Introduction
The foundations of maintenance of the adult
circulatory system are based on hematopoietic cells, particularly
hematopoietic stem cells (HSCs), which are responsible for the
lifelong production of all blood lineages. Accumulating evidence
indicates that the number and functional properties of
hematopoietic cells in aged animals are altered to become the
opposite of those in younger counterparts (1). The loss of function of bone
hematopoietic cells leads to myeloid skewing of differentiation at
the expense of lymphopoiesis (2,3).
Disruption of the functions of normal bone hematopoietic cell
functions is responsible for diverse leukemia subtypes and immune
deficiencies due to aging and other physiological and pathological
stimuli (4).
It well known that HSCs express c-kit and Sca-1
(Ly-6A/E) cell surface molecules and lack lineage markers
(Sca-1+ Lin− c-kit+ cells). Sca-1
is a glycosylphosphatidylinositol-linked cell surface protein found
on hematopoietic stem cells in the mouse (5). Sca-1 has been reported to be
necessary for normal HSC activity and is involved in determining
the fate of hematopoietic progenitor/stem cells, as Sca-1-knockout
mice have defects in short-term competitive transplantation and
serial transplantation (6,7). Further evidence has revealed that the
proliferative response of Thy-1low Sca-1+Lin−
c-kit+ cells from aged mice to the FMS-like tyrosine
kinase 3 ligand and thrombopoietin is markedly decreased (8,9). It
may be considered that Sca-1+ cells, to a certain
extent, represent a population cells involved in hematopoiesis and
blood homeostasis.
Panax ginseng is used in traditional Chinese
medicine to enhance stamina and overcome physical stress over past
decades. The beneficial effects of ginseng and its constituents in
terms of its anticancer and immunomodulatory effects have also been
reported (10). It has been
demonstrated that ginsenoside, one of >25 constituents derived
from ginseng, has various functions, including anti-aging,
antioxidant and immunomodulatory effects (11,12).
Ginsenoside Rg1(Rg1), one of the neutral saponins of Ginseng root,
is purified using its crystalline decaacetate (13). The structure of this saponin has
been established as 6,20-di-O-β-glucosyl-20S-protopanax
atriol (14). It has been
demonstrated that Rg1 exerts protective effects on human
endothelial cells and PC12 cells (15,16).
Rg1 also increases neural plasticity in efficacy and structure, and
the proliferation and differentiation of neural progenitor cells in
the dentate gyrus of the hippocampus of normal adult mice and a
global ischemia model in gerbils (17). It has also been reported that Rg1
in P. ginseng increases CD4+ T-cell activity and
the proportion of T helper cells among the total number of T cells,
and promotes the gene expression of IL-2 in murine CD (13,18).
Based on this information, the present study
hypothesized that Rg1 has a protective effect on aged hematopoietic
cells. The present study investigated the effects of Rg1 on
Sca1+ hematopoietic cells using in vitro exposure
experiments. As a positive control, tert-butyl hydroperoxide
(t-BHP) was selected, owing to pervious study demonstrating that
100 µmol/l t-BHP effectively induced Sca-1+ cell
senescence following 6 h co-culture in vitro (19). Replicative senescent
Sca1+ cells were from serial transplanted C57BL/6 mice
were also used to investigate the effects of Rg1 on the aging
process of Sca1+ cell subpopulation in vivo. The
present study also examined changes in the chromosome end telomere
system of Sca1+ cells and other senescence-associated
biomarkers. The aim of these investigations was to determine the
effects of Rg1 in the maintenance of hematopoietic cell function
and tissue homeostasis.
Materials and methods
The study was approved by the Ethics Committee of
Chongqing Medical University (Chongqing, China). Rg1 (purity, 96%
of P. ginseng) was purchased from Jinlin Hongjiu Co., Ltd.
(Changchun, China), Iscove's modified Dulbecco's medium (IMDM)
culture medium was provided by Gibco Life Technologies (Carslbad,
CA, USA), fetal bovine serum (FBS) and horse serum were purchased
from Sijiqing Co, Ltd. (Hangzhou, China), an Anti-Sca-1+
Micro Bead kit was obtained from Miltenyi Biotec GmbH (Berhisch
Gladbach, Germany), t-BHP and Ficoll separation solution were
obtained from Sigma-Aldrich (St. Louis, MO, USA), methylcellulose
was obtained from (Stemcell Technologies, Inc., Vancouver, Canada),
telomere probes were obtained from Invitrogen Life Technologies
(Carlsbad, CA, USA), a genomic DNA extraction kit, DNA Ladder and
Hinf I endonucleases were obtained from Sangon Biotech, Co., Ltd.
(Shanghai, China), the telomere repeat amplification
protocol-quantitative polymerase chain reaction (TRAP-qPCR) silver
stain telomerase activity detection kit was obtained from KeyGEN
Co., Ltd. (Shanghai, China), an ECL kit was obtained from Pierce
Biotechnology (Rockford, IL, USA). Rabbit anti-CDKN2A/p16-INK4a
(cat. no. bs-0740R), rabbit anti-CDK2 (cat. no. bs-10726R), rabbit
anti-phospho-Rb (Ser780; cat. no. bs-1347R), rabbit anti-CDK4 (cat.
no. bs-0633R), goat anti-Mouse IgG/RBITC (cat. no. bs-0296G-RBITC)
and horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG
(1:500) antibodies were obtained from Bioss (Beijing, China), and
were diluted with sterile phosphate-buffered saline (PBS). The
western blotting kit and the SA-β-Gal staining kit was from
Beyotime Institute of Biotechnology (Shanghai, China).
Animals and cell culture
C57BL/6 mice (6–8 weeks; 20–25 g) were bred in-house
in a pathogen-free environment at a temperature of 22–25°C. The
Institutional Animal Care and Use Committee of Chongqing Medical
University (Chongquing, China) approved all animal experiments,
which were performed according to Guide for the Care and Use of
Laboratory Animals (20). The bone
marrow suspension, obtained from the femur and tibia of the C57BL/6
mice with a needle was subjected to Ficoll-Paque density gradient
separation to isolate bone mononuclear cells, followed by
Sca-1+ cell separation using magnetic-activated cell
sorting (MACS). The Sca-1+ cells were cultured in IMDM
supplemented with 10% FBS at 37°C in a humidified atmosphere
containing 5% CO2 prior to drug exposure. Each group
contained 5 mice unless otherwise specified.
Serial transplantation assay
Briefly, the Sca-1+ cells were isolated
and purified from male C57BL/6 mice bone marrow mono-nucleated
cells using MACS and cultured in IMDM supplemented with 10% fetal
bovine serum. A total of 10,000 harvested Sca-1+ cells
from male C57BL/6 mice were injected into the lateral tail veins of
lethally irradiated (8.5 Gy Co60γ) female C57BL/6 mice.
Mice were irradiated to 8.5 Gy in an Cammacell-40 Exposure
Instrument (Atomic Energy of Canada Limited, Gloucester, Canada) at
a dose-rate of 1.0 Gy per minute for 8.5 min. At 4 weeks
post-transplantation, the recipients were used as donors for a
subsequent transplantation cycle and for in vitro assays.
Serial transplants were performed in both male and female C57BL/6
mice strains. Engraftment efficiency in the C57BL/6 mice recipients
was monitored by detecting the Sry gene of the Y-chromosome using
PCR for donor contribution (35 cycles of 94°C for 4 min, 94°C for
20 sec, 60°C for 30 sec and 72°C for 30 sec; primer sequences
(Bio-Rad, Hercules, CA, USA), forw ard 5′-GAAAAGCCTTACAGAAGCCGA-3′
and reverse 5′-G TATGTGATGGCATGTGGGTTC-3′; 211 base pairs). Serial
transplantation assays were performed three times in independent
experiments, with five recipient mice/group/experiment. The animals
were monitored daily for the presence of disease, and were
sacrificed by decapitation under diethyl ether (Tianhao Chemical
Industry, Guangdong, China) anesthetics at designated times-points
following transplantation, or when moribund. The experiments were
performed following dividing of the 20 adult female C57BL/6 mice
into the four following groups, each containing five mice: Group 1,
mice were irradiated with total 8.5 Gy Co60γ and
injected with 0.2 ml PBS via the lateral tail vein, designated the
control); group 2, mice were irradiated with total 8.5 Gy
Co60γ followed by intraperitoneal administration with 20
mg/kg/d Rg1 for 4 weeks as the positive control (designated Rg1);
group 3, mice were irradiated with total 8.5 Gy Co60γ
followed by injection with 10,000 Sca-1+ cells via tail
vein transplantation, designated the negative control (T) group;
group 4, mice were administered intraperitoneally with 20 mg/kg/d
Rg1 for 1 month following Sca-1+ cell transplantation,
designated T+Rg1. The Sca-1+ cells isolated from each
group of mice were then assessed for aging biomarkers.
Senescence-associated β-galactosidase
(SA-β-Gal) activity assay
The cells (10,000) were fixed with 0.5%
glutaraldehyde for 15 min, followed by washing twice with PBS and
incubation with 1 ml SA-β-Gal staining solution at 37°C without
CO2 for 24 h. The number of positively stained cells
were stochastically counted within 100 cells using a charge-coupled
device camera attached to a phase-contrast microscope (CKX41-A32RC;
Olympus, Tokyo, Japan). All counting was performed in
triplicate.
Colony formation assay
The cells from all the groups were respectively
harvested and plated (3×103/well) in a 96-well plate,
with each well containing 1.1 ml 0.8% methylcellulose
(Sigma-Aldrich) in an environment of 37°C, 5% CO2 for 2
weeks prior to fixation with 4% paraformaldehyde and staining with
0.1% crystal violet. The colony numbers were counted under an
optical microscope (CX22; Olympus, Tokyo, Japan). A cell sphere
containing >50 cells was considered one colony.
Western blot analysis
For western blot analysis, cellular proteins were
obtained from the total cell lysates resuspended in double
distilled H2O. The cellular proteins (30 µg) were
electrophoresed using SDS-poly-acrylamide gel electrophoresis and
transferred onto a polyvinylidene fluoride membrane (GE Healthcare
Life Sciences, Chalfont, UK). The membrane was blocked with 5% skim
milk in PBS for 2 h at room temperature and then incubated with
specific antibodies, anti-P16, anti-Rb, anti-CDK2, anti-CDK4 and
anti-cyclin E (Bioss) all at 1:200 dilutions at 4°C overnight.
HRP-conjugated goat anti-rabbit antibodies were used as a secondary
antibody, at 1:5,000 dilution for 2 h at room temperature. The
specific proteins were detected using enhanced chemiluminescent
reagents (Pierce Biotechnology). Densitometry quantification was
performed using the Image-Pro Plus v 6.0 software (Media
Cybernetics, Inc., Bethesda, MD, USA).
Southern blot analysis
Genomic DNA from the six treatment groups were
digested with Hinf/RsaI enzyme at 37°C for 2 h. Electrophoresis of
the digested genomic DNA (20 µg) was performed in 0.7%
agarose gels (Yeasen, Shanghai, China) at 1 V/cm. Following
electrophoresis, the gels were denatured and neutralized; and the
DNA was transferred in 20X SSC onto nylon membrane filters. The
membrane was prehybridization at 42°C for 1 h in 10 ml
prehybridization solution, containing 3 m 20X SSC, 1 ml 50X
Denhardt solution, 0.5 ml 10% SDS, and 5.5 ml H2O
(Klamar, Shanghai, China), followed by incubated at 42°C overnight
following the addition of telomere probe (100 ng/ml). Finally, the
membrane was washed twice in 2X SSC containing 0.1% SDS (Klamar)
for 30 min, blocked for 15 min, and mixed by agitation with
streptavidin-HRP (1:400; Boster, Wuhan, China) buffer at 37°C for
40 min. The membrane was then washed in PBS three times, followed
by 10 min incubation with 3,3′-diaminobenzidine and capture of
images. The intensity of the signals were determined using
AlphaView System software (Alpha Innotech Corporation, St. San
Leandro, CA, USA) and the mean lengths of the TRFs were calculated
with L=Σ (ODi:l:Li)=Σ (ODi).
Target region amplified polymorphism
(TRAP)-quantitative (q)PCR assay
A total of 1×106 cells were washed once
in ice-cold wash buffer, resuspended and centrifuged at 800 x g for
5 min at 4°C. The precipitation was homogenized with 40 µl
cold lysis buffer and lysad for 30 min in ice, followed by
centrifugation at 1,500 rpm for 30 min at 4°C and collection of the
supernatant (optical density 260/280 of RNA: 1.8–2.0). To the
extension reaction, 50 µl of a solution, containing 5
µl 10XTRAP buffer, 1 µl dNTPs, 1 µl Taq-DNA
polymerase, 1 µl TS primer, 2 µl telomerase
extraction, 39 µl DEPC H2O and 1 µl CX
primer was added. Prior to qPCR, solution without CX primer was
pre-incubated at 23°C for 30 min. The PCR for the TRAP assay was
performed using the following program: 94°C for 5 min; 35 cycles at
94°C for 30 sec, 50°C for 30 sec, 72°C for 90 sec; 72°C for 10 min.
SYBR Green dye (1 µl) was added to the PCR tube, mixed well,
and incubated for 10 min at room temperature. PCR was conducted
using a Bio-Rad sequence detection system (cfx96; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The fluorescence intensity
was determined using a fluorospectrophotometer (Bio-Rad
Laboratories, Inc.), and the ratio of telomerase
activity/fluorescence intensity:protein concentration was
determined.
Data analysis
All values are expressed as the mean ± standard
deviation. Analyses were performed using repeated measures of
analysis of variance and an unpaired two-tailed Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Ginsenoside Rg1 has a protective effect
on normal and aged Sca-1+ cells following in vitro
exposure
To observe the effects of Rg1 on Sca-1+
cells in vitro, the Sca-1+ cells were treated
with an achievable plasma concentration of 10 µmol/l Rg1. To
enable direct comparison with the effect of Rg1 on aging cells,
side by side investigations were performed using t-BHP, owing to
the fact that 100 µmol/l t-BHP effectively induces
Sca-1+ cells aging following 6 h in vitro
co-culture (19). The results of
the SA-β-Gal staining assay demonstrated that there was a marked
increase following the cells treated with 100 µmol/l t-BHP,
compared with the control group cells. Otherwise, no significantly
difference in the number of positively-stained Sca-1+
were observed cells between the groups treated with or without 10
µmol/l Rg1. To determine whether Rg1 had protective effects
on the aging Sca-1+ cells, the Sca-1+ cells
were primarily cultured with 100 µmol/l t-BHP for 6 h, and
then washed with sterile PBS and transferred into 10 µmol/l
Rg1 medium for another 12 h co-culture. Following the SA-β-Gal
staining assay, the numbers of positively-stained Sca-1+
cells were counted. As shown in Fig.
1, the higher rate of positive staining of the
Sca-1+ cells caused by t-BHP exposure decreased
following treatment with Rg1 (8.33±2.41, 7.04±2.53, 68.50±4.95 and
30.08±2.44 for the control, Rg1, t-BHP and Rg1+t-BHP groups,
respectively).
To determine whether Rg1 functionally affected the
ability of Sca-1+ cells to form colonies,
methylcellulose colony-forming unit (CFU) assays were performed.
The results revealed that Sca-1+ cells exposed to 10
µmol/l Rg1 exhibited a minor/moderate increase in CFUs. By
contrast, the Sca-1+ cells exposed to 100 µmol/l
t-BHP exhibited a significant decrease in CFUs. The decreased
abilities of Sca-1+ cells to form colonies caused by
t-BHP was apparently compensated for by treatment with 10
µmol/l Rg1 (15.04±1.78, 18.25±2.27, 3.34±1.58 and 9.34±2.23
CFUs in the control, Rg1, t-BHP and Rg1+t-BHP groups, respectively;
Fig. 1). These data indicated that
Rg1 had a protective effect on normal and on t-BHP-induced aged
Sca-1+ cells in vitro.
Rg1-mediated Sca-1+cell
senescence alleviation is dependent on the regulation of p16 and
downstream cell cycle regulators
It is known that the
p16INK4a/pRb signaling pathway is
important in the process of replicative senescence in fibroblasts.
To gain insight into the molecular basis of Rg1-mediated senescence
alleviation in Sca-1+ cells, western blot analysis was
performed to examine the expression patterns of the important
senescence-asssociated proteins, P16 and Rb, in Sca-1+
cells exposed to different agents. The results demonstrated that
the protein expression levels of P16 and Rb are markedly increased
following t-BHP exposure in vitro. However, the higher
protein expression levels of P16 and Rb in t-BHP-mediated
Sca-1+ cells was decreased by 10 µmol/l Rg1 in
vitro. Cell senescence is linked with specific changes in the
expression of cell cycle regulators, contributing to cell
proliferation arrest. To confrim the protective effects of Rg1, the
present study further analyzed the expression levels of CDK2, CDK4
and cyclin E in the Sca-1+ cells. Rg1 was observed to
significantly restore the higher protein expression levels of CDK2,
CDK4 and cyclin E in the t-BHP-induced Sca-1+ cells. No
other detectable differences were observed in the levels of protein
expression between the Sca-1+ cells with or without Rg1
exposure in vitro (Fig. 2).
These results implied that Rg1 substantially alleviated the cell
cycle arrest, which was induced by t-BHP in vitro, and was
associated with the regulation of
p16INK4α/pRb signaling.
 | Figure 2Rg1-mediated alleviation of
Sca-1+ cell senescence is dependent on the regulation of
p16 and downstream cell cycle regulators. (A) Protein expression
levels of P16, Rb, CDK4, Cyclin E and CDK2 of the Sca-1+
cells in different treatment groups. 1, control; 2, t-BHP; 3, Rg1;
4, t-BHP+Rg1. (B) Quantification of respective proteins expression
levels in Sca-1+ cells in different groups is
represented as histograms. Data are expressed as the mean ±
standard deviation (n=3). *P<0.05. Rg1, ginsenoside Rg1; t-BHP,
tert-butyl hydroperoxide; control, untreated cells. |
Ginsenoside Rg1 restores the aging
profile of Sca-1+ cells in mice
To validate the protective effects of Rg1 on
Sca-1+ hematopoietic cells in mice, a serial
transplantation assaywas performed and Sca-1+ cells were
collected from male mice and transplanted into lethally irradiated
female mice to reconstitute the hematopoietic compartment. At 4
weeks post-transplantatation, the functional changes of the
Sca-1+ cells were assessed using a colony forming units
assay. These results indicated that the Rg1-treated
Sca-1+ cells had higher colony-forming capacity, leading
to an increased number of CFUs, compared with the control group of
untreated cells (Fig. 3). The
first indication of increased colony-forming capacity in the
Rg1-treated Sca-1+ cells transplanted into the primary
recipient mice was evident from the evaluated level of
colony-forming capacity in Sca-1+ cells obtained from
secondary recipients. At 8 weeks post-transplantation, the
Rg1-treated Sca-1+ cells from secondary recipient mice
exhibited higher colony-forming capacity compared with the cells in
the control group. A similar tendency was also observed in the
colony-forming capacity of the Sca-1+ cells from the
third recipient mice.
To determine the aging profile of the
Sca-1+ cells during the process of transplantation, BM
Sca-1+ cells from the Rg1-treated or control group
recipient mice were analyzed using an SA-β-Gal staining assay. The
results revealed an increased number of positively stained cells
within the transplant process, indicating that the regulation of
replicative senescence was, at least in part, responsible for
Sca-1+ cell aging during the process of serial
transplantation. The beneficial effects of Rg1 on Sca-1+
cells aging was evidenced by the return of the aging cells close to
normal levels following Rg1 treatment.
p16-Rb signaling is responsible for
Rg1-mediated Sca-1+ cells aging alleviation in mice
To gain insight into the basis for Rg1 on
Sca-1+ cells in mice, Sca-1+ cells from the
tibial and femoral bone marrow of the third recipient mice were
collected and analyzed using western blotting to observe the
age-associated changes in the protein expression levels of
p16-Rb signaling pathways. The results demonstrated that
there were decreases in the levels of P16 and Rb, but increases in
the levels of CDK2, CDK4 and cyclin E in the Rg1-treated mice,
compared with the control group (Fig.
4). These findings correlated with a significant change in the
expression levels of P16, Rb, CDK2, CDK4 and cyclin E in the
Sca-1+ cells in vitro assays. These results
implied that p16INK4a/pRb signaling was
responsible for the regulation of Rg1 on Sca-1+ cells
aging alleviation in mice.
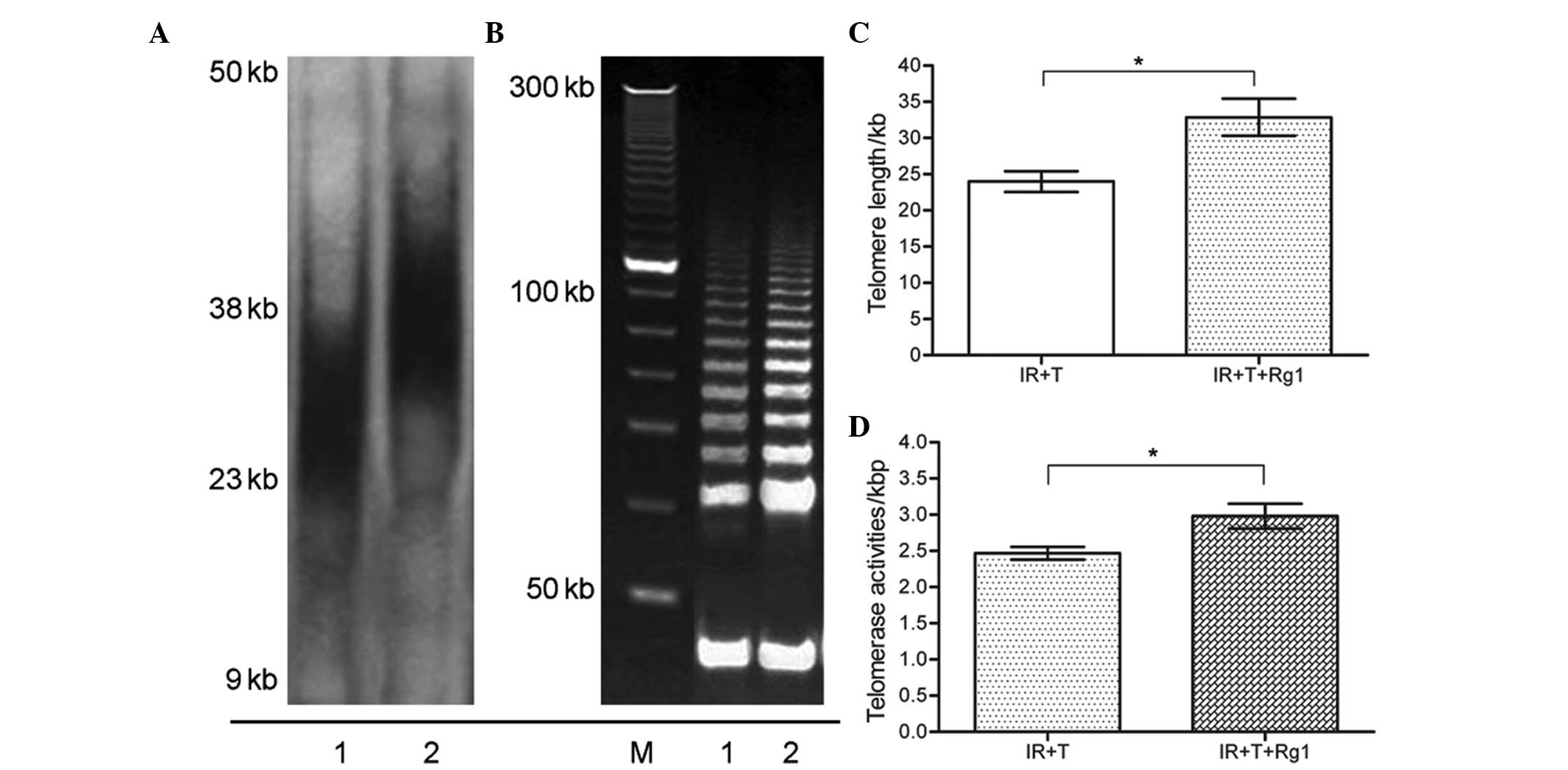
Rg1 functions as telomere elongation and
telomerase maintenance of aged Sca-1+ cells in mice
Telomeres are formed by tandem repeats of the TTAGGG
sequence, which has a base attrition loss during each cell
division. Telomere shortening progressively occurs during cell
replicative senescence. Abnormalities in the telomere length and
telomerase activities of the recipient mice further promoted an
examination of the senescence-associated p16-Rb signaling
pathways (21). To analyze whether
Rg1-mediated Sca-1+ cells aging alleviation is linked to
telomere changes, the present study performed southern blotting to
observe changes in telomere length in Sca-1+ cells from
the third recipient mice. As shown in Fig. 5, the telomere lengths of the
Sca-1+ cells from recipient mice were marginally
decreased, compared with the control group cells, whereas a marked
restoration in the telomere length of the Sca-1+ cells
was identified following Rg1 treatment. This recovery was observed
at similar time-points in three independent experiments. Telomeres
are important in the aging of an organism and in the regulation of
cell senescence, which protects chromosome telomeres from
degradation (22). To further
confirm that the protective effects of Rg1 on Sca-1+
cells aging is consistent with telomere length compensation, the
present study compared the telomerase activities of the Rg1-treated
ad control group Sca-1+ cells from third recipient mice.
In agreement with the effects of Rg1 on telomere length restoration
in the Sca-1+ cells, these results indicated that the
telomerase activities of the Sca-1+ cells improved
following Rg1 administration, compared with the control group cells
in the third recipient mice, as is shown in Fig. 5.
Discussion
In the present study, Rg1 was found to selectively
act on aged Sca-1+ cell and normal Sca-1+
cell function to alleviate aging, which was possibly regulated by
the p16-Rb signaling pathways. These findings were evidenced
by an increase in the telomerase activities and telomere lengths of
the aged Sca-1+ cells following Rg1 treatment in
vitro. Secondly, the serial transplantation assay revealed that
Rg1 reconstituted the telomerase activities and elongated the
telomere lengths of the aged Sca-1+ cells in the
recipient mice.
Panax ginseng has traditionally been
administered to treat 'deficiency' conditions associated with
symptoms, including fatigue, irritability or respiratory tract
symptoms in traditional Chinese medicine (23). Rg1, one of the various ginsenosides
from Panax ginseng, has been demonstrated as a potential
anti-aging agent on neural progenitor cells in the dentate gyrus of
the hippocampus of normal adult mice and in a model of global
ischemia in gerbils (17).
Cellular senescence is a stress response, which is characterized by
permanent cell proliferation arrest and is triggered by various
factors including oncogenes, oxidative stress and persistent DNA
damage (24). Loss of immune
function and an increased incidence of myeloid leukemia are two of
the most clinically significant consequences of aging of
hematopoietic cells (25). The
anti-aging effect of Rg1 on hematopoietic cell was demonstrated in
the present study by the fact that the aging profile of the aged
Sca-1+ cells was alleviated following treatment with
Rg1. The results also revealed prolonged telomere lengths of the
Sca-1+ cells following Rg1 treatment. The results of the
present study also demonstrated that high levels of telomerase
activities were responsible for stem cell telomere maintenance,
which was consistent with a previous report that telomere
maintenance is the predominant mechanism underlying the anti-aging
phenotype of telomerase-overexpressing transgenic mice (26).
Cells in a state of senescence exhibit specific
features, including an enlarged and fattened morphology, increased
β-galactosidase activity and cell cycle arrest (27). The activity of SA-β-Gal can be
detected at pH 6.0 in several types of cultured cells undergoing
replicative and stress-induced senescence (28). It has been used widely as a marker
of cellular senescence in vivo and in vitro (29). This is consistent with the results
of the present study, in which the number of positively stained
Sca-1+ cells decreased following Rg1 treatment in
vitro.
Telomere shortening is attributed to the
accumulation of DNA single-strand breaks, induced by oxidative
damage. Excessive telomere shortening and severe telomere uncapping
owing to telomerase deficiency trigger a DNA damage response at the
chromosome ends, which subsequently upregulates cell cycle-negative
modulators and involves activation of the P16 and Rb proteins
(30). The results of the present
study demonstrated that the expression levels of the cell cycle
negative regulators, P16 and Rb, in the normal and aged
Sca-1+ cells were reduced following treatment with Rg1.
This implied that the anti-aging effect of Rg1 on the
Sca-1+ cells was mediated by cell cycle regulation,
which wa involved in telomere and telomerase function. Replicative
senescence is another mechanism of senescence that share the same
feathers with stress-induced senescence (31). In the present study, the
Sca-1+ cells exhibited marked senescence feathers
following three serial generation transplantations, indicating that
replicative senescence of the Sca-1+ cells was achieved
in mice. The aging phenotype of the Sca-1+ cells was
retrieved following Rg1 treatment, in a generation-dependent
manner.
Taken together, the result of the present study
confirmed that Rg1 had protective effects on aged Sca-1+
cells from mice in vitro and in vivo, in which the
p16-Rb signaling pathways were responsible for regulating
the alleviation of senescence. These findings demonstrate the
potential for the anti-aging function of Rg1 on adult stem and
progenitor cells, particularly on hematopoietic stem and progenitor
cells, to be achieved using external telomerase intervention.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant. nos. 81202785 and 81173398) and
the Natural Science Foundation of Chongqing municipality (grant.
no. 2009BA5038).
References
|
1
|
Geiger H, de Haan G and Florian MC: The
ageing haematopoietic stem cell compartment. Nat Rev Immunol.
13:376–389. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sudo K, Ema H, Morita Y and Nakauchi H:
Age-associated characteristics of murine hematopoietic stem cells.
J Exp Med. 192:1273–1280. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gekas C and Graf T: CD41 expression marks
myeloid-biased adult hematopoietic stem cells and increases with
age. Blood. 121:4463–4472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cho RH, Sieburg HB and Muller-Sieburg CE:
A new mechanism for the aging of hematopoietic stem cells: aging
changes the clonal composition of the stem cell compartment but not
individual stem cells. Blood. 111:5553–5561. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ito CY, Li CY, Bernstein A, Dick JE and
Stanford WL: Hematopoietic stem cell and progenitor defects in
Sca-1/Ly-6A-null mice. Blood. 101:517–523. 2003. View Article : Google Scholar
|
|
6
|
Welm BE, Tepera SB, Venezia T, Graubert
TA, Rosen JM and Goodell MA: Sca-1+cells in the mouse
mammary gland represent an enriched progenitor cell population. Dev
Biol. 245:42–56. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bradfute SB, Graubert TA and Goodell MA:
Roles of Sca-1 in hematopoietic stem/progenitor cell function. Exp
Hematol. 33:836–843. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morrison SJ, Wandycz AM, Akashi K,
Globerson A and Weissman IL: The aging of hematopoietic stem cells.
Nat Med. 2:1011–1016. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Henckaerts E, Langer JC and Snoeck HW:
Quantitative genetic variation in the hematopoietic stem cell and
progenitor cell compartment and in lifespan are closely linked at
multiple loci in BXD recombinant inbred mice. Blood. 104:374–379.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schlag EM and McIntosh MS: The
relationship between genetic and chemotypic diversity in American
ginseng (Panax quinquefolius L). Phytochemistry. 93:96–104. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liao LM, Zhang Y, Lin SF, Hong SB and Lin
Y: Enzymatic Transformation from protopanaxadiol ginsenoside Rb1
into rare ginsenoside CK and its anti-cancer activity. Advanced
Materials Res. 641:752–755. 2013. View Article : Google Scholar
|
|
12
|
Shen L, Xiong Y, Wang DQ, Howles P,
Basford JE, Wang J, Xiong YQ, Hui DY, Woods SC and Liu M:
Ginsenoside Rb1 reduces fatty liver by activating AMP-activated
protein kinase in obese rats. J Lipid Res. 54:1430–1438. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kenarova B, Neychev H, Hadjiivanova C and
Petkov VD: Immunomodulating activity of ginsenoside Rg1 from Panax
ginseng. Jpn J Pharmacol. 54:447–454. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagai Y, Tanaka O and Shibata S: Chemical
studies on the oriental plant drugs-XXIV: Structure of
ginsenoside-Rg1, a neutral saponin of ginseng root. Tetrahedron.
27:881–892. 1971. View Article : Google Scholar
|
|
15
|
Chen WF, Zhou LP, Chen L, Wu L, Gao QG and
Wong MS: Involvement of IGF-I receptor and estrogen receptor
pathways in the protective effects of ginsenoside Rg1 against
Aβ25-35-induced toxicity in PC12 cells. Neurochem Int.
62:1065–1071. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan J, Liu Q, Dou Y, Hsieh Y, Liu Y, Tao
R, Zhu D and Lou Y: Glucocorticoid receptor-ERK pathway contributes
to ginsenoside Rg1 protection against β-amyloid peptide-induced
human endothelial cells apoptosis. J Ethnopharmacol. 147:456–466.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng Y, Shen LH and Zhang JT:
Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and
its mechanism of action. Acta Pharmacol Sin. 26:143–149. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee EJ, Ko E, Lee J, Rho S, Ko S, Shin MK,
Min BI, Hong MC, Kim SY and Bae H: Ginsenoside Rg1 enhances
CD4+ T-cell activities and modulates Th1/Th2
differentiation. Int Immunopharmacol. 4:235–244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Y, Yang B, Yao X and Wang Y:
Establishment of an aging model of Sca-1+ hematopoietic stem cell
and studies on its relative biological mechanisms. In Vitro Cell
Dev Biol Anim. 47:149–156. 2011. View Article : Google Scholar
|
|
20
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals. Guide for the Care and Use of Laboratory Animals. 8th
Edition. National Academies Press (US); Washington, DC: 2011
|
|
21
|
Janzen V, Forkert R, Fleming HE, Saito Y,
Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE and
Scadden DT: Stem-cell ageing modified by the cyclin-dependent
kinase inhibitor p16INK4a. Nature. 443:421–426. 2006.PubMed/NCBI
|
|
22
|
Martínez P and Blasco MA: Telomeric and
extra-telomeric roles for telomerase and the telomere-binding
proteins. Nat Rev Cancer. 11:161–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kiefer D and Pantuso T: Panax ginseng. Am
Fam Physician. 68:1539–1542. 2003.PubMed/NCBI
|
|
24
|
Ohtani N and Hara E: Roles and mechanisms
of cellular senescence in regulation of tissue homeostasis. Cancer
Sci. 104:525–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Snoeck HW: Aging of the hematopoietic
system. Curr Opin Hematol. 20:355–361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tomás-Loba A, Flores I, Fernández-Marcos
PJ, et al: Telomerase reverse transcriptase delays aging in
cancer-resistant mice. Cell. 135:609–622. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao H and Darzynkiewicz Z: Biomarkers of
cell senescence assessed by imaging cytometry. Methods Mol Biol.
965:83–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bandyopadhyay D, Gatza C, Donehower LA and
Medrano EE: Analysis of cellular senescence in culture in vivo: The
senescence-associated beta-galactosidase assay. Curr Protoc Cell
Biol Chapter. 18:Unit 18. 92005.
|
|
29
|
Itahana K, Campisi J and Dimri GP: Methods
to detect biomarkers of cellular senescence: the
senescence-associated beta-galactosidase assay. Methods Mol Biol.
371:21–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sperka T, Wang J and Rudolph KL: DNA
damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol
Cell Biol. 13:579–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Beauséjour CM, Krtolica A, Galimi F,
Narita M, Lowes W, Yaswen P and Campisi J: Reversal of human
cellular senescence: Roles of the p53 and p16 pathways. EMBO J.
22:4212–4222. 2003. View Article : Google Scholar : PubMed/NCBI
|