVEGF; cat. no. 676472; Chemicon, Billerica, MA USA), 100 U/ml
penicillin and 100 µg/ml streptomycin (Beyotime Institute of
Biotechnology, Shanghai, China) at 37°C with 5% CO2 in a
humidified incubator. Confluent HUVECs were used for experiments
between passages 3 and 5.
Assays for cell viability
A methyl thiazolyl tetrazolium (MTT) assay was
performed in order to determine the cell viability of HUVECs at
various concentrations of glucose. Cells were seeded at a density
of 5×103 cells/well into 96-well plates for 24 h.
Confluent cells were then incubated in serum-free cell culture
medium with various concentrations of glucose (5.5, 10, 20, 30 and
40 mmol/l) for 24, 48 or 72 h at 37°C with 5% CO2. MTT
solution (20 µl/well; Sigma-Aldrich, St. Louis, MO, USA) was
then added and the samples were incubated for a further 4 h at 37°C
with 5% CO2. The absorbance of the samples was read at
490 nm (Model 1450; Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
HUVECs were treated with various concentrations of
glucose and for different durations as follows: 30 mmol/l glucose
for 0, 4, 8, 12, 24, 48 or 72 h; 5.5, 10, 20, 30 and 40 mmol/l
glucose for 24 h; and control (5.5 mmol/l), high (30 mmol/l) and
hypertonic mannitol (25 mmol/l; Shijiazhuang Siyao Co., Ltd.,
China) concentrations for 0, 4, 7, 12, 24, 48 and 72 h. Mannitol
was used to exclue te effect of high glucose osmotic pressiure on
the cells. Total RNA was extracted from HUVECs using TRIzol reagent
(Invitrogen Life Technologies, Waltham, MA, USA). qPCR with cDNA
was performed using a Real-Time Fluorescent SYBR Green I PCR kit
(Takara Bio, Inc., Dalian, China). The cDNA primers (Shanghai Boya
Biotechnology Co., Ltd., Shanhai, China) used were as follows:
TRIB1 sense, 5′-GCTGTGCATCCACACTGGAC-3′ and antisense,
5′-GCGATGGCAGCTGGATGTAA-3′; TRIB2 sense,
5′-CTCAAGCTGCGGAAATTCATCTTTA-3′ and antisense,
5′-TGGTGTTCAAGATCTCTGGGCTTAC-3′; TRIB3 sense,
5′-GTCTGGTCCTGCGTGATCTCAA-3′ and antisense, 5′-GTATG
AGGCCCGTGAGCTGAGT-3′; β-actin sense, 5′-TGGACATCCGCAAAGAC-3′ and
antisense, 5′-GAAAGGGTGTAACGCAACTA-3′ (Shanghai Boya Biotechnology
Co., Ltd., Shanghai, China). mRNA expression levels were calculated
relative to those of β-actin.
Immunofluorescence staining and western
blot analysis of TIRB3 protein levels
In order to investigate effect of glucose on TRIB3
protein levels, immunofluorescence staining and western blot
analysis were performed. For immunofluores-cence staining, HUVECs
were treated with high glucose (HG; 30 mmol/l) for 24 or 48 h at
37°C with 5% CO2. Cells were grown on glass coverslips
and fixed with 4% paraformaldehyde (Tianjin Guangcheng Chemical
Reagents Co., Ltd., Tianjin, China) for 30 min at room temperature,
then permeabilized with a blocking solution containing 0.3% Triton
X-100 and 5% bovine serum albumin (both Tianjin Lisheng
Pharmaceutical Co., Ltd.) in phosphate-buffered saline (PBS).
Following incubation with rabbit polyclonal immunoglobulin (Ig)G
TRIB3 primary antibodies (1:50; cat. no. NB100-56398; Imgenex,
Littleton, CO, USA) overnight at 4°C, fluorescein-isothiocyanate
(FITC)-conjugated anti-rabbit secondary antibodies (1:100; cat. no.
35552; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing China) were added and incubated for 1 h at room
temperature. Nuclei were counterstained with DAPI (Nanjing KGI
Biotechnology Development Co., Ltd, Nanjing, China) for 1 min.
Cells were then observed and fluorescence images were captured
using an inverted fluorescence microscope (IX71-A12FL/PH; Olympus
Corp., Tokyo, Japan).
For western blot analysis, HUVECs were exposed to HG
(30 mmol/l) for 24, 48 or 72 h. TRIB3 protein concentrations were
determined using a Bicinchoninic Acid Protein Assay Kit (Beyotime
Institute of Biotechnology) according to the manufacturer’s
instructions. Protein samples were electrotransferred onto 20%
SDS-polyacrylamide gels and then immobilized to nitrocellulose
membranes (Bio-Rad Laboratories, Inc.). The membranes were blocked
and incubated with rabbit polylclonal IgG TRIB3 primary antibodies
(1:800; cat. no. NB100-56398; Imgenex) overnight at 4°C. Following
washing three times with 1X Tris-buffered saline containing
tween-20 for 5 mins, the membranes were incubated with goat
anti-rabbit IgG secondary antibodies conjugated to horseradish
peroxidase (1:100; cat. no. ZF-0315; Pierce Biotechnology, Inc.,
Rockford, IL, USA). The immuno-reactive bands were quantified using
a FluorChem 9900-50 gel documentation imaging system (Alpha
Innotech, San Leandro, CA, USA).
Small interfering (si)RNA and
transfection
siRNA against human TIRB3 (sense,
5′-CGAGCUCGAAGUGGGCCCCTT-3′ and antisense,
5′-GGGGCCCACUUCGAGCUCGTT-3′) were designed and synthesized by
Zimmer Medical International Trading (Shanghai, China). A
nonspecific siRNA duplex (sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and
antisense, 5′-ACGUGACACGUUCGGAGAATT-3′) (Zimmer Medical
International Trading) was used as a control oligonucleotide.
HUVECs were grown to 50% confluence in 12-well plates and
Opti-Minimal Essential Medium (Thermo Fisher Scientific, Waltham,
MA, USA). Cells were transfected with 400 pmol siRNA molecules (at
a concentration of 150 pmol/10cm2) using Lipofectamine
2000 (Invitrogen Life Technologies). Following 6 h of incubation at
37°C with 5% CO2, the medium was replaced with complete
cell culture medium or HG (30 mmol/l) medium for 48 h at 37°C with
5% CO2.
FITC-Annexin V and propidium iodide (PI)
double staining
Cells (0.5×106/well) were seeded into
6-well plates, which were treated with or without TRIB3 or control
siRNA in the presence of normal glucose (5.5 mmol/l) or HG (30
mmol/l), followed by incubation for 48 h at 37°C with 5%
CO2. Following 0.125% trypsin-EDTA (Gibco Life
Technologies, Carlsbad, CA, USA) digestion, the cell pellet was
washed twice with ice-cold PBS. Subsequently, 400 µl binding buffer
(Sigma-Aldrich) and 5 µl FITC-labeled Annexin V (Bipec
Biopharma Corporation, Cambridge, MA, USA) were added and cells
were then incubated in the dark at 4–8°C for 15 min. The PI
solution (10 µl; Bipec Biopharma Corporation, Cambridge, MA,
USA) was added and cells were incubated in the dark for a further 5
min. A FACScan flow cytometer (Becton Dickinson, Franklin Lakes,
NJ, USA) was then used to determine the apoptotic rate of cells.
The ratio of PI-positive to Annexin V-positive cells was used as a
negative control.
Statistical analysis
Values are presented as mean ± standard deviation.
An unpaired t-test and one-way analysis of variance followed by the
least significant difference post hoc test were used to evaluate
the data. Statistical analyses were performed using SPSS 15.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference between
values.
Results
Effect of various glucose concentrations
on HUVEC survival rates
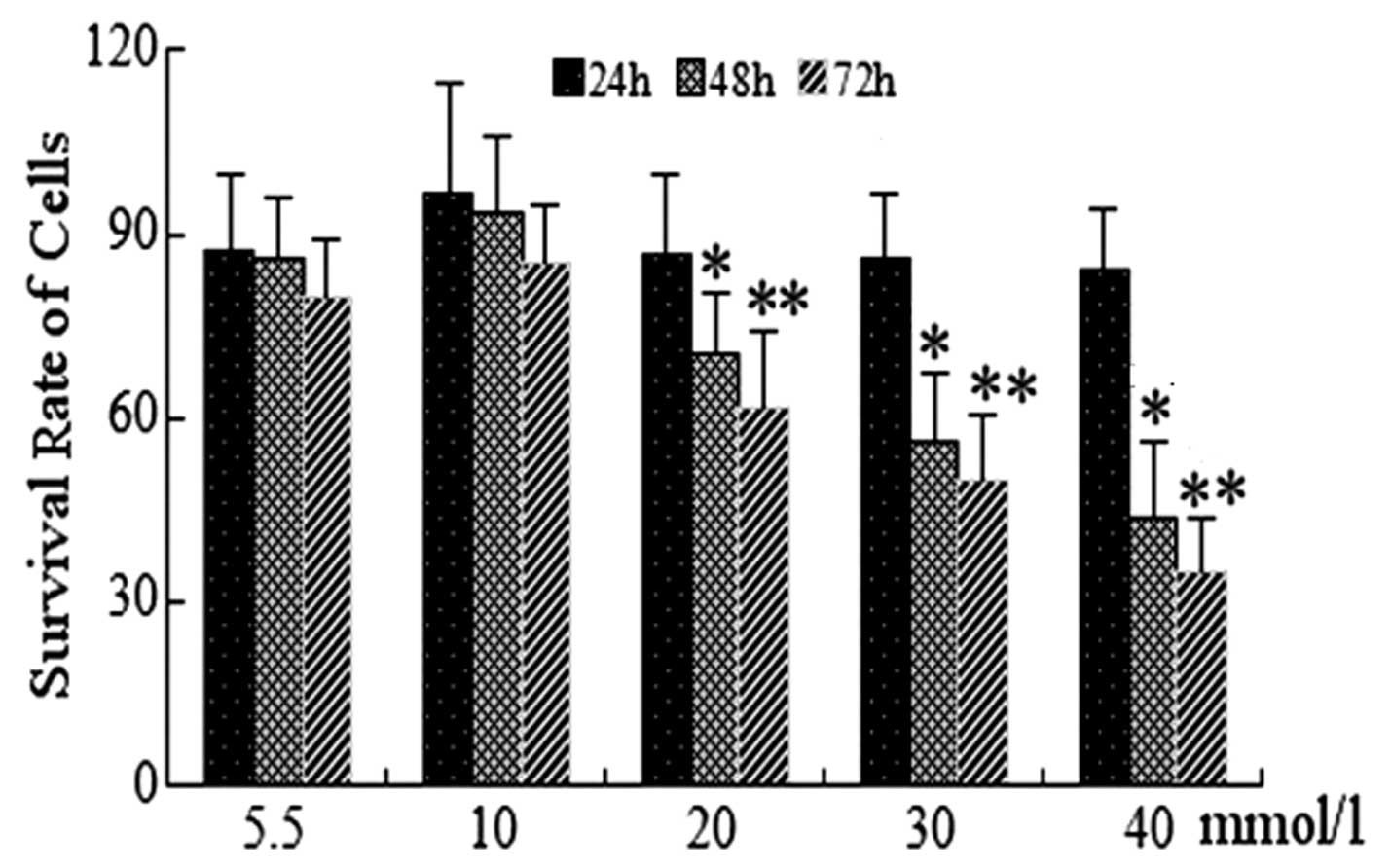
An MTT assay was used to determine the survival rate
of cells following treatment with various glucose concentrations
(5.5, 10, 20, 30 and 40 mmol/l) for 24, 48 and 72 h. As shown in
Fig. 1, at 24 h no significant
differences were identified in the cell viability of HUVECs treated
with 30 or 40 mmol/l glucose compared with that of the normal
glucose control group (5.5 mmol/l; P>0.05). Under identical
conditions, HUVECs treated with 30 and 40 mmol/l for 48 and 72 h
demonstrated significantly reduced survival rates compared with
that of the control group (P<0.05). No significant differences
were observed in cell viability at glucose concentrations of 10 and
20 mmol/l at 24, 48 or 72 h compared with that of the control
group. Overall, the survival rates of HUVECs were found to be
decreased in a concentration- and time-dependent manner following
treatment with glucose (Fig. 1).
Of note, the cell survival rate of HUVECs was <50% at 40 mmol/l
glucose; therefore, the HG groups were treated with 30 mmol/l
glucose for subsequent experiments.
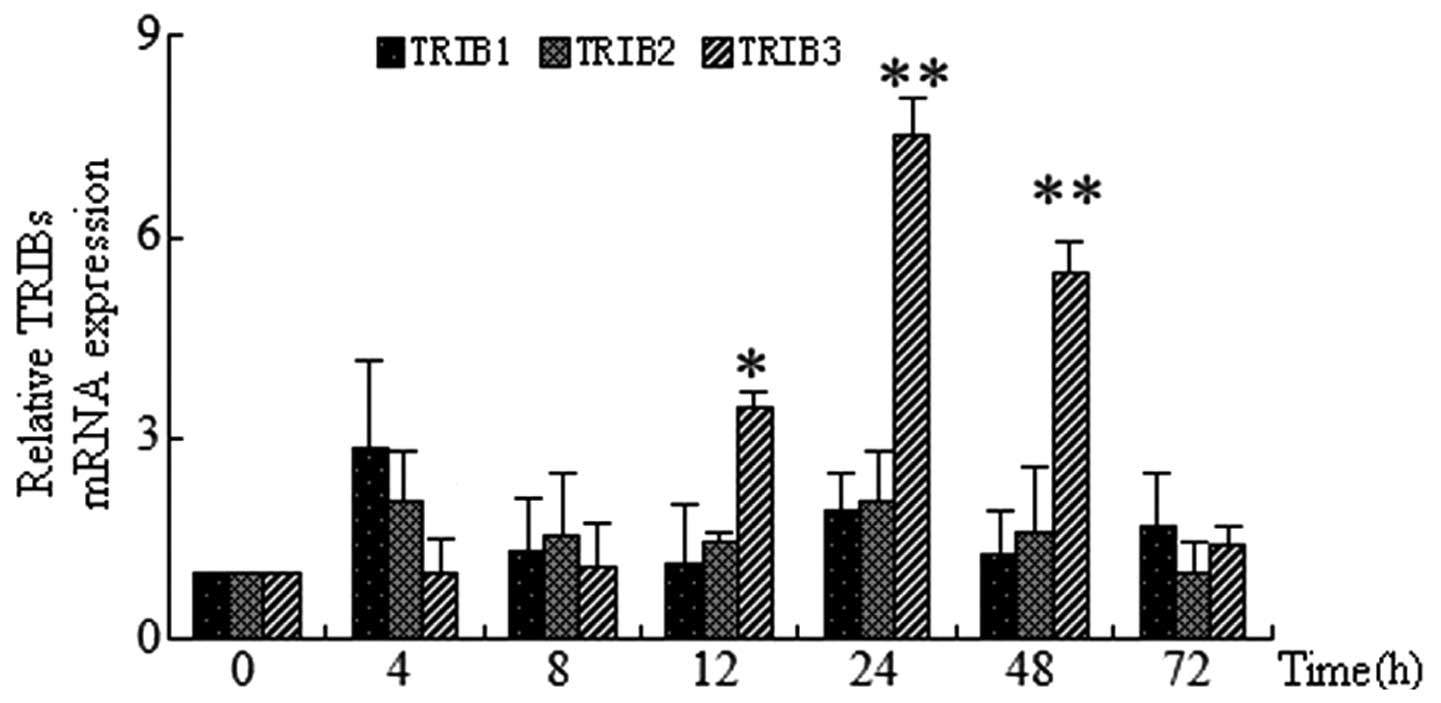
Effects of HG on TRIB mRNA
expression
HUVECs were treated with 30 mmol/l glucose for 4, 8,
12, 24, 48 or 72 h. RT-qPCR was then used to determine the mRNA
expression of TRIB1, TRIB2 and TRIB3. The results indicated that
mRNAs of each TRIB gene were present in endothelial cells. In
addition, TRIB3 gene expression was found to increase in a
time-dependent manner in response to HG. TRIB3 expression peaked at
24 h (7.2-fold vs. control; P<0.01), which then decreased by 48
h; at 72 h TRIB3 expression was not significantly increased. By
contrast, under identical conditions no significant increases were
observed for TRIB1 and TRIB2 mRNA expression in HUVECs at any
time-point (Fig. 2).
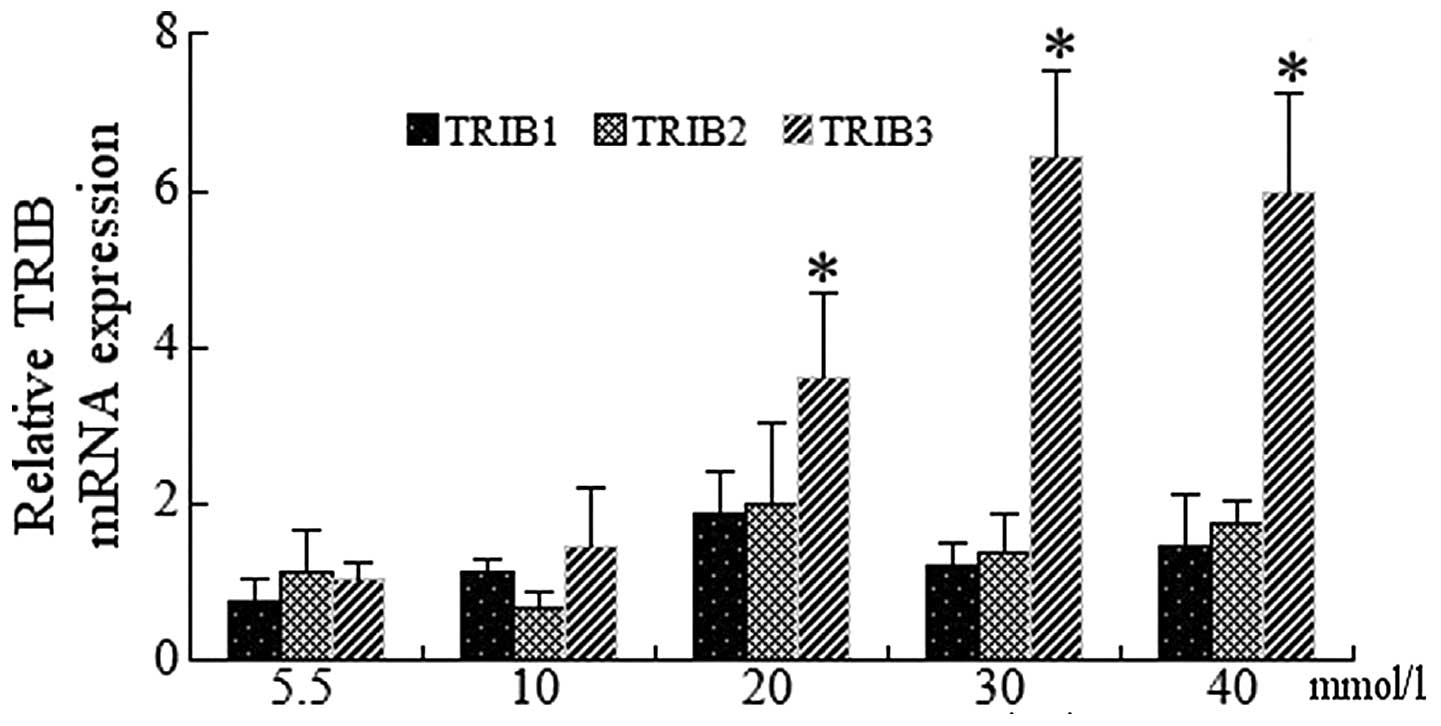
Effect of various glucose concentrations
on TRIB mRNA
mRNA expression of the TRIB genes was examined in
HUVECs incubated with 5.5, 10, 20, 30 and 40 mmol/l glucose for 24
h. RT-qPCR analysis of endothelial cells revealed significant
increases in TRIB3 expression at 30 and 40 mmol/l glucose compared
with that of the control (P<0.01); of note, TRIB3 expression was
highest following treatment with 30 mmol/l glucose (Fig. 3). Although mRNA expression of TRIB1
and TRIB2 showed slight increases, the values did not reach
statistical significance (data not shown).
Effect of hypertonic glucose on mRNA
expression of TRIB3
In order to determine whether high glucose is
responsible for high glucose-induced TRIB3 expression, TRIB3 mRNA
expression was examined in HUVECs treated with HG, hypertonia and
normal glucose conditions for 4, 8, 12, 24, 48 and 72 h. RT-qPCR
analysis revealed that TRIB3 mRNA expression was significantly
increased following 12, 24 and 48 h of HG treatment compared with
that of the normal glucose group at each time-point, with the
highest TRIB3 expression observed at 24 h post treatment (Fig. 4). By contrast, cells grown in
hypertonic glucose showed no significant differences in TRIB3 mRNA
expression levels compared with those of the normal glucose group
at each time-point (Fig. 4).
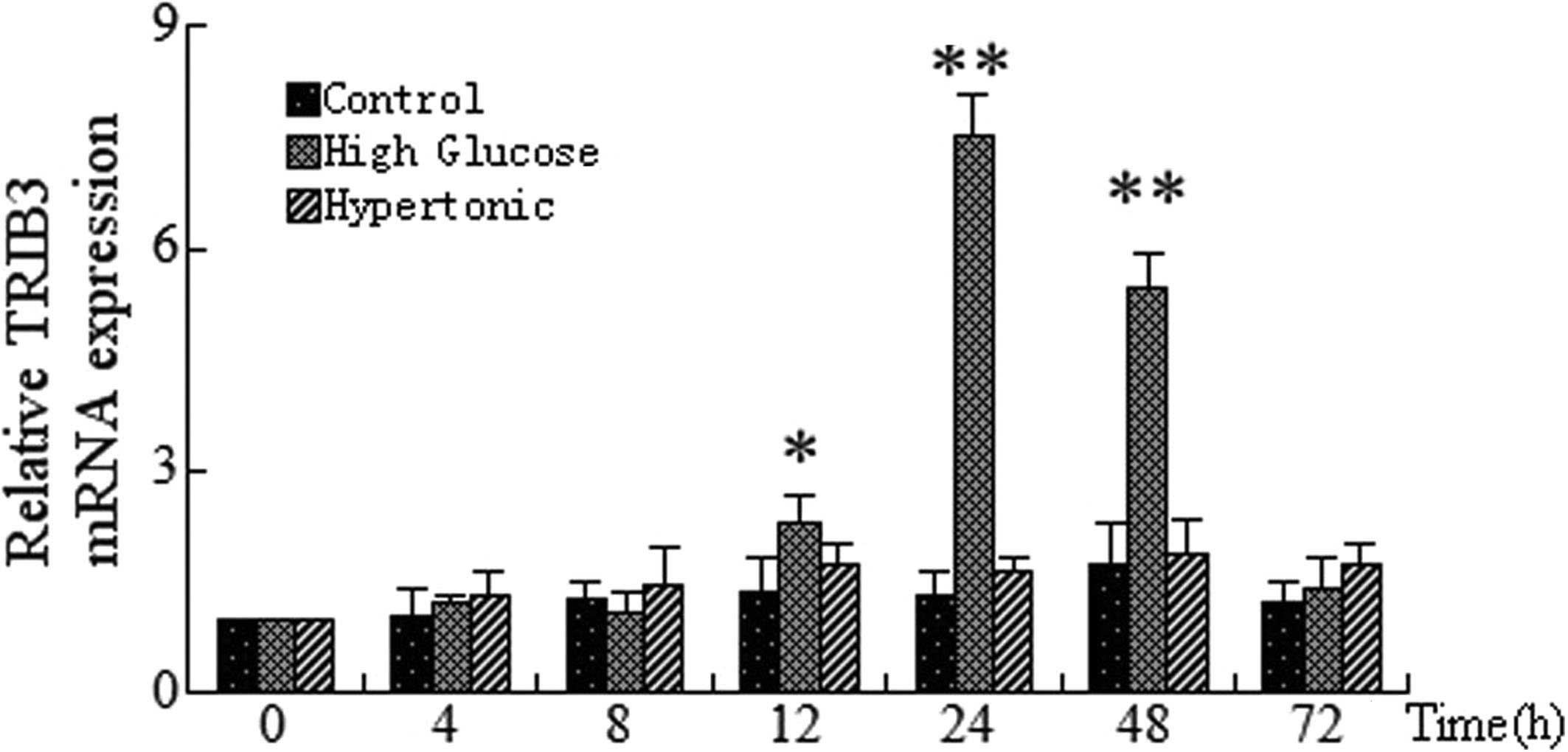 | Figure 4Time-dependent expression of TRIB3
mRNA in HUVECs incubated under control, high glucose and hypertonic
conditions. HUVECs were incubated with control (5.5 mmol/l), high
(30 mmol/l) and hypertonic mannitol concentrations for 0, 4, 7, 12,
24, 48 and 72 h. Reverse transcription polymerase chain reaction
was then used to analyzed the mRNA expression of TRIB3. Values were
calculated relative to the expression of β-actin and are presented
as the mean ± standard deviation. *P<0.05 and
**P<0.01, as compared with at 0 h. TRIB3, tribbles
homolog 3 gene; HUVECs, human umbilical vein endothelial cells;
Control, normal glucose. |
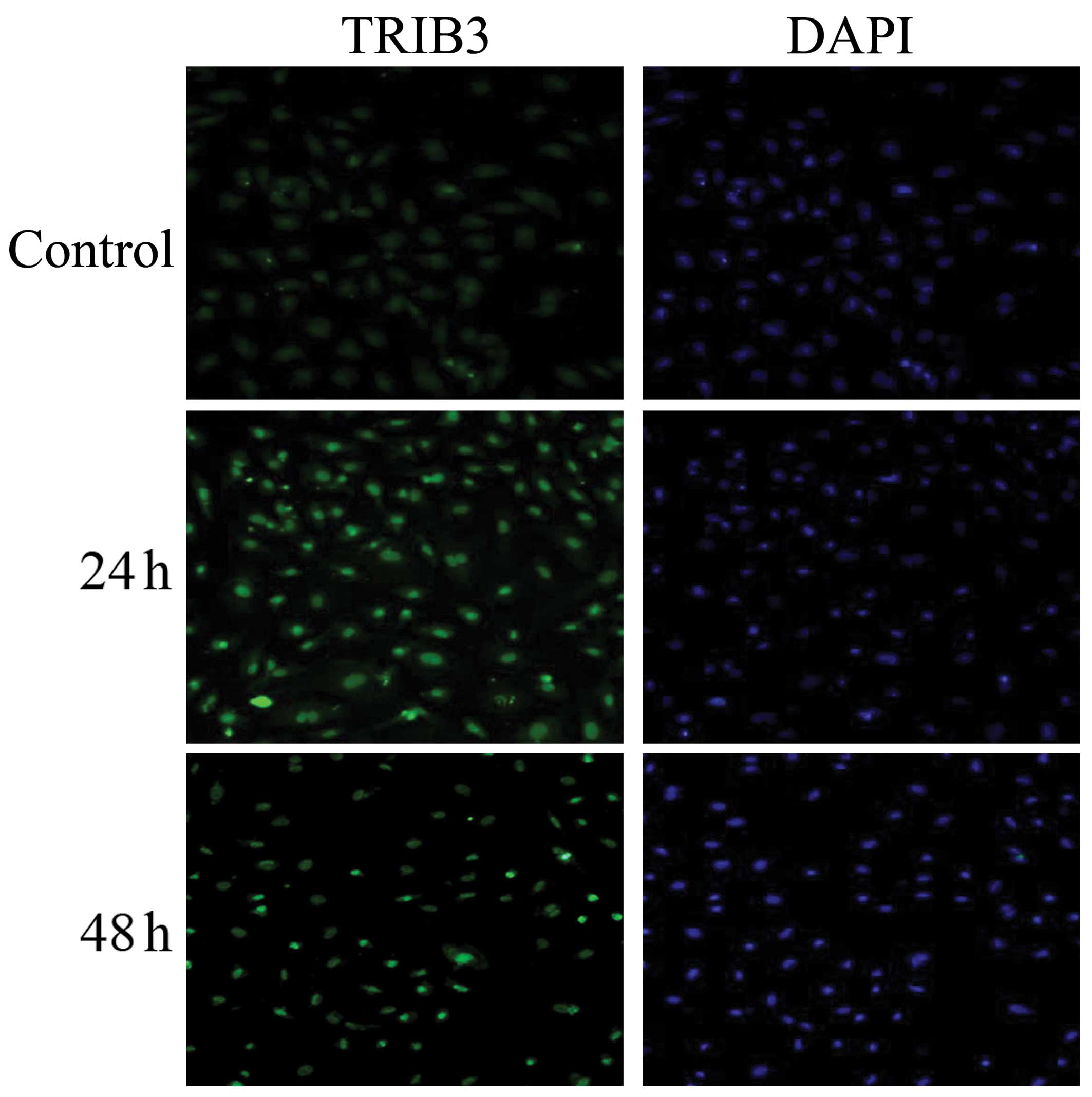
Effects of HG on subcellular localization
and protein expression of TRIB3
The distribution and localization of TRIB3 in the
endothelial cells was assessed using immunofluorescence staining.
As shown in Fig. 5, the
immunostaining intensity of TRIB3 showed increased expression in
the nuclei of cells cultured in HG medium compared with that of
cells treated with normal glucose. However, TRIB3 expression was
not observed in the cytoplasm of HUVECs. Furthermore, these
immunostaining assays confirmed that expression of TRIB3, as
measured by nucleolar fluorescence and immunofluorescence staining,
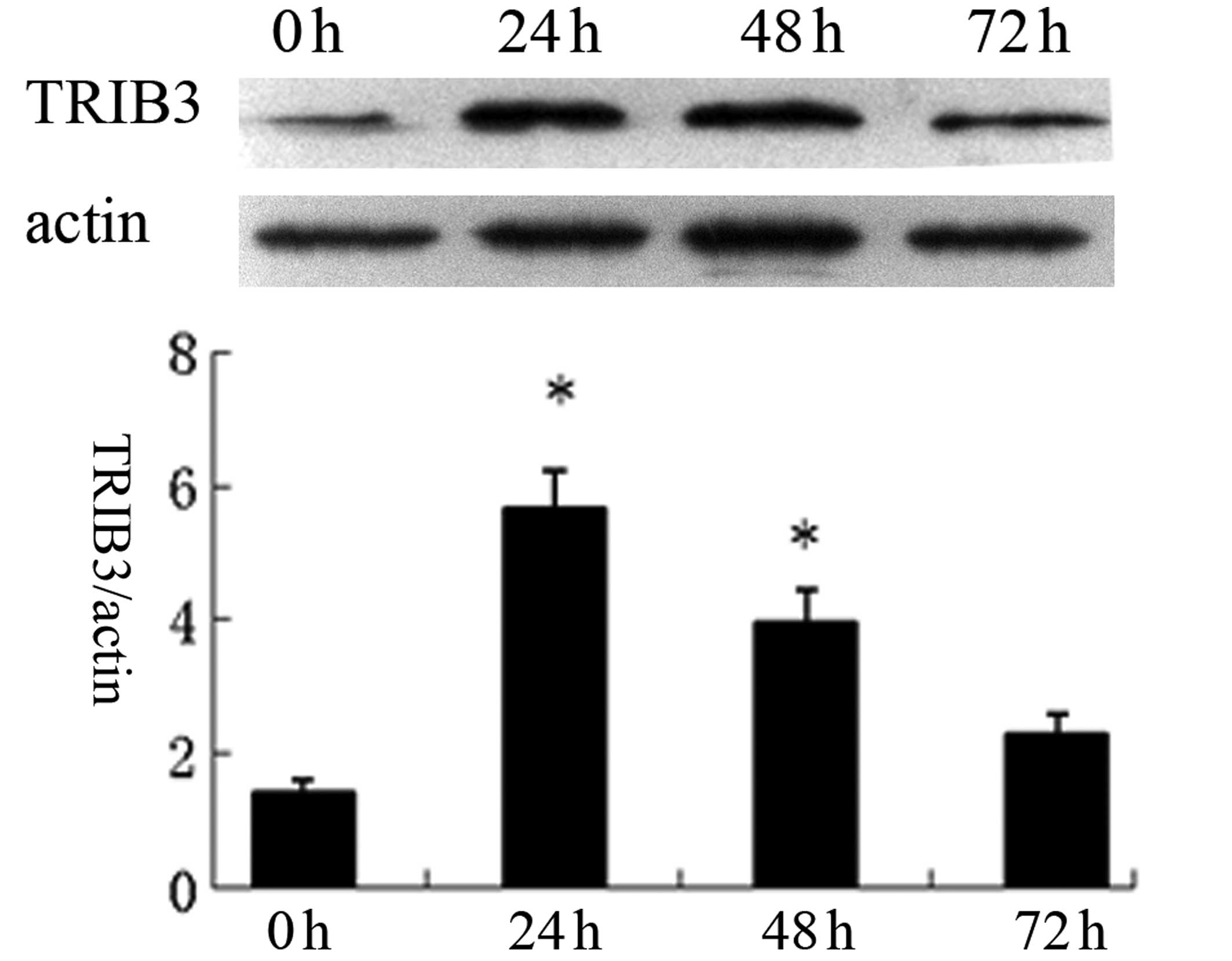
was strongest at 24 h, followed by exposure for 48 h. Western blot
analysis revealed that HUVECs grown in HG medium for 24 h exhibited
a 5.44-fold increase in TRIB3 protein levels, which then decreased,
although expression was still significant compared with the control
group, following exposure for 48 h. However, exposure of the cells
to HG for 72 h had no significant effect on TRIB3 expression
compared with that of the control (Fig. 6). These results were consistent
with the results of the immunofluorescence experiment.
Effect of TRIB3 siRNA on HUVEC apoptosis
following HG treatment
As shown in Fig. 7,
the effect of TRIB3 siRNA on HUVEC apoptosis was examined under
high glucose conditions for 48 h. In the present study, siRNA
exhibited an efficient inhibitory effect on TRIB3 expression and
cell apoptosis at a concentration of 150 pmol/10 cm2.
TRIB3 gene expression was reduced by 90% at 48 h post-transfection.
In addition, cell apoptosis was reduced by 42% in the transfected
cells grown in HG medium for 48 h, as compared with the cells grown
in normal medium. The apoptotic fraction of cells grown in HG
medium was significantly reduced, as compared with the cells grown
in normal glucose medium (P<0.05). In addition, TRIB3
siRNA-transfected HUVECs cultured in HG medium had a significantly
higher rate of cell apoptosis compared with that of HG-treated
cells transfected with control siRNA (P<0.05) (Fig. 7).
Discussion
The aim of present study was to obtain insights into
the role of human TRIB genes, particularly TRIB3, in endothelial
cell dysfunction in vitro and provide evidence for the role
of these genes in diabetes-associated atherosclerosis. In the
current study, it was demonstrated that the expression of TRIB1,
TRIB2 and TRIB3 were detected in endothelial cells following
culture in high glucose medium. In addition, the results showed
that genetic ablation of endogenous TRIB3 by siRNA reduced
endothelial cell apoptosis and promoted cell survival. Therefore,
the present study demonstrated that increased TRIB3 expression may
regulate HG- or diabetes-induced endothelial cell apoptosis.
In one study of a cell model, high ambient glucose
concentrations were shown to modulate the mRNA expression of
fibronectin, collagen, tissue-type plasminogen activator and
plasminogen activator inhibitor; in addition, high glucose was
demonstrated to induce delayed replication and excess cell death in
cultured vascular endothelial cells (15). Endothelial dysfunction has been
hypothesized to have an important role in the progression and
pathogenesis of vascular complications in diabetes. Numerous
studies have reported that the apoptosis of endothelial cells was
prominent in models of hyperglycemia in vitro (16,17).
In the present study, high glucose concentrations were found to
induce apoptosis in HUVECs, which was in line with previous
findings (15–17). Furthermore, HUVEC survival rates
were found to decrease in a concentration- and time-dependent
manner. However, the specific mechanisms of high glucose-induced
apoptotic endothelial cell death remains to be fully
elucidated.
The human homolog of Drosophila tribbles
(TRIB) was first identified using a genome-wide functional screen
for components of inflammatory signaling networks (18). Drosophila tribbles was
reported to be involved in the coordination of entry into mitosis
as well as morphogenesis and cell fate determination during early
embryogenesis (19).
Overexpression of tribbles was reported to slow the cell cycle,
while loss of tribbles function was associated with increased
proliferation (20). In humans
there are three mammalian tribbles-like proteins, TRIB1, TRIB2 and
TRIB3 (21). TRIBs appear to
function in a cell-type- and stimulus-specific manner. In response
to inflammatory stimuli, it was demonstrated that TRIB1 was rapidly
and transiently upregulated in aortic smooth muscle cells and
monocytes, whilst a profound but delayed activation was observed in
synoviocytes (22). In addition,
TRIB2 expression was reported to be upregulated at 6 h post
inflammatory stimuli (interleukin-1) in monocytes, whereas TRIB2
expression was significantly decreased in response to identical
stimuli in synovial fibroblasts (22). Furthermore, TRIB3 expression was
differentially regulated in the various cell types examined; low
TRIB3 mRNA levels were detected at 3 and 6 h following inflammatory
stimuli in synovial fibroblasts and vascular smooth muscle cells;
by contrast, in THP-1 cells, TRIB3 expression was significantly
upregulated, with the highest levels observed at 10 h following
stimuli. However, it remained to be elucidated whether the TRIB
genes were involved in the regulation of endothelial cell apoptosis
in response to high glucose or diabetes. The results of the present
study indicated that increasing concentrations of glucose were able
to stimulate TRIB1, TRIB2 and TRIB3 mRNA expression in HUVECs. Of
note, TRIB1 and TRIB2 genes were transiently, but not
significantly, upregulated in response to high glucose levels
compared with that of the normal glucose group. However, TRIB3
expression was significantly upregulated in HUVECs in response to
high glucose concentrations, with the highest levels observed
following 24 h of culture or at 30 mmol/l glucose. The pattern of
TRIB3 regulation was observed to be time- and
concentration-dependent. Furthermore, immunofluorescence staining
following HG treatment revealed that TRIB3 protein was
predominantly localized in the nuclei of endothelial cells.
Comparable results regarding the subcellular localization of TRIB3
were reported in a study by Ord et al (23). This previous study demonstrated
that the TRIB3-green fluorescent protein fusion protein resided
primarily in the nuclei of transfected cells, including cos-7,
GT1–7, CHO, HeLa and HEK293 cells (23).
A previous study demonstrated that TRIB3 was
upregulated in response to fasting and diabetes; therefore, it was
proposed that TRIB3 may have a major role in hepatic insulin
resistance (10). Analyses of
TRIB3 expression demonstrated that TRIB3 levels were highest in
liver tissues; however, TRIB3 was also detected in the heart,
kidney, lung, skin, small intestine and stomach, although it was
not found to be located in skeletal muscle (24). Altered TRIB3 expression induced by
various stimuli was reported to be highly cell- and/or species-type
specific. In PC-3 prostate cancer cells, a glucose or amino acid
deficiency resulted in a substantial increase in TRIB3 protein
levels and this increase was reversed following the addition of
fresh nutrients (25). In
addition, hypoxia, osmotic stress or serum starvation did not exert
a significant effect on TRIB3 expression (25). In another study, TRIB3 mRNA was
found to be elevated in 3T3-L1 adipocytes and L6 myotubes exposed
to low glucose or glucose-free medium and in 3T3-L1 adipocytes
exposed to dexamethasone (26).
However, in the present study, the results of the in vitro
analysis of TRIB3 expression in HUVECs following glucose
stimulation were not consistent with these previous findings. TRIB3
expression was analyzed using RT-qPCR, western blot analysis and
immunofluorescence staining, the results of which revealed that
TRIB3 expression was highest in cells incubated in HG medium for 24
h, followed by that of exposure for 48 h. Furthermore, the present
study reported that the increased expression of TRIB3 in response
to high glucose was not mediated by glucose-associated hypertonia.
This therefore indicated that rapid, high glucose and endoplasmic
stress may induce the changes in the expression of TRIB3 in
different histological types, including liver, adiopose tissue,
heart, kidney, lung, skin, small intestine and stomach (24).
A previous study suggested that TRIB3 has important
roles in the coordination of entry into mitosis as well as
morphogenesis and cell fate determination through regulating the
degradation of the CDC25 mitotic activator String (19). TRIB3 has been reported to be
involved in cell death during endoplasmic reticulum stress due to
the downregulation of its own induction through the repression of
CCAAT/enhancer binding protein homologous protein/activating
transcription factor 4 function (27,28).
Therefore, in the present study, a loss of function experiment was
performed using TRIB3 siRNA knockdown to evaluate the effects of
TRIB3 on the regulation of HUVEC apoptosis. siRNA is used to
regulate gene expression through entering a multimeric nuclease
complex that identifies target mRNA (29). In the present study, siRNA
efficiently exhibited a pronounced inhibitory effect on TRIB3
expression and cell apoptosis at a concentration of 150 pmol/10
cm2. TRIB3 gene expression was reduced by 90% at 48 h
following transfection. In addition, cell apoptosis was reduced by
42% in transfected cells grown in HG medium for 48 h compared with
those grown in normal medium. Therefore the silencing of the TRIB3
gene by siRNA revealed that endogenous levels of TRIB3 protected
HUVECs cells from apoptosis in response to high glucose.
In conclusion, the present study identified that
TRIB1, TRIB2 and TRIB3 were present in HUVECs cells. In addition,
these findings suggested that TRIB3 was associated with HG-induced
HUVECs apoptosis and may partially mediate the formation and/or
pathogenesis of atherosclerosis under diabetic conditions.
Therefore, TRIB3 may be a potential therapeutic target for
attenuating the progression of atherosclerosis in diabetes.
Acknowledgments
The present study was supported by research grants
from the Independent Innovation Foundation of Shandong University
(no. 2012JC034), the Research Award Fund for Outstanding
Middle-aged and Young Scientist of Shandong Province (no.
BS2010YY029), the Natural Science Foundation of Shandong Province
(nos. ZR2009CM022, ZR2009CM025 and BS2009YY026), the National
Natural Science Foundation of China (nos. 30971215, 81070141,
81100605, 81270352 and 81270287) and the National Basic Research
Program of China (973 Program; no. 2013CB530700).
References
|
1
|
O’Neill MS, Veves A, Zanobetti A, et al:
Diabetes enhances vulnerability to particulate air
pollution-associated impairment in vascular reactivity and
endothelial function. Circulation. 111:2913–2920. 2005. View Article : Google Scholar
|
|
2
|
Davignon J and Ganz P: Role of endothelial
dysfunction in atherosclerosis. Circulation. 109(21 Suppl 1):
III27–III32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Norata GD, Tonti L, Roma P and Catapano
AL: Apoptosis and proliferation of endothelial cells in early
atherosclerotic lesions: possible role of oxidised LDL. Nutr Metab
Cardiovasc Dis. 12:297–305. 2002.
|
|
4
|
Guangda X and Yuhua W: Apolipoprotein e4
allele and endothelium-dependent arterial dilation in Type 2
diabetes mellitus without angiopathy. Diabetologia. 46:514–519.
2003.PubMed/NCBI
|
|
5
|
Lorenzi M, Cagliero E and Toledo S:
Glucose toxicity for human endothelial cells in culture. Delayed
replication, disturbed cell cycle, and accelerated death. Diabetes.
34:621–627. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lorenzi M, Montisano DF, Toledo S and
Barrievx A: High glucose induces DNA damage in cultured human
endothelial cells. J Clin Invest. 77:322–325. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lorenzi M, Nordberg JA and Toledo S: High
glucose prolongs cell-cycle traversal of cultured human endothelial
cells. Diabetes. 36:1261–1267. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kageyama S, Yokoo H, Tomita K, et al: High
glucose-induced apoptosis in human coronary artery endothelial
cells involves up-regulation of death receptors. Cardiovasc
Diabetol. 10:732011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sheu ML, Ho FM, Yang RS, et al: High
glucose induces human endothelial cell apoptosis through a
phosphoinositide 3-kinase-regulated cyclooxygenase-2 pathway.
Arterioscler Thromb Vasc Biol. 25:539–545. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Du K, Herzig S, Kulkarni RN and Montminy
M: TRIB3: a tribbles homolog that inhibits Akt/PKB activation by
insulin in liver. Science. 300:1574–1577. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Radovits T, Lin LN, Zotkina J, et al:
Poly(ADP-ribose) polymerase inhibition improves endothelial
dysfunction induced by reactive oxidant hydrogen peroxide in vitro.
Eur J Pharmacol. 564:158–166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Prudente S, Hribal ML, Flex E, et al: The
functional Q84R polymorphism of mammalian Tribbles homolog TRIB3 is
associated with insulin resistance and related cardiovascular risk
in Caucasians from Italy. Diabetes. 54:2807–2811. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qi L, Heredia JE, Almrejos JY, et al: TRB3
links the E3 ubiquitin ligase COP 1 to lipid metabolism. Science.
312:1763–1766. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gong HP, Wang ZH, Jiang H, et al: TRIB3
functional Q84R polymorphism is a risk factor for metabolic
syndrome and carotid atherosclerosis. Diabetes Care. 32:1311–1313.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baumgartner-Parzer SM, Wagner L,
Pettermann M, et al: High-glucose-triggered apoptosis in cultured
endothelial cells. Diabetes. 44:1323–1327. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakagami H, Morishita R, Yamamoto K, et
al: Phosphorylation of p38 mitogen-activated protein kinase
downstream of bax-caspase-3 pathway leads to cell death induced by
high D-glucose in human endothelial cells. Diabetes. 50:1472–1481.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zou MH, Shi C and Cohen RA: High glucose
via peroxynitrite causes tyrosine nitration and inactivation of
prostacyclin synthase that is associated with
thromboxane/prostaglandin H(2) receptor-mediated apoptosis and
adhesion molecule expression in cultured human aortic endothelial
cells. Diabetes. 51:198–203. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kiss-Toth E, Wyllie DH, Holland K, et al:
Functional mapping of Toll/interleukin-1 signalling networks by
expression cloning. Biochem Soc Trans. 33(Pt 6): 1405–1406. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mata J, Curado S, Ephrussi A and Rørth P:
Tribbles coordinates mitosis and morphogenesis in Drosophila by
regulating string/CDC25 proteolysis. Cell. 101:511–522. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grosshans J and Wieschaus EA: A genetic
link between morphogenesis and cell division during formation of
the ventral furrow in Drosophila. Cell. 101:523–531. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hegedus Z, Czibula A and Kiss-Toth E:
Tribbles: a family of kinase-like proteins with potent signalling
regulatory function. Cell Signal. 19:238–250. 2007. View Article : Google Scholar
|
|
22
|
Sung HY, Francis SE, Crossman DC and
Kiss-Toth E: Regulation of expression and signalling modulator
function of mammalian tribbles is cell-type specific. Immunol Lett.
104:171–177. 2006. View Article : Google Scholar
|
|
23
|
Ord D and Ord T: Mouse NIPK interacts with
ATF4 and affects its transcriptional activity. Exp Cell Res.
286:308–320. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okamoto H, Latres E, Liu R, et al: Genetic
deletion of Trb3, the mammalian Drosophila tribbles homolog,
displays normal hepatic insulin signaling and glucose homeostasis.
Diabetes. 56:1350–1356. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schwarzer R, Dames S, Tondera D, et al:
TRB3 is a PI 3-kinase dependent indicator for nutrient starvation.
Cell Signal. 18:899–909. 2006. View Article : Google Scholar
|
|
26
|
Yacoub Wasef SZ, Robinson KA, Berkaw MN
and Buse MG: Glucose, dexamethasone and the unfolded protein
response regulate TRB3 mRNA expression in 3T3-L1 adipocytes and L6
myotubes. Am J Physiol Endocrinol Metab. 291:E1274–E1280. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ord D, Meerits K and Ord T: TRB3 protects
cells against the growth inhibitory and cytotoxic effect of ATF4.
Exp Cell Res. 313:3556–3567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohoka N, Hattori T, Kitagawa M, et al:
Critical and functional regulation of CHOP (C/EBP homologous
protein) through the N-terminal portion. J Biol Chem.
282:35687–35694. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Agrawal N, Dasaradhi PV, Mohmmed A, et al:
RNA interference: biology, mechanism, and applications. Microbiol
Mol Biol Rev. 67:657–685. 2003. View Article : Google Scholar : PubMed/NCBI
|