Introduction
With the increase in population age, senile dementia
has become a social problem worldwide (1). The central nervous system damage
caused by type 2 diabetes mellitus (T2DM) is attracting increasing
attention (2). Several studies
have demonstrated the association between T2DM and cognitive
dysfunction, with the central nervous system damage secondary to
T2DM termed 'diabetes-associated cognitive decline (DACD)'
(3,4).
At present, the precise mechanism underlying DACD
remains to be elucidated. A variety of factors, including abnormal
glucose metabolism, oxidative stress, T2DM complications and the
inflammatory response, are involved in DACD, and there is
overlapping among the pathogenic factors (5). Several investigations have been
performed on the association between oxidative stress and cognitive
dysfunction. Free radicals are highly reactive, and are involved in
oxidization, biomolecular damage and toxicity to nerve cells, thus
leading to lipofuscin deposition, increased age spots and vacuolar
degeneration (6). Malardé et
al (7) demonstrated that
fermented soy permeate exhibited antioxidant and anti-inflammatory
properties in streptozoticin (STZ)-induced diabetic rats. In
addition, Wang et al (8)
reported that chronic treatment with oxymatrine alleviates DACD,
which is associated with oxidative stress, inflammation and
apoptosis in rats.
Previous evidence has demonstrated that inflammation
is involved in pathological damage via multiple mechanisms, which
damages vascular function integrity in DACD (9). In treating the pathological
mechanisms, reducing the inflammatory reaction in the brain can
significantly improves neuronal damage and nerve fiber
degeneration, thereby improving learning and memory (10). Mao et al (11) reported that Huperzine A ameliorates
DACD via oxidative stress, inflammation and apoptosis. In addition,
Li et al (9) reported that
chrysin markedly alleviates DACD via oxidative stress, inflammation
and apoptosis (9).
Peroxisome proliferator-activated receptor γ (PPARγ)
belongs to the superfamily of nuclear receptors and is a
ligand-dependent transcription factor (12). PPARs regulate the gene expression
of various start regions containing PPAR response elements,
intracellular transcription levels and fatty acid and glucose
metabolism, and inhibit the inflammatory response (12). Tharaheswari et al (13) reported that trigonel-line and
diosgenin attenuate endoplasmic reticulum stress and enhance
adipose tissue PPARγ activity in T2DM rats. Capobianco et al
(14) demonstrated that PPAR
activation regulates nitric oxide production, lipid concentration
and lipoperoxidation in the placenta of patients with T2DM. In
addition, pioglitazone ameliorates memory deficits in diabetic mice
via the activation of PPARγ (15).
Naringenin is a natural dihydro flavonoid, which is
widely distributed in grapefruit and other citrus fruits and may
also be chemically synthesized (16). Previous studies have demonstrated
that naringenin exhibits various pharmacological effects, including
anti-tumor, anti-mutagenic and anti-atherosclerotic effects
(17–19). Studies have suggested that
naringenin may reverse the liver damage caused by drugs or toxic
chemical compounds, including alcohol, cadmium, carbon
tetrachloride, oxytetracycline and dimethyl nitrosamine (20–23).
The present study aimed to investigate the effects of naringenin on
DACD. In addition, the correlation between nuclear oxidative
stress, proinflammatory factors, PPARγ and DACD was
investigated.
Materials and methods
Animals
A total of 32 male 6-week-old Sprague-Dawley rats
(weight, 270±20 g) obtained from the experimental center of the
Dalian Medical University (Dalian, China) were selected for the
experimental procedures in the present study. All animal procedures
were performed in accordance with the guidelines of the First
Affiliated Hospital of the Dalian Medical University and were
approved by the Ethics Committee of Dalian Medical University
(Dalian, China). Prior to experimentation, the rats were provided
with ad libitum access to food and water, and were housed in
a laboratory animal room at 23±1°C in 50–70% humidity on a 12 h
light/dark cycle.
Drugs and chemicals
Naringin (purity, ≥98%) and STZ were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Glutathione peroxidase (GSH-Px;
S0056), glutathione (GSH; S0053), superoxide dismutase (SOD;
S0038), malondialdehyde (MDA; S0131), ELISA kits, bicinchoninic
acid (BCA; P0006) protein assay kits and caspase-3 (C1115) and
caspase-9 (C1157) activity test kits were purchased from Beyotime
Institute of Biotechnology (Haimen, China), and tumor necrosis
factor α (TNF-α; RT029) and interleukin (IL)-6 (RI001) were
purchased from Shanghai Gefan Biological Technology Co., Ltd.
(Shanghai, China).
Establishment of the diabetic rat
model
At the onset of the experiment, the body weights of
the experimental rats were measured. Plasma glucose levels were
then detected using an enzymatic glucose oxidase peroxidase
diagnostic kit. Fasting blood glucose levels >250 mg/dl were
considered diabetic and were used for further experimentation. The
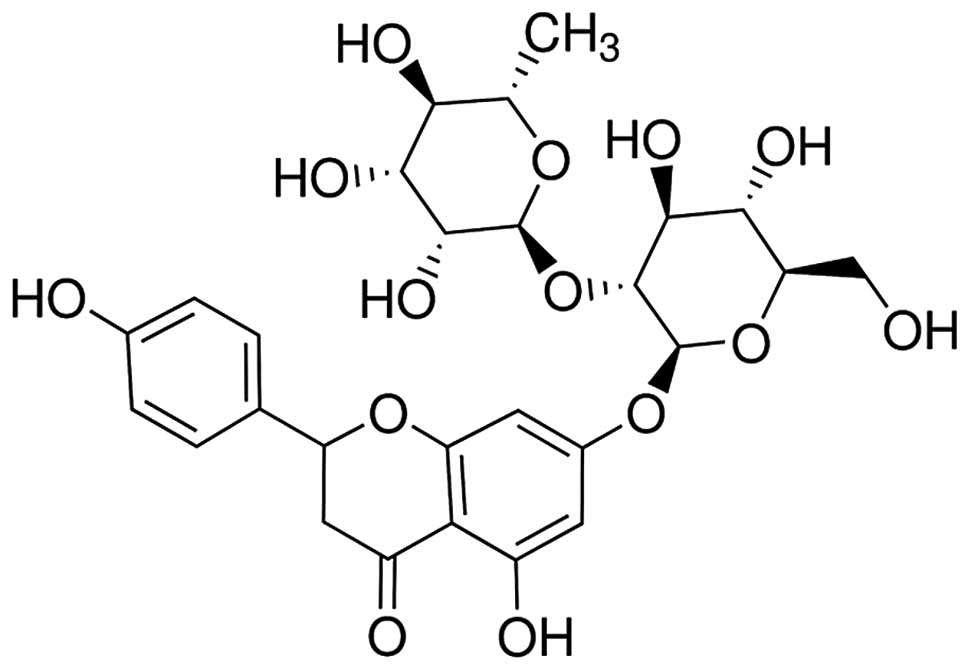
chemical structure of naringin is shown in Fig. 1. After 1 week of acclimatization,
the rats received a single intraperitoneal injection of STZ (50
mg/kg) to induce T2DM, with the exception of the normal healthy
controls, as previously described (24). At the end of the experiment (48 h
following injection), the body weight and blood glucose levels of
the experimental rats were measured.
Experimental design
In total, eight normal rats were injected with
physiological saline, defined as the control group; eight diabetic
rats were injected with physiological saline, defined as the DM
group; 24 diabetic rats were randomly divided into three groups,
and were treated with naringin at doses of 100 mg/kg and 200 mg/kg
into the caudal vein, as previously described (25). Rats from each group (4/group) were
immediately sacrificed by cervical dislocation under 30 mg/kg
pentobarbital (Sigma-Aldrich), and brain tissue and blood samples
were collected under anesthesia (300 mg/kg intraperitoneally). The
samples were stored at −80°C for further experimentation.
Morris water maze (MWM) assessment
Following treatment with naringin (100 and 200
mg/kg) for 16 weeks, MWM assessments were performed, as previously
described (26,27). Rats from each group (4/group) were
trained to swim freely with a circular Plexiglas platform (14 cm
diameter) submerged 1.5 cm beneath the surface of the water prior
to performing the MWM test. The platform was located in a fixed
position, equidistant from the center and the wall of the tank. The
rats were subjected to four training trials per day. The rats were
placed into the tank at one of the four designated start points per
day, in a pseudorandom order, and trained for as many days as
required to reach the criterion of 25 sec to reach the platform. If
the rats failed to find the platform within 60 sec, they were
manually guided to the platform and allowed to remain there for 5
sec. Following the final training session, a probe trial was
performed after 24 h, consisting of a 60 sec free swim in the pool
in the absence of the platform. The MWM assessments were recorded
via video capture and analyzed using a SMARTW system (PanLab,
Barcelona, Spain).
Oxidative stress assessment
Tissue sections (~5 mg) of the cerebral cortex and
hippocampus were added to 100 µl tissue lysis buffer
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) on ice, and incubated for 10 min. The homogenates were then
centrifuged at 20,000 × g for 15 min at 4°C. The clear supernatant
was collected and analyzed for oxidative stress. This was achieved
by quantifying the levels of GSH-Px, GSH, SOD and MDA in the
cerebral cortex and hippocampus, using commercially-available kits
(Beyotime Institute of Biotechnology), according to the
manufacturer's instructions.
TNF-α and IL-6 assessment
Tissue sections (~5 mg) of the cerebral cortex and
hippocampal samples were added to 100 µl tissue lysis buffer
on ice, and incubated for 10 min. The homogenates were then
centrifuged at 20,000 × g for 15 min at 4°C. TNF-α and IL-6 ELISA
kits (Beyotime Institute of Biotechnology) were used to quantify
the protein levels, according to the manufacturer's instructions,
and the samples were analyzed spectrophotometrically (Model 550;
Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 450 nm
absorbance.
Western blot analysis
Tissue sections (~5 mg) of the cerebral cortex and
hippocampal tissue samples were added to 100 µl tissue lysis
buffer on ice and incubated for 10 min. The homogenates were then
centrifuged at 20,000 × g for 15 min at 4°C. The supernatant was
collected and the protein concentration was measured using a BCA
protein assay kit. The protein was separated by 8–12% SDS-PAGE
(Sigma-Aldrich), and electrotransferred to polyvinyldene difluoride
membranes (0.22 mm; Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd., Beijing, China). The membranes were blocked with
tris-buffered saline (TBS; Wuhan Boster Biological Technology,
Ltd., Wuhan, China) containing 5% non-fat milk for 2 h. The
membranes were subsequently incubated with monoclonal mouse
anti-human PPARγ (sc-7273; 1:2,000; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and polyclonal mouse anti-human β-actin (bs-0061R;
1:500; BIOSS, Beijing, China) primary antibodies, overnight at 4°C.
The membranes were washed three times with TBS with Tween 20 (1%;
Sigma-Aldrich) for 2 h, and then incubated with anti-mouse
horseradish peroxidase-conjugated IgG (sc-2370; 1:3,000; Santa Cruz
Biotechnology, Inc.), for 2 h. The bands were visualized using an
ECL kit (Bio-Rad Laboratories, Inc.) and quantified using
densitometry (Image Quant LAS 4000 software; GE Healthcare Life
Sciences, Chalfont, UK).
Caspase-3 and caspase-9 activity level
quantification
Tissue sections (~5 mg) of the cerebral cortex and
hippocampal tissue samples were added to 100 µl tissue lysis
buffer on ice and incubated for 10 min. The homogenates were then
centrifuged at 20,000 × g for 15 min at 4°C. The activity levels of
caspase-3 and caspase-9 were measured using a caspase-3 and
caspase-9 activity test kit, according to the manufacturer's
instructions, and incubated at 37°C for 120 min. The levels of
caspase-3 and caspase-9 were measured using the Model 550
spectrophotometer at 405 nm.
Statistical analysis
Statistical analyses were performed using one-way
analysis of variance followed by Dunnett's test. Statistical
analyses were performed using SPSS 17.0 (SPSS, Inc., Chicago, IL,
USA), and the data are expressed as the mean ± standard deviation.
P<0.05 was considered to indicated a statistically significant
difference.
Results
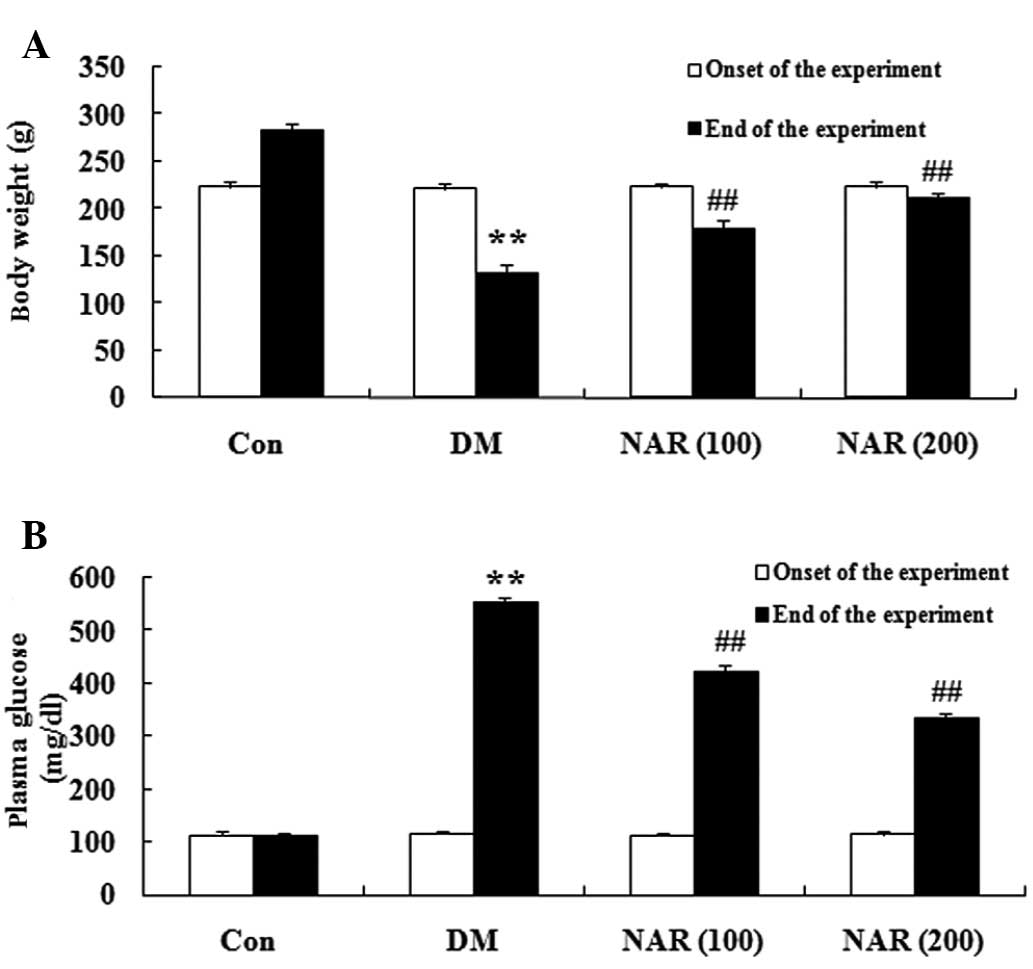
Effects of naringin on body weight and
blood glucose levels
The chemical structure of naringin is shown in
Fig. 1. The DM rats exhibited a
significant decrease in body weight and an increase in blood
glucose levels, compared with the normal control group. Following
treatment with naringin (100 and 200 mg/kg) for 16 weeks, the body
weights and blood glucose levels of the DM rats were significantly
increased and decreased, respectively, compared with those of the
untreated DM group (Fig.
2A–B).
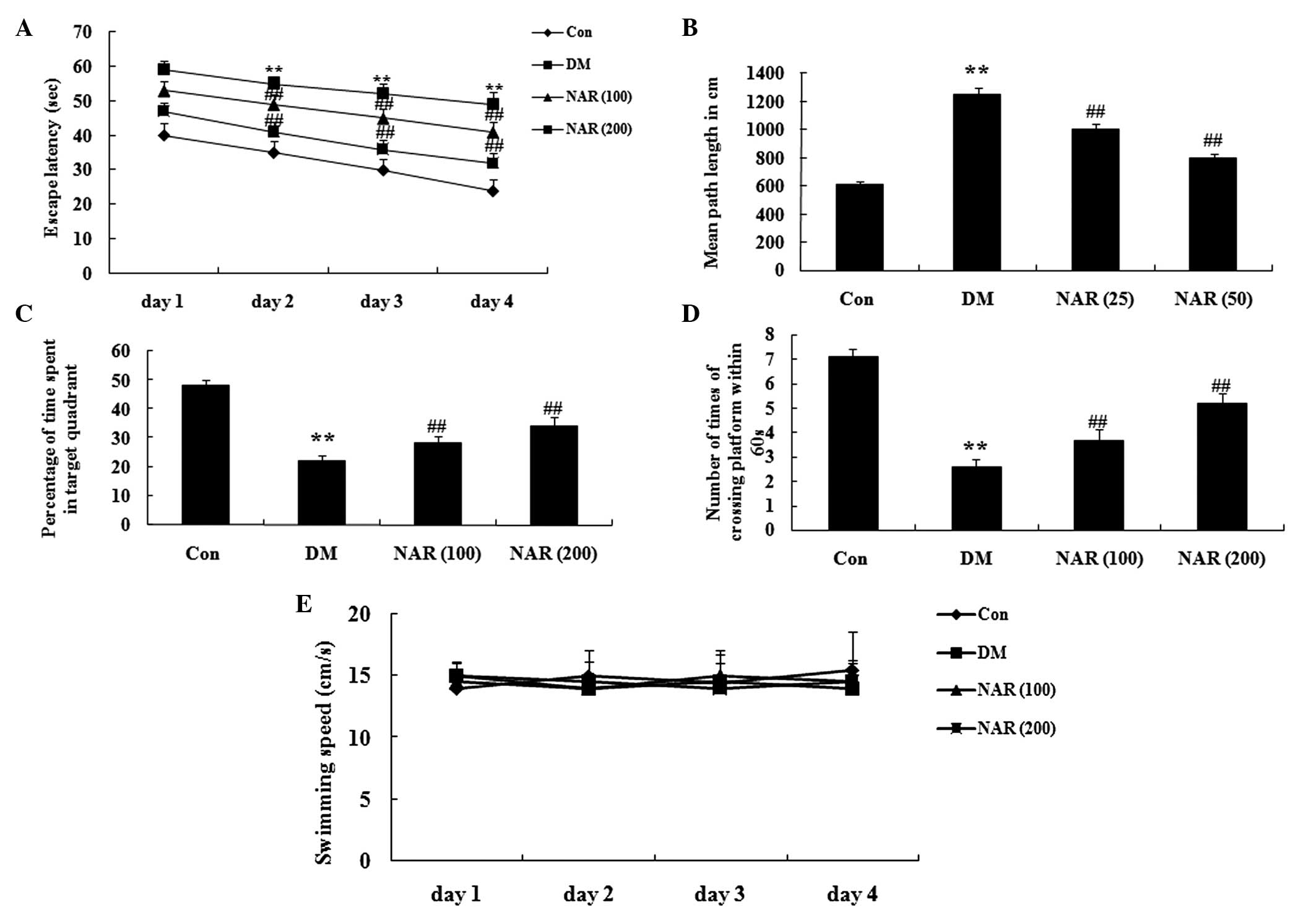
Effects of naringin on cognitive
deficit
Following 16 weeks treatment with naringin (100 and
200 mg/kg), a MWM test was used to assess cognitive function. The
escape latency markedly increased in the DM group, compared with
the control group (Fig. 3A).
Following treatment with naringin, the escape latency was reduced,
compared with that of the DM group (Fig. 3A). The mean path length
significantly increased in the DM rats, compared with the control
group. The mean path length following treatment with naringin was
significantly reduced, compared with that of the DM group (Fig. 3B). The duration spent in the target
quadrant and the number of times the rats crossed the former
platform location were significantly reduced in the DM group,
compared with those observed in the control group (Fig. 3C–D). Treatment with naringin
markedly reversed these effects in the DM rats (Fig. 3C–D). However, the swimming speed of
the rats in the control group was similar to those observed in the
other groups (Fig. 3E).
Effects of naringin on diabetes-induced
changes in oxidative stress
To examine the effects of naringin on oxidative
stress in the brain tissue, the expression levels of GSH-Px, GSH,
SOD and MDA were measured in the cerebral cortex and hippo-campus
tissue samples. As shown in Fig.
4A–C, the expression levels of GSH-Px, GSH and SOD were
significantly decreased in the cerebral cortex and hippocampus of
the DM group, compared with those of the control group. Treatment
of the STZ-induced diabetic rats with naringin (100 and 200 mg/kg)
significantly increased the expression levels of GSH-Px, GSH and
SOD in the cerebral cortex and hippocampus (Fig. 4A–C). In addition, the expression
levels of MDA in the DM group were significantly increased,
compared with those of the control group (Fig. 4D). Following treatment with
naringin (100 and 200 mg/kg) for 16 weeks, these expression levels
were significantly reduced (Fig.
4D).
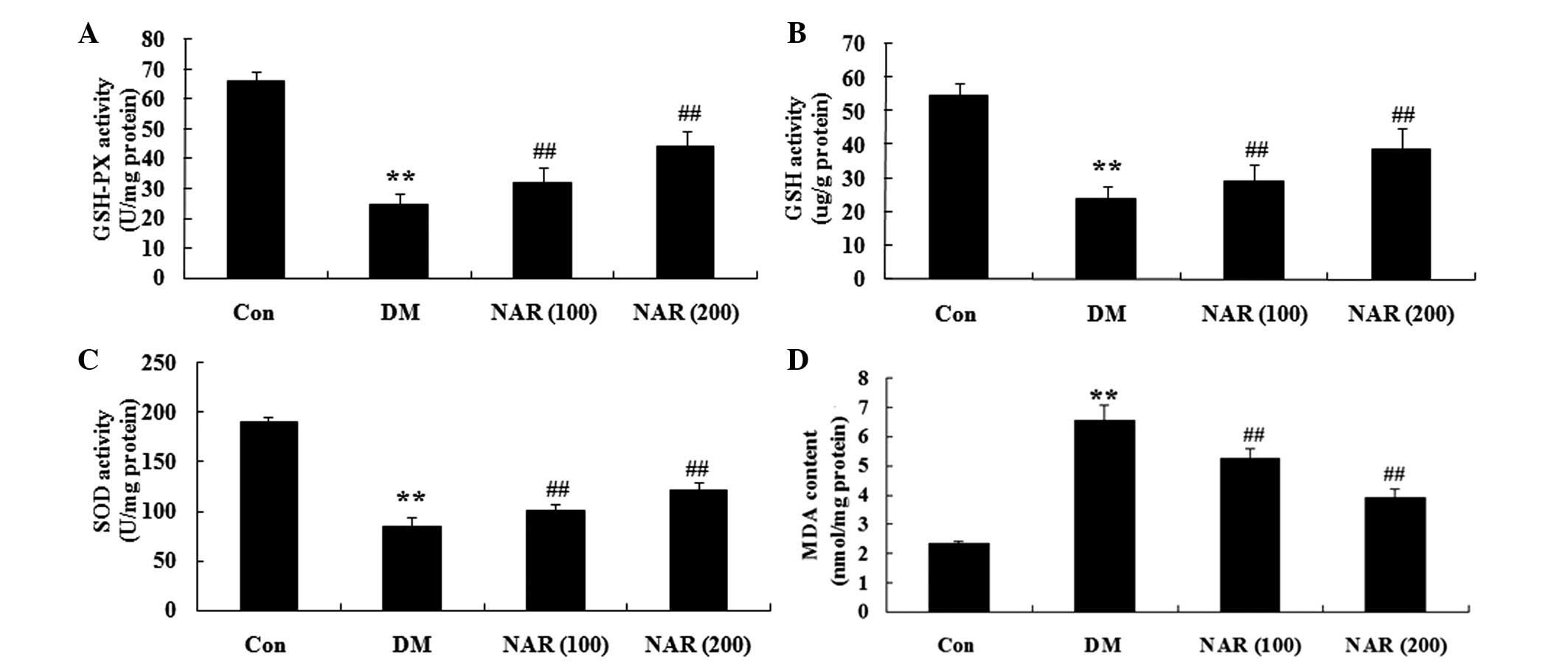 | Figure 4Effects of naringin on
diabetes-induced changes in oxidative stress. The effects of
naringin on the expression levels of (A) GSH-Px, (B) GSH, (C) SOD
and (D) MDA in the rats. Data are expressed as the mean ± standard
deviation. **P<0.01, vs the Con group;
##P<0.01, vs. the DM group. Con, control group; DM,
diabetes group; NAR (100), naringin (100 mg/kg)-treated group; NAR
(200), naringin (200 mg/kg)-treated group; GSH, glutathione;
GSH-Px, GSH peroxidase; SOD, superoxide dismutase; MDA,
malondialdehyde. |
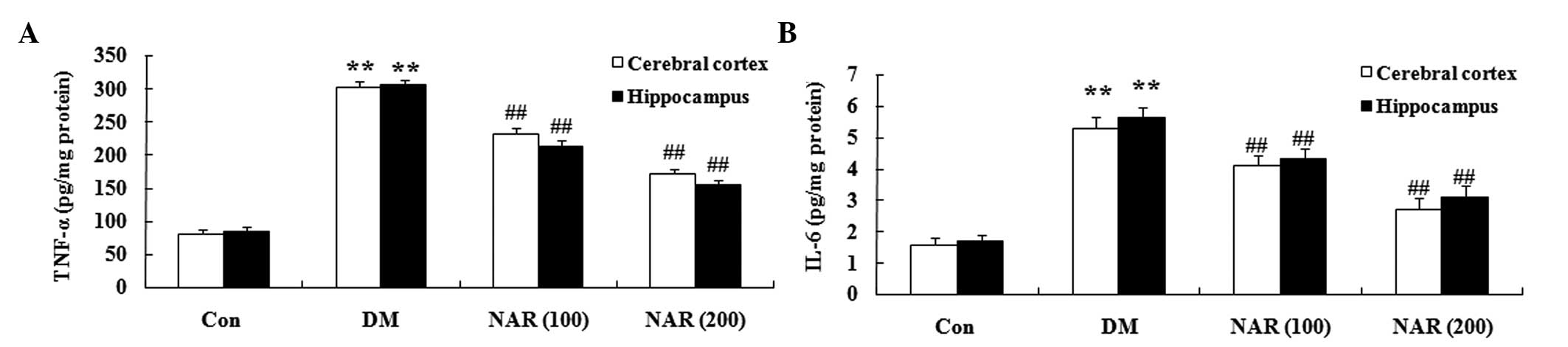
Effects of naringin on DM-induced changes
in proinflammatory cytokines
To determine the effects of naringin on the brain
proinflammatory cytokines in the DM rat, the expression levels of
TNF-α and IL-6 were measured in the cerebral cortex and
hippocampus. Compared with those of the control group, the
expression levels of TNF-α and IL-6 were significantly increased in
the cerebral cortex and hippocampus tissues of the STZ-induced DM
rats (Fig. 5A and B). Treatment
with naringin (100 and 200 mg/kg) significantly reversed the
elevated expression levels of TNF-α and IL-6 in the cerebral cortex
and hippocampus, compared with the DM group (Fig. 5A and B).
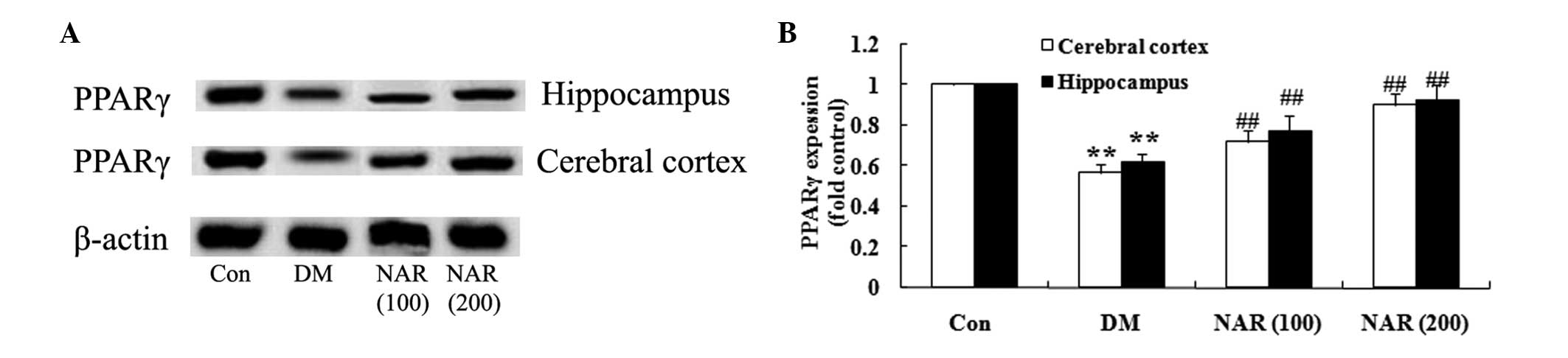
Effects of naringin on the expression
levels of PPARγ
To investigate whether naringin exerts its effects
through upregulation of the expression of PPARγ, the expression
levels of PPARγ were measured in the cerebral cortex and
hippo-campus tissues using western blotting. As shown in Fig. 6A and B, the expression levels of
PPARγ were significantly decreased in the cerebral cortex and
hippocampus of the DM group, compared with those of the control
group. Treatment of the STZ-induced DM rats with naringin (100 and
200 mg/kg) significantly increased the protein expression levels of
PPARγ in the cerebral cortex and hippocampus, compared with the DM
rats without naringin (Fig.
6A–B).
PPARγ inhibitor regulates the effects of
naringin on DACD
To confirm the observed results that the PPARγ
GW9662 inhibitor (0.3 mg/kg) regulated the effects of naringin on
DACD, a MWM test was performed. The PPARγ inhibitor significantly
decreased the protein expression levels of PPARγ (Fig. 7A–B). In addition, PPARγ inhibitor
reversed the effects of naringin on DACD following treatment with
naringin (100 and 200 mg/kg) for 16 weeks (Fig. 7C–G).
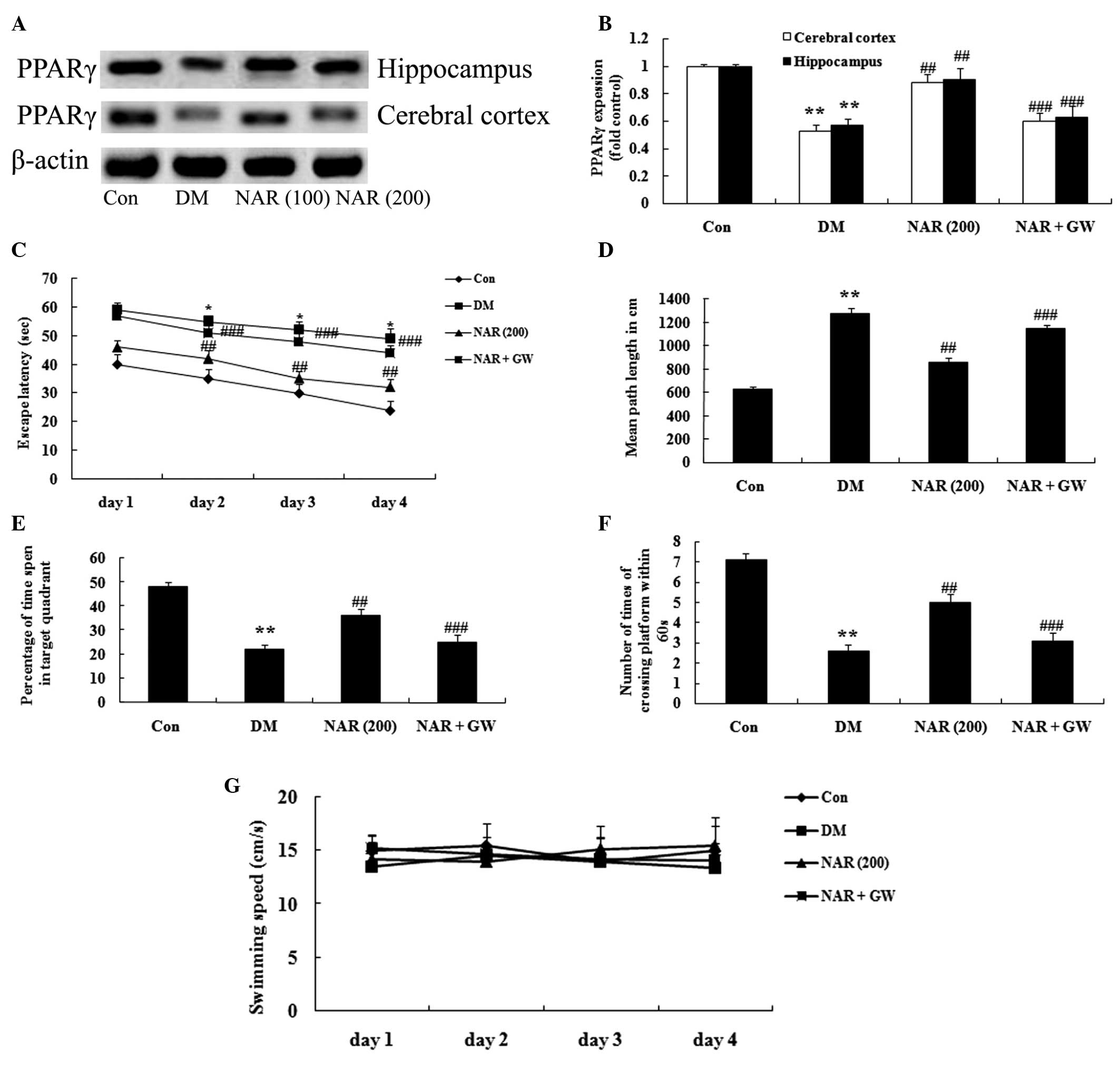 | Figure 7PPARγ inhibitor regulates the effects
of naringin on DACD. (A) Representative western blots and (B)
quantitative results of the protein expression of PPARγ in the
cerebral cortex and hippocampus. Effects of naringin on the (C)
escape latency, (D) mean path length, (E) mean percentage of time
spent in the target quadrant, (F) number of times of platform
crossing, and (G) swimming speed in the rats. Data are expressed as
the mean ± standard deviation. **P<0.01, vs. the Con
group; ##P<0.01, vs. the DM group; and
###P>0.05, vs. the DM group. Con, control group; DM,
diabetes group; NAR (200), naringin (200 mg/kg)-treated group;
NAR+GW (naringin, 200 mg/kg+GW9662, 0.3 mg/kg)-treated group.
PPARγ, peroxisome proliferator-activated receptor γ. |
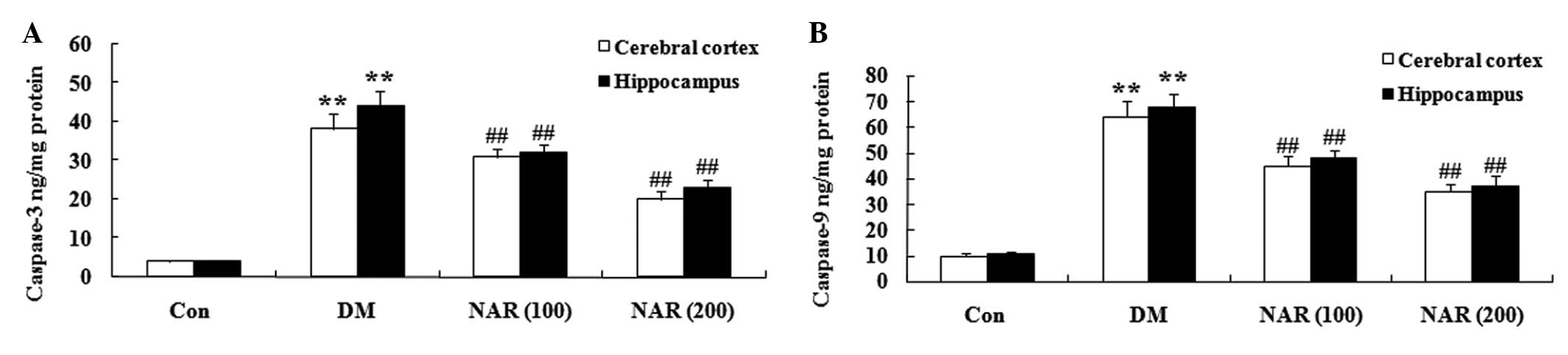
Effects of naringin on the expression
levels of caspase-3 and caspase-9
To examine whether naringin affected the levels of
caspase-3 and caspase-9, the expression levels of caspase-3 and
caspase-9 were measured in the cerebral cortex and hippo-campus
tissues using ELISA assays. As shown in Fig. 8A and B, naringin notably increased
the expression levels of caspase-3 and caspase-9 in the cerebral
cortex and hippocampus of the DM group, compared with the control
group. Treatment with naringin (100 and 200 mg/kg) decreased the
expression levels of caspase-3 and caspase-9 in the cerebral cortex
and hippo-campus, compared with the control group (Fig. 8A and B).
Discussion
With changes in lifestyles and aging of the
population, T2DM incidence is increasing year by year (28). As a systemic disease, T2DM can
cause a variety of organizational, structural and functional
changes in organs, and the lesions can affect the whole body.
Therefore, early recognition and treatment of DACD can delay and
reduce the incidence of dementia, and improve the life quality of
patients with T2DM (29). In the
present study, naringin significantly increased the body weight and
reduced the levels of blood glucose in DM rats. Oršolić et
al (30) reported that the
DNA-protective effects of naringenin increased the body weight of
alloxan-induced diabetic mice. Naringenin has also been observed to
markedly normalize the body weights of albino mice (31). Priscilla et al (32) demonstrated that naringenin reduces
the postprandial glycemic response in DM rats. The results of the
present study demonstrated that naringen effectively improved the
cognitive deficit of DM rats.
The biological basis of DACD in cognitive impairment
may be associated with abnormal brain development, in which a large
number of reactive oxygen species produced by oxidative stress and
secondary cell injury are important (33). Under normal circumstances, the body
has an effective antioxidant defense mechanism, and SOD is one of
the most important antioxidant enzymes (34). An increase in the levels of
reactive oxygen species can cause lipid peroxidation, DNA damage
and cell-associated molecule or gene regulation, thereby inducing
the nerve cell damage characteristic of certain cognitive deficits
(35). The compensatory activity
of antioxidant enzyme SOD is then increased. Therefore, the
possible biological basis of DACD may be associated with SOD.
Oxidized low density lipoprotein is highly cytotoxic, which can
lead to vascular endothelial cell necrosis, and it closely
associated with the occurrence of atherosclerosis and other
cardiovascular and cerebrovascular diseases (36). MDA is a major metabolite involved
in the biological membrane damage by free radicals, and can cause
disorders of protein synthesis, which results in memory and mental
decline (36). The levels of MDA
reflect the degree of lipid peroxidation in the body (36). The results of the present study
further determined that the effects of naringin markedly increased
the expression levels of GSH-Px, GSH and SOD in the cerebral cortex
and hippocampus of the DM rats. In addition, the expression levels
of MDA were significantly reduced following treatment with naringin
in the DM rats. Previous studies have demonstrated that naringenin
has a similar protective effects to L-arginine in
monocrotaline-induced pulmonary hypertension through oxidative
stress, inflammation and nitric oxide in rats (37). Jeon et al (38) reported that naringenin increases
the expression levels of GSH-Px, GSH and SOD in rats fed a
high-cholesterol diet. Hermenean et al (39) demonstrated that naringenin
increases the expression of MDA and decreases the expression level
of SOD, catalase, GSH and GSH-Px in the mouse kidney (39).
TNF-α is a type of cytokine produced by activated
monocytes, which exhibits a wide range of activities (40). TNF-α may be involved in nerve
damage, and a previous study on the brain neuroinflammatory
response of patients with dementia demonstrated that the primary
deposit of TNF-α is β-amyloid, which is one of the causes of
neurodegenerative dementia (41).
IL-6 is produced by monocytes or macrophages, and is a stimulating
factor produced by several cells in vivo; IL-6 exhibits a
wide range of biological activities, and is an important member in
the complex network of cytokines in the body, which are involved in
various pathophysiological processes (42). An increase in the expression levels
of IL-6 in the plasma of elderly patients is closely associated
with cognitive impairment, which is a risk factor for cognitive
dysfunction, and the rise of inflammatory factors in the plasma of
patients occurs prior to the clinical diagnosis of dementia,
suggesting that inflammatory cytokines of the peripheral blood may
be involved in the pathophysiology of dementia (43). The results of the present study
demonstrated that naringin decreased the expression levels of TNF-α
and IL-6 in the cerebral cortex and hippocampus of DM rats. A
previous study demonstrated that naringin significantly decreased
the production and expression levels of IL-1β and IL-6 in diabetic
mice (44). The inhibition of
TNF-α and IL-6 by naringenin may contribute to its
anti-inflammatory activity in rats with ethanol-induced liver
injury (45). Bodet et al
(46) also reported that naringin
exhibits anti-inflammatory properties in macrophages.
PPARs are a type of ligand-activated nuclear
transcription factor that regulate the expression of several key
genes, including those involved in glucose and lipid metabolism
(47). There are three subtypes of
PPARs: PPAR-α, PPAR-γ and PPAR-δ. PPAR-γ is an important
transcription factor in lipogenesis, and promotes adipocyte
differentiation, increased insulin sensitivity and lower blood
sugar levels (48). PPAR-γ reduces
the levels of blood fat and increases insulin sensitivity.
Therefore, PPAR-γ has become the focus of investigations on anti-DM
drugs (49). In the cerebral
cortex and hippocampus, naringin activated the protein expression
of PPARγ in DM rats. Sharma et al (50) provided evidence that naringin
ameliorates insulin resistance, hepatic steatosis and kidney damage
in T2DM rats by regulating oxidative stress, inflammation and
upregulation of PPARγ. Naringin reduces ethanol intake and
ethanol-conditioned place preference in mice (51). The present study demonstrated that
the PPARγ inhibitor, GW9662, decreased the protein expression of
PPARγ, as well as the effects of naringin on the cognitive deficit
of the DM rats. These results suggested that naringin enhanced the
cognitive deficit of DM rats via the upregulation of PPARγ.
The results of the present study demonstrated that
naringin reduced the expression levels of caspase-3 and caspase-9
in the cerebral cortex and hippocampus of DM rats, and decreased
cell apoptosis in the brain tissues of the DM rats. Treatment with
naringin improves functional recovery via the inhibition of
caspase-3 following spinal cord injury in rats (52). In addition, naringin reduces the
levels of 3-nitropropionic acid-induced apoptosis through decreased
caspase-3 activation (53).
In conclusion, the present study demonstrated that
naringin significantly ameliorated cognitive deficits via oxidative
stress, and the proinflammatory and PPARγ signaling pathways in
T2DM rats. Future research will focus on the characterization of
naringin, and aim to investigate the therapeutic effects of
naringin on DACD in vitro and in vivo.
Acknowledgments
The current study was supported by the National
Natural Scientific Foundation Project (grant no. 81371454; 2013);
the Natural Scientific Foundation Project of Liaoning Province
(grant no. 2014023021; 2014); the Dalian Natural Scientific
Foundation Project (grant no. 2014E145F176); and the Natural
Science Foundation of China (grant no. 30872665 to Mr. Bin Dong;
2008).
References
|
1
|
Lin Z, Gu J, Xiu J, Mi T, Dong J and
Tiwari JK: Traditional chinese medicine for senile dementia. Evid
Based Complement Alternat Med. 2012:6926212012. View Article : Google Scholar
|
|
2
|
Wang YB, Wang S, Bai R, Du JL, Xing Q, Ba
Y, Yang Y, Zhang XY, Shi CH and Yao JJ: Efficacy of switching from
premixed insulin to insulin glargine regimen in Type 2 diabetes
mellitus patients with different islet functions. Mol Med Rep.
10:1096–1102. 2014.PubMed/NCBI
|
|
3
|
Tonoli C, Heyman E, Roelands B, Pattyn N,
Buyse L, Piacentini MF, Berthoin S and Meeusen R: Type 1
diabetes-associated cognitive decline: A meta-analysis and update
of the current literature. J Diabetes. 6:499–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen J, Liang L, Zhan L, Zhou Y, Zheng L,
Sun X, Gong J, Sui H, Jiang R, Zhang F and Zhang L: ZiBuPiYin
recipe protects db/db mice from diabetes-associated cognitive
decline through improving multiple pathological changes. PLoS One.
9:e916802014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang XH, Liang LN, Zhan LB, Lu XG, Shi X,
Qi X, Feng ZL, Wu MJ, Sui H, Zheng LP, et al: The effect of Chinese
Jinzhida recipe on the hippocampus in a rat model of
diabetes-associated cognitive decline. BMC Complement Altern Med.
13:1612013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuhad A and Chopra K: Effect of sesamol on
diabetes-associated cognitive decline in rats. Exp Brain Res.
185:411–420. 2008. View Article : Google Scholar
|
|
7
|
Malardé L, Groussard C, Lefeuvre-Orfila L,
Vincent S, Efstathiou T and Gratas-Delamarche A: Fermented soy
permeate reduces cytokine level and oxidative stress in
streptozotocin-induced diabetic rats. J Med Food. 18:67–75. 2015.
View Article : Google Scholar
|
|
8
|
Wang SB and Jia JP: Oxymatrine attenuates
diabetes-associated cognitive deficits in rats. Acta Pharmacol Sin.
35:331–338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li R, Zang A, Zhang L, Zhang H, Zhao L, Qi
Z and Wang H: Chrysin ameliorates diabetes-associated cognitive
deficits in Wistar rats. Neurol Sci. 35:1527–1532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maczurek A, Hager K, Kenklies M, Sharman
M, Martins R, Engel J, Carlson DA and Münch G: Lipoic acid as an
anti-inflammatory and neuroprotective treatment for Alzheimer's
disease. Adv Drug Deliv Rev. 60:1463–1470. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mao XY, Cao DF, Li X, Yin JY, Wang ZB,
Zhang Y, Mao CX, Zhou HH and Liu ZQ: Huperzine A ameliorates
cognitive deficits in streptozotocin-induced diabetic rats. Int J
Mol Sci. 15:7667–7683. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Umemoto T and Fujiki Y: Ligand-dependent
nucleocytoplasmic shuttling of peroxisome proliferator-activated
receptors, PPARα and PPARγ. Genes Cells. 17:576–596. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tharaheswari M, Jayachandra Reddy N, Kumar
R, Varshney KC, Kannan M and Sudha Rani S: Trigonelline and
diosgenin attenuate ER stress, oxidative stress-mediated damage in
pancreas and enhance adipose tissue PPAR γ activity in type 2
diabetic rats. Mol Cell Biochem. 396:161–174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Capobianco E, Martinez N, Fornes D, Higa
R, Di Marco I, Basualdo MN, Faingold MC and Jawerbaum A: PPAR
activation as a regulator of lipid metabolism, nitric oxide
production and lipid peroxidation in the placenta from type 2
diabetic patients. Mol Cell Endocrinol. 377:7–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu LP, Yan TH, Jiang LY, Hu W, Hu M, Wang
C, Zhang Q, Long Y, Wang JQ, Li YQ, et al: Pioglitazone ameliorates
memory deficits in streptozotocin-induced diabetic mice by reducing
brain β-amyloid through PPARγ activation. Acta Pharmacol Sin.
34:455–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi Y, Tan Y, Mao S and Gu W: Naringenin
inhibits allergen-induced airway remodeling in a murine model of
asthma. Mol Med Rep. 9:1204–1208. 2014.PubMed/NCBI
|
|
17
|
Arul D and Subramanian P: Naringenin
(citrus flavonone) induces growth inhibition, cell cycle arrest and
apoptosis in human hepatocellular carcinoma cells. Pathol Oncol
Res. 19:763–770. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimizu T, Lin F, Hasegawa M, Okada K,
Nojiri H and Yamane H: Purification and identification of
naringenin 7-O-methyltransferase, a key enzyme in biosynthesis of
flavonoid phytoalexin sakuranetin in rice. J Biol Chem.
287:19315–19325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee S, Lee CH, Moon SS, Kim E, Kim CT, Kim
BH, Bok SH and Jeong TS: Naringenin derivatives as anti-atherogenic
agents. Bioorg Med Chem Lett. 13:3901–3903. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jayaraman J, Veerappan M and Namasivayam
N: Potential beneficial effect of naringenin on lipid peroxidation
and antioxidant status in rats with ethanol-induced hepatotoxicity.
J Pharm Pharmacol. 61:1383–1390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Renugadevi J and Prabu SM: Cadmium-induced
hepatotoxicity in rats and the protective effect of naringenin. Exp
Toxicol Pathol. 62:171–181. 2010. View Article : Google Scholar
|
|
22
|
Yen FL, Wu TH, Lin LT, Cham TM and Lin CC:
Naringenin-loaded nanoparticles improve the physicochemical
properties and the hepatoprotective effects of naringenin in
orally-administered rats with CCl (4)-induced acute liver failure.
Pharm Res. 26:893–902. 2009. View Article : Google Scholar
|
|
23
|
Pari L and Gnanasoundari M: Influence of
naringenin on oxytetracycline mediated oxidative damage in rat
liver. Basic Clin Pharmacol Toxicol. 98:456–461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Siddiqui O, Sun Y, Liu JC and Chien YW:
Facilitated transdermal transport of insulin. J Pharm Sci.
76:341–345. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu W, Wu J, Cai F, Xiang J, Zha W, Fan D,
Guo S, Ming Z and Liu C: Curcumin alleviates diabetic
cardiomyopathy in experimental diabetic rats. PLoS One.
7:e520132012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Liu H, Zhang Y, Li J, Teng X, Liu
A, Yu X, Shan Z and Teng W: Effects of isolated positive maternal
thyroglobulin antibodies on brain development of offspring in an
experimental autoimmune thyroiditis model. Thyroid. 25:551–558.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stemper BD, Shah AS, Pintar FA, McCrea M,
Kurpad SN, Glavaski-Joksimovic A, Olsen C and Budde MD: Head
rotational acceleration characteristics influence behavioral and
diffusion tensor imaging outcomes following concussion. Ann Biomed
Eng. 43:1071–1088. 2015. View Article : Google Scholar
|
|
28
|
Uslu S, Kebapçi N, Kara M and Bal C:
Relationship between adipocytokines and cardiovascular risk factors
in patients with type 2 diabetes mellitus. Exp Ther Med. 4:113–120.
2012.PubMed/NCBI
|
|
29
|
Fang H, Luo X, Wang Y, Liu N, Fu C, Wang
H, Fang Y, Shi W, Zhang Y, Zeng C and Wang X: Correlation between
single nucleotide polymorphisms of the ACTA2 gene and coronary
artery stenosis in patients with type 2 diabetes mellitus. Exp Ther
Med. 7:970–976. 2014.PubMed/NCBI
|
|
30
|
Oršolić N, Gajski G, Garaj-Vrhovac V,
Dikić D, Prskalo ZŠ and Sirovina D: DNA-protective effects of
quercetin or naringenin in alloxan-induced diabetic mice. Eur J
Pharmacol. 656:110–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roy A, Das A, Das R, Haldar S,
Bhattacharya S and Haldar PK: Naringenin, a citrus flavonoid,
ameliorates arsenic-induced toxicity in swiss albino mice. J
Environ Pathol Toxicol Oncol. 33:195–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Priscilla DH, Roy D, Suresh A, Kumar V and
Thirumurugan K: Naringenin inhibits α-glucosidase activity: A
promising strategy for the regulation of postprandial hyperglycemia
in high fat diet fed streptozotocin induced diabetic rats. Chem
Biol Interact. 210:77–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, He H, Li D, Zhu W, Duan K, Le Y,
Liao Y and Ou Y: The role of the TLR4 signaling pathway in
cognitive deficits following surgery in aged rats. Mol Med Rep.
7:1137–1142. 2013.PubMed/NCBI
|
|
34
|
Zhu X, Su B, Wang X, Smith MA and Perry G:
Causes of oxidative stress in Alzheimer disease. Cell Mol Life Sci.
64:2202–2210. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kuhad A, Sethi R and Chopra K: Lycopene
attenuates diabetes-associated cognitive decline in rats. Life Sci.
83:128–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu YW, Zhu X, Yang QQ, Lu Q, Wang JY, Li
HP, Wei YQ, Yin JL and Yin XX: Suppression of methylglyoxal
hyperactivity by mangiferin can prevent diabetes-associated
cognitive decline in rats. Psychopharmacology (Berl). 228:585–594.
2013. View Article : Google Scholar
|
|
37
|
Ahmed LA, Obaid AA, Zaki HF and Agha AM:
Naringenin adds to the protective effect of L-arginine in
monocrotaline-induced pulmonary hypertension in rats: Favorable
modulation of oxidative stress, inflammation and nitric oxide. Eur
J Pharm Sci. 62:161–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jeon SM, Kim HK, Kim HJ, Do GM, Jeong TS,
Park YB and Choi MS: Hypocholesterolemic and antioxidative effects
of naringenin and its two metabolites in high-cholesterol fed rats.
Transl Res. 149:15–21. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hermenean A, Ardelean A, Stan M, Herman H,
Mihali CV, Costache M and Dinischiotu A: Protective effects of
naringenin on carbon tetrachloride-induced acute nephrotoxicity in
mouse kidney. Chem Biol Interact. 205:138–147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lichte P, Grigoleit JS, Steiner EM,
Kullmann JS, Schedlowski M, Oberbeck R and Kobbe P: Low dose LPS
does not increase TLR4 expression on monocytes in a human in vivo
model. Cytokine. 63:74–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li J, Wang Y, Zhou Y and Liu J: Gastric
bypass surgery alters the mechanisms of insulin resistance in the
adipose tissue of GK rats. Mol Med Rep. 6:1111–1116.
2012.PubMed/NCBI
|
|
42
|
Ross JH, Hardy DC, Schuyler CA, Slate EH,
Mize TW and Huang Y: Expression of periodontal interleukin-6
protein is increased across patients with neither periodontal
disease nor diabetes, patients with periodontal disease alone and
patients with both diseases. J Periodontal Res. 45:688–694. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Serlin Y, Levy J and Shalev H: Vascular
pathology and blood-brain barrier disruption in cognitive and
psychiatric complications of type 2 diabetes mellitus. Cardiovasc
Psychiatry Neurol. 2011:6092022011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tsai SJ, Huang CS, Mong MC, Kam WY, Huang
HY and Yin MC: Anti-inflammatory and antifibrotic effects of
naringenin in diabetic mice. J Agric Food Chem. 60:514–521. 2012.
View Article : Google Scholar
|
|
45
|
Jayaraman J, Jesudoss VA, Menon VP and
Namasivayam N: Anti-inflammatory role of naringenin in rats with
ethanol induced liver injury. Toxicol Mech Methods. 22:568–576.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bodet C, La VD, Epifano F and Grenier D:
Naringenin has anti-inflammatory properties in macrophage and ex
vivo human whole-blood models. J Periodontal Res. 43:400–407. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pejcić T, Stanković I, Petković TR,
Borovac DN, Djordjević I and Jeftović-Stoimenov T: Peroxisome
proliferator-activated receptor gamma as modulator of inflammation
in pulmonary sarcoidosis. Srp Arh Celok Lek. 141:705–709. 2013.
View Article : Google Scholar
|
|
48
|
Grygiel-Gorniak B: Peroxisome
proliferator-activated receptors and their ligands: Nutritional and
clinical implications - a review. Nutr J. 13:172014. View Article : Google Scholar
|
|
49
|
Liu Q, Chen L, Hu L, Guo Y and Shen X:
Small molecules from natural sources, targeting signaling pathways
in diabetes. Biochim Biophys Acta. 1799:854–865. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sharma AK, Bharti S, Ojha S, Bhatia J,
Kumar N, Ray R, Kumari S and Arya DS: Up-regulation of PPARγ, heat
shock protein-27 and -72 by naringin attenuates insulin resistance,
β-cell dysfunction, hepatic steatosis and kidney damage in a rat
model of type 2 diabetes. Br J Nutr. 106:1713–1723. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bahi A, Nurulain SM and Ojha S: Ethanol
intake and ethanol-conditioned place preference are reduced in mice
treated with the bioflavonoid agent naringin. Alcohol. 48:677–685.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rong W, Wang J, Liu X, Jiang L, Wei F, Hu
X, Han X and Liu Z: Naringin treatment improves functional recovery
by increasing BDNF and VEGF expression, inhibiting neuronal
apoptosis after spinal cord injury. Neurochem Res. 37:1615–1623.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gopinath K, Prakash D and Sudhandiran G:
Neuroprotective effect of naringin, a dietary flavonoid against
3-nitropropionic acid-induced neuronal apoptosis. Neurochem Int.
59:1066–1073. 2011. View Article : Google Scholar : PubMed/NCBI
|