Introduction
Oxidative stress is a mechanism commonly implicated
in neurodegenerative diseases, including Alzheimer's disease,
Parkinson's disease and amyotrophic lateral sclerosis (1–3).
There is increasing evidence that the production of reactive oxygen
species (ROS) during oxidative stress leads to mitochondrial
dysfunction and apoptosis (4–7). A
previous study demonstrated that numerous chemical and
physiological inducers of oxidative stress result in apoptosis
(7). Among them,
H2O2 has been extensively used to induce
oxidative stress in vitro (8). The products of
H2O2, superoxide and hydroxyl radicals, are
the major components of ROS.
A crucial balance between ROS generation and
antioxidant defence is important in disease prevention.
Antioxidants are able to help reduce neuronal degeneration by
preventing the generation of free radicals (9–14).
However, the synthetic antioxidants are associated with toxicity
and are potential carcinogens (15). Therefore, the development of
non-toxic and highly active antioxidant compounds is important.
Luteolin (3,4,5,7-tetrahydroxylflavone) is a
component of numerous traditional Chinese medicines, and is a
flavonoid compound derived from Lonicera japonica Thunb.
Luteolin has been demonstrated to possess numerous biological
effects, including anti-inflammatory, anti-oxidative and
anticarcinogenic activity (16–19).
Luteolin has been previously used in pharmacological and clinical
practice (20,21). The current study investigated
whether luteolin has protective effects against
H2O2-induced apoptosis in rat
pheochromocytoma cells (PC12) cells, and the potential signaling
pathways involved were explored.
Materials and methods
Materials
PC12 cells were obtained from the American Type
Culture Collection (Manassas, VA, USA). All cell culture medium
components were purchased from Invitrogen Life Technologies
(Carlsbad, CA, USA). H2O2 was purchased from
Sigma-Aldrich (St. Louis, MO, USA). LY294002 was supplied by EMD
Millipore (Billerica, MA, USA). Luteolin was obtained from Chengdu
Must Biotechnology Co., Ltd. (Chengdu, China) and the purity of the
chemical was >98.0%.
Cell culture and treatment
PC12 cells (1×105) were grown (100
µl/well in 96-well plates) in Dulbecco's modified Eagle's
medium supplemented with 10% fetal calf serum, 1% penicillin and
streptomycin at 37°C and 5% CO2 and 95% air for 24 h.
Cells were used for experiments during the exponential growth
phase. PC12 cells were preconditioned with different concentrations
of luteolin (10, 25 and 50 µg/ml) for 1 h, whereas the
control cells received 0.9% saline (Beyotime Institute of
Biotechnology, Nantong, China) instead. Subsequently, PC12 cells
were exposed to H2O2 (400 µM, final
concentration) for 6 h.
Cell viability assay
Cell viability was determined using the MTT
(3-(4,5)-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide) reduction assay. Following H2O2 (400
µM) treatment alone or with different concentrations of
luteolin for 6 h, cells were incubated with 20 µl MTT
(Beyotime Institute of Biotechnology) for 4 h. Cells were
pretreated with phosphoinositide 3-kinase (PI3K) inhibitor LY294002
(60 µM) for 1 h at 37°C to investigate the role of protein
kinase B (Akt) in the effect of luteolin (50 µg) on PC12
cells. Absorbance was measured at 570 nm (iMark; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and used to calculate the
relative ratio of cell viability.
Cytotoxicity assay
Cell death was assessed by measuring LDH release
into the medium (22). Following
H2O2 (400 µM) treatment alone or with
different concentrations of luteolin for 6 h, the medium was
collected. LDH release was measured according to the manufacturer's
instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China).
Measurement of intracellular ROS
generation
Intracellular ROS levels were determined using
fluorescent 2′,7′-dichlorofluorescein (DCF) derived from
cell-permeable dichlorodihydrofluorescein diacetate (DCFH-DA) from
Nanjing Jiancheng Bioengineering Institute (Nanjing, China)
(23). Following treatment with
H2O2 (400 µM) alone or with different
concentrations of luteolin for 6 h, PC12 cells were incubated with
200 µl medium containing 2 µl 20 mM DCFH-DA solution
for 30 min in the dark at 37°C and 5% CO2. Subsequently,
cells were washed twice with normal medium (PBS; pH 7.4; Beyotime
Institute of Biotechnology) and DCF fluorescence was measured with
excitation/emission wavelengths of 485/530 nm (BX50-FLA; Olympus
Corporation, Tokyo, Japan).
Measurement of superoxide dismutase
(SOD), glutathione peroxidase (GSH-Px) and malondialdehyde (MDA)
levels
Cells were harvested by centrifugation at 1,380 × g
at 4°C for 5 min, washed with cold phosphate-buffered saline (PBS;
Gibco Life Technologies; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) twice and homogenized in lysis buffer containing 20 mM
Tris (pH 7.5), 150 mM NaCl, 1% Triton X-100 and 1 mM PMSF. The
supernatant was then collected. The levels of SOD, GSH-Px and MDA
were measured according to the manufacturer's instructions of the
respective kits (Nanjing Jiancheng Bioengineering Institute).
Western blotting
Following H2O2 (400 µM)
treatment alone or with different concentrations of luteolin for 6
h, PC12 cells were washed with cold PBS and homogenized in lysis
buffer containing proteinase inhibitors. Following measurement of
protein levels using a Bicinchoninic Acid Protein Assay kit
(Beyotime Institute of Biotechnology), protein was mixed with 5X
SDS sample buffer. Subsequently proteins were separated using 10%
SDS-PAGE and transferred onto polyvinylidene difluoride membranes
(EMD Millipore, Billerica, MA, USA). Following blocking with 5%
fat-free milk for 2 h at room temperature, the membranes were
incubated overnight at 4°C with polyclonal antibodies specific to
Akt (anti-mouse; 1:1,000 dilution; cat. no. SAB4500797;
Sigma-Aldrich), phosphorylated Akt (p-Akt; anti-mouse, 1:1,000
dilution; cat. no. SAB4301414; Sigma-Aldrich), Bcl-2 (anti-mouse;
1:1,000 dilution; cat. no. SAB1305653; Sigma-Aldrich), Bax
(anti-mouse; 1:1,000 dilution; cat. no. B3428; Sigma-Aldrich) and
β-actin (anti-mouse; 1:1,000 dilution; cat. no. A1978;
Sigma-Aldrich). Subsequently, the membranes were incubated with the
corresponding secondary antibodies (anti-rabbit; 1:1,000 dilution;
cat. no. SE7; Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) at room temperature for 2 h. The blots were
visualized using enhanced chemiluminescence-plus reagent (EMD
Millipore), and analyzed using LabImage software, version 2.7.1
(Kapelan GmbH, Halle, Germany).
Statistical analysis
All the experiments were performed a minimum of
three times. Values are presented as the mean ± standard deviation.
Differences between groups were analyzed using a one-way analysis
of variance with SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA),
followed by Dunnett's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
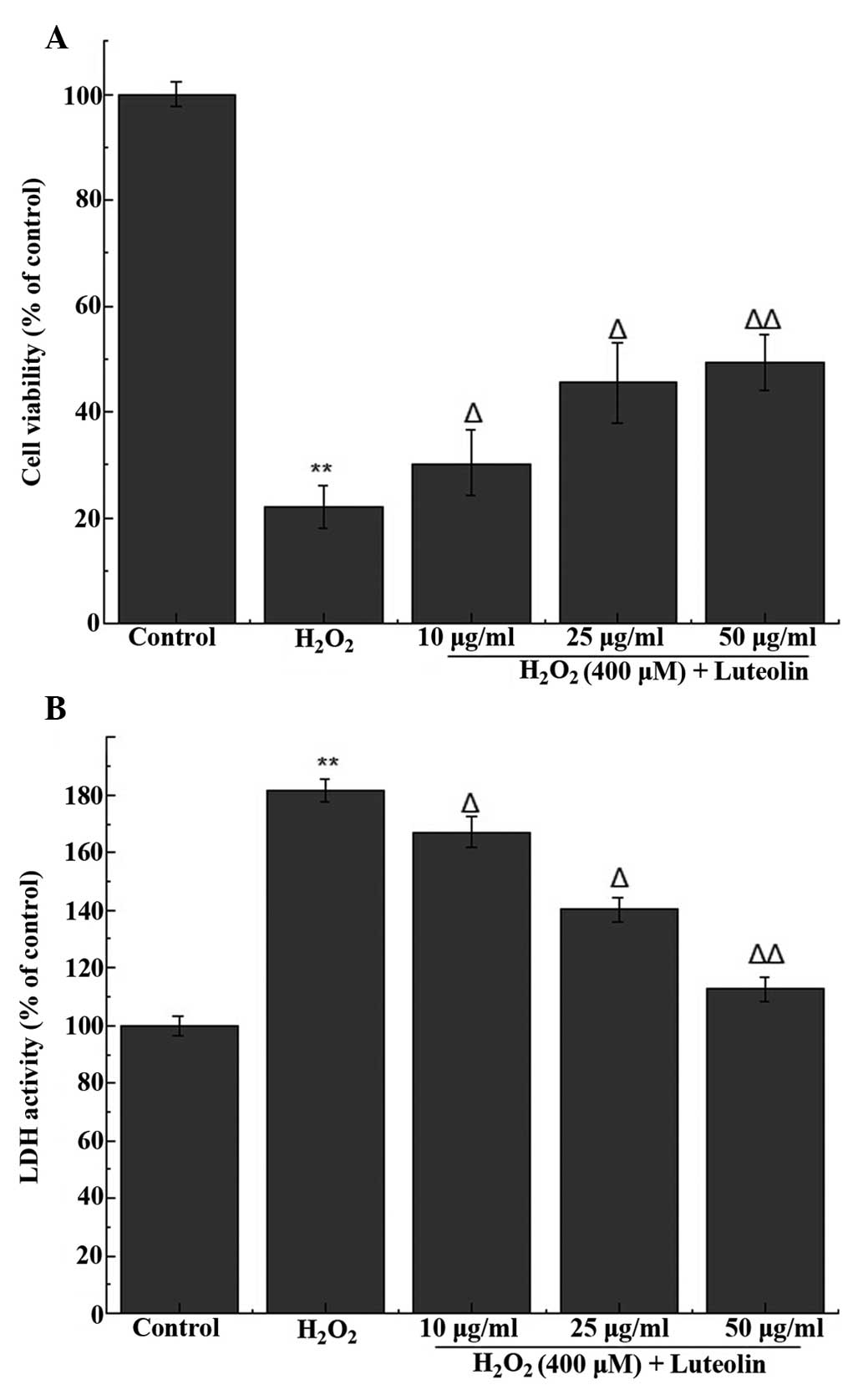
Effect of luteolin on cell viability in
PC12 cells
In order to determine the working concentration of
luteolin, PC12 cells were treated with luteolin, from which three
concentrations of luteolin (10, 25 and 50 µg/ml) were
selected for subsequent experiments. The MTT assay indicated that
the percentage of viable cells following treatment with 400
µM H2O2 was 22.2±3.1% (Fig. 1A). Following pretreatment with 10,
25 and 50 µg/ml luteolin, cell viability was 30.29±2.1,
45.6±4.7% and 49.4±5.3, respectively. These results indicate that
luteolin is able to attenuate H2O2-induced
cytotoxicity in PC12 cells.
Effect of luteolin on LDH release in PC12
cells
LDH release was used to measure the level of cell
death, and compared with the control group, LDH release from cells
treated with 400 µM H2O2 was
181.5±4.2%. Following pretreatment with different concentration of
luteolin, LDH release was 167.2±3.3, 140.3±2.7% and 112.6±5.1,
respectively, compared with the control group. These results
indicate that luteolin is able to attenuate
H2O2-induced cytotoxicity in PC12 cells
(Fig. 1B).
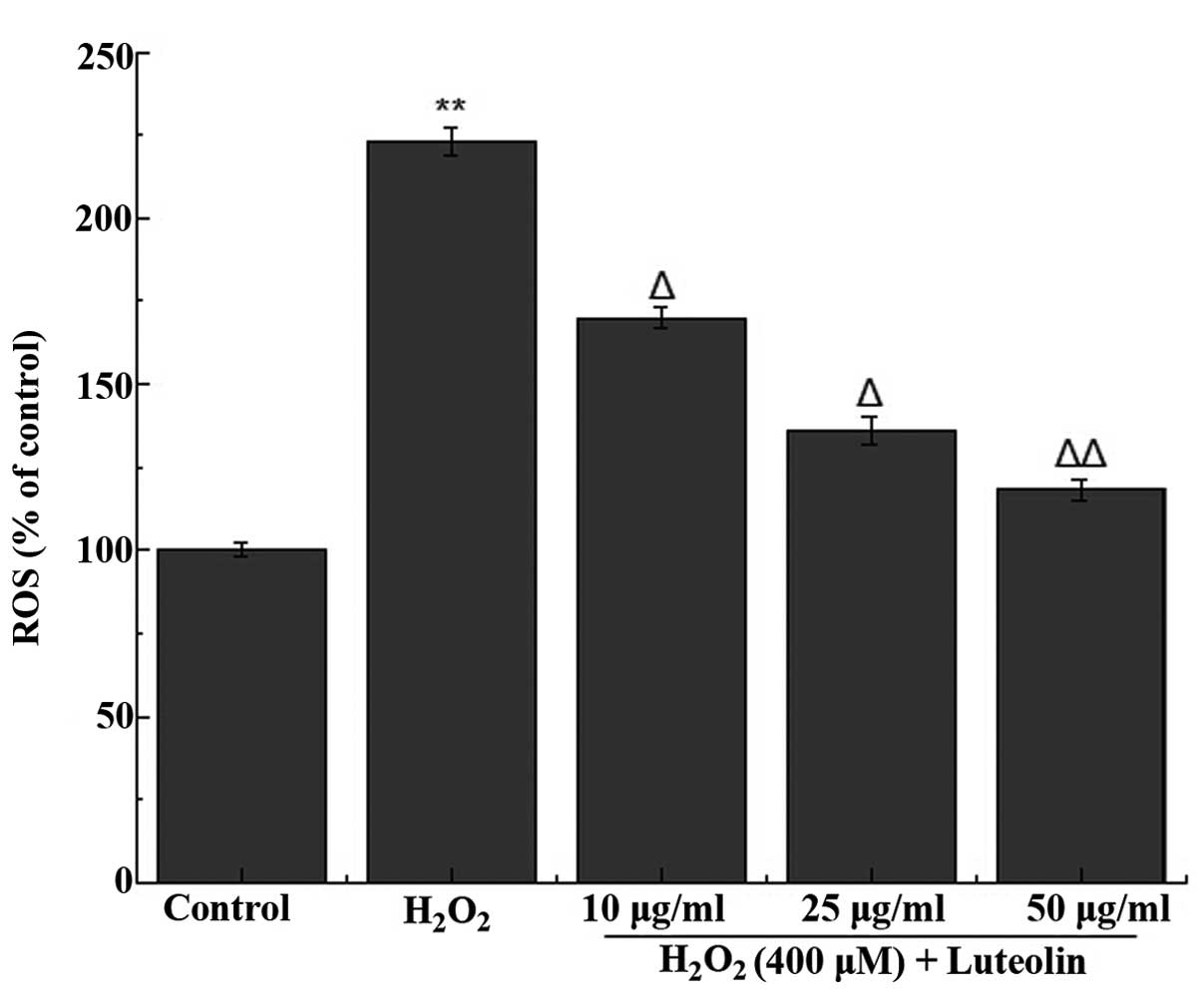
Effect of luteolin on ROS generation in
PC12 cells
The effect of luteolin on
H2O2-induced ROS generation in PC12 cells was
measured. It was observed that treatment of the cells with 400
µM H2O2 increased the generation of
ROS (Fig. 2). However, the
increased ROS generation was significantly reduced following
pretreatment of the cells with different concentrations of
luteolin.
Effect of luteolin on SOD, GSH-Px and MDA
levels in PC12 cells
The activity of the antioxidant enzymes (SOD and
GSH-Px) and the end product of oxidation (MDA) were measured in the
PC12 cells. The results indicated a significant reduction in the
activity levels of SOD and GSH-Px, in addition to an increase in
the level of MDA following treatment with 400 µM
H2O2. The reduced SOD and GSH-Px activity was
attenuated following pretreatment with luteolin, with 25 and 50
µg/ml luteolin significantly ameliorating the increased MDA
levels following H2O2 treatment (Fig. 3).
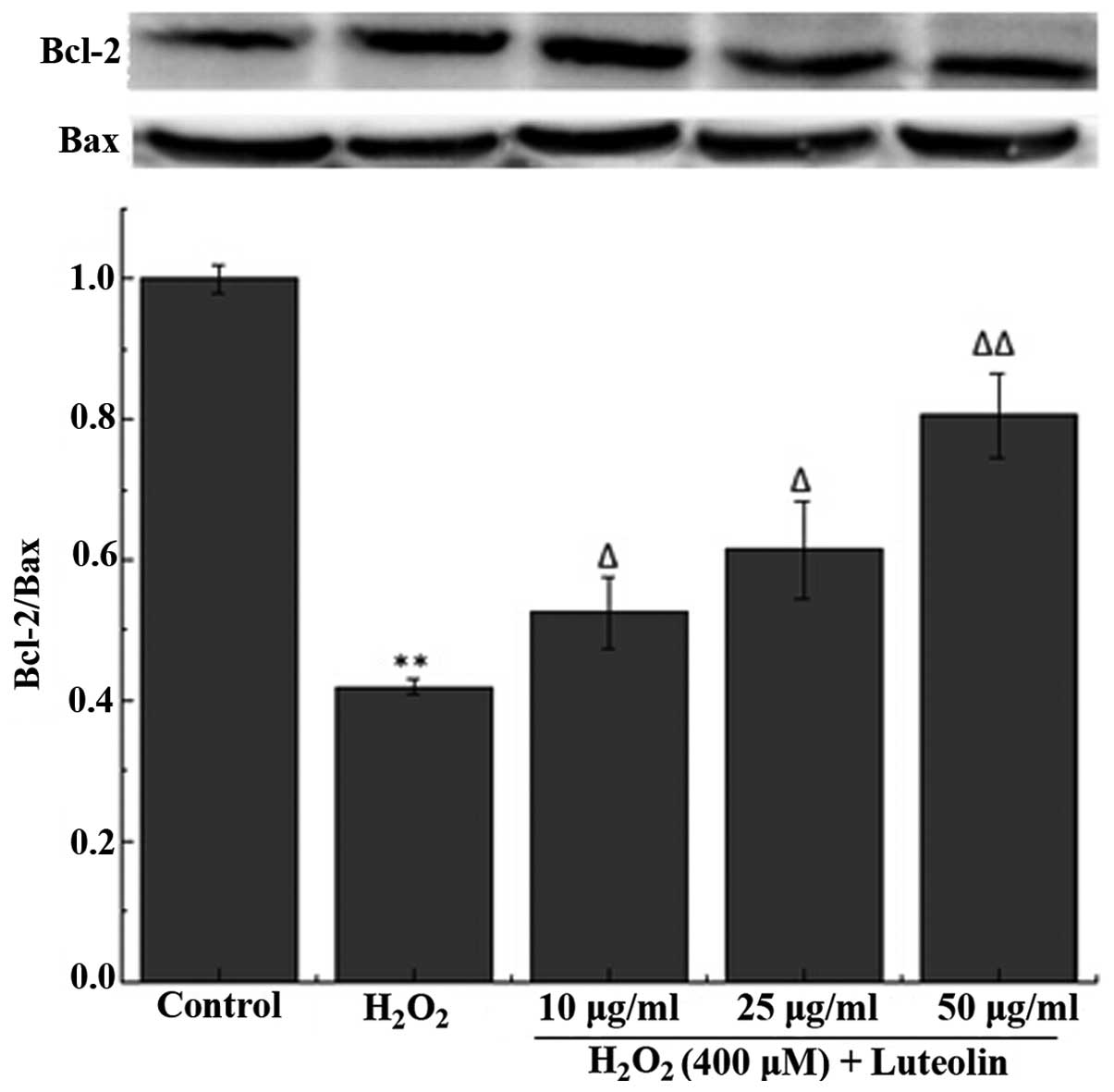
Effect of luteolin on the Bcl-2/Bax ratio
in PC12 cells
To further investigate the effect of luteolin on
H2O2-induced PC12 cell apoptosis, the
Bcl-2/Bax ratio was measured. The western blotting results
demonstrated that the Bcl-2/Bax ratio was reduced in PC12 cells in
the H2O2-treated group compared with the
control group (Fig. 4). However,
pretreatment with luteolin significantly attenuated this
reduction.
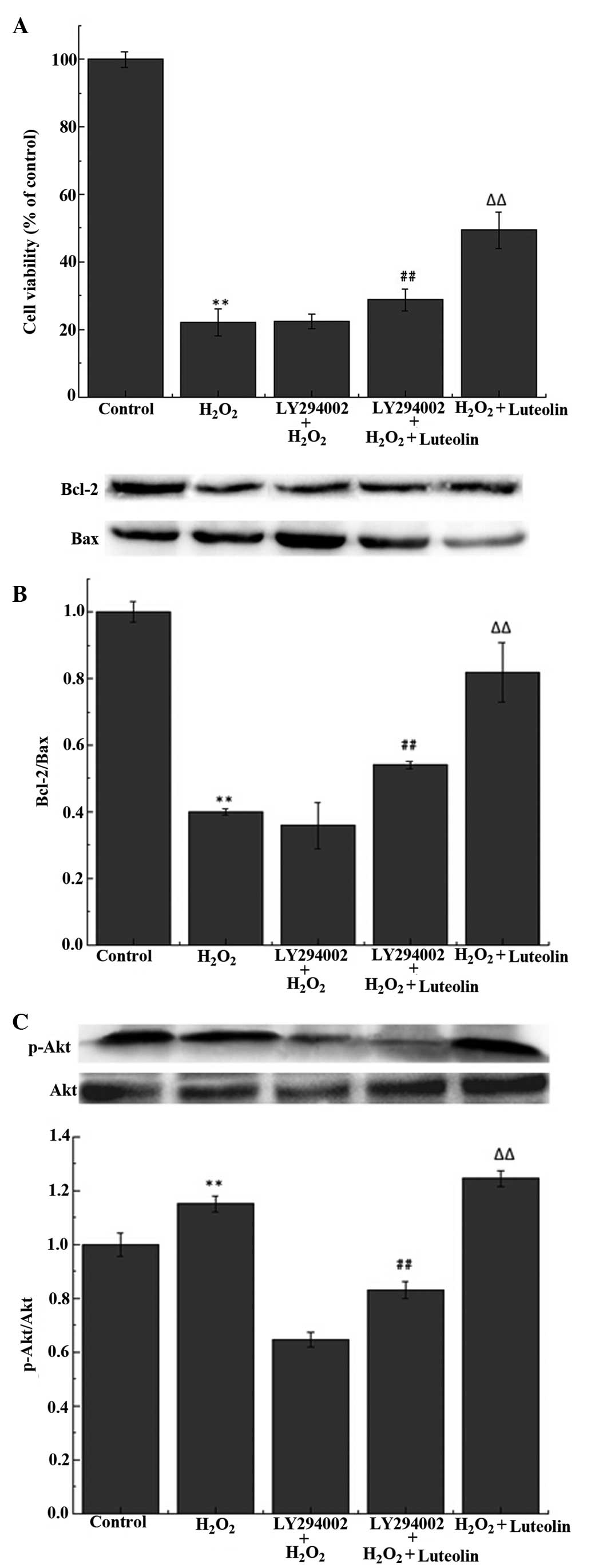
Effect of luteolin on the PI3K/Akt
pathway in PC12 cells
The western blot analysis demonstrated that the
luteolin treatment significantly increased the levels of p-Akt
(Fig 5A). To investigate whether
the protective effects of luteolin were mediated through the
PI3K/Akt pathway, PC12 cells were pretreated with LY294002, a
PI3K/Akt inhibitor. The results demonstrated that the effects of
luteolin on p-Akt levels (Fig.
5A), cell viability (Fig. 5B)
and the Bcl-2/Bax ratio (Fig. 5C)
were reduced following the pretreatment with LY294002.
Discussion
Previous studies have demonstrated that oxidative
stress is important in the activation of apoptosis and neuronal
cell death in neurodegenerative diseases (24–26).
H2O2 generates superoxide and hydroxyl
radicals, the major components of ROS, and has been extensively
used to induce oxidative stress in vitro (8). PC12 cells are commonly used for
neurobiological and neurochemical studies (12,27,28).
Therefore, in the current study H2O2-induced
cytotoxicity was investigated in PC12 cells. Luteolin has been
demonstrated to exhibit anti-inflammatory, anti-oxidative and
anti-carcinogenic effects (16–18).
The current study investigated whether luteolin has protective
effects against H2O2-induced apoptosis in
PC12 cells, and therefore whether it may be of clinical
importance.
LDH is an enzyme involved in glycolysis, and cell
damage results in the release of LDH, therefore the activity levels
of LDH are used as an indicator of cellular integrity. ROS are a
product of the aerobic metabolism, and the excess generation of ROS
results in lipid peroxidation (29). Cells possess endogenous
antioxidants such as GSH-Px and SOD, which scavenge ROS to prevent
cell damage. The predominant physiological functions of GSH-Px are
free radical scavenging, antioxidant activity and anti-aging
activity (30). SOD is able to
transform intracellular superoxide anions into
H2O2. MDA is the end-product of
oxygen-derived free radicals and lipid oxidation, and may be used
as an indicator of oxidative damage (31). The current study demonstrated that
luteolin was able to inhibit the reduction in cell viability
induced by H2O2. In addition, luteolin was
able to reduce ROS formation and LDH release in
H2O2-treated PC12 cells. SOD and GSH-Px
activity were observed to increase following treatment with
luteolin, while MDA was reduced. Together, this demonstrates that
luteolin was able to increase antioxidant defense, reduce the
production of ROS and cellular damage, indicating that luteolin has
protective effects against H2O2-induced
damage in PC12 cells.
The Bcl-2 and Bax genes have been demonstrated to
serve a key role in determining whether a cell survives or
undergoes apoptosis (32). Bcl-2
and Bax are Bcl-2 family members, and Bcl-2 is involved in the
maintenance of cell survival, while Bax serves to accelerate
apoptosis. Bcl-2 and Bax have been suggested to be implicated in
apoptosis induced by ROS-generating agents (33). In the current study, following
pretreatment with luteolin the expression of Bcl-2 was increased,
while the expression of Bax was reduced. These alterations resulted
in an increase in the Bcl-2/Bax ratio, which indicates that
apoptosis was inhibited. These results indicated that luteolin was
able to attenuate H2O2-induced apoptosis in
PC12 cells.
Akt is a central node in cell signaling downstream
of growth factors, cytokines and additional cellular stimuli. It
promotes cell survival and protects against apoptosis through its
ability to phosphorylate and inactivate apoptotic factors (34). Previous studies have indicated that
in response to oxidants such as H2O2, Akt was
rapidly activated (35,36). Futhermore, a previous study
demonstrated that Bcl-2 acts downstream of the PI3K/Akt signaling
pathway, and that upregulation of Bcl-2 serves an important role in
cell survival (37). In the
present study, the results demonstrated that luteolin enhanced the
PI3K/Akt pathway in response to H2O2.
LY294002 is a selective inhibitor of PI3K, which was
demonstrated in the current study to attenuate the effect of
luteolin on cell viability, Akt phosphorylation and the Bcl-2/Bax
ratio. These results suggest that luteolin was able to protect the
PC12 cells against H2O2-induced apoptosis via
reducing ROS levels and activating the PI3K/Akt signaling
pathway.
In conclusion, the current study demonstrated that
luteolin protected PC12 cells from
H2O2-induced apoptosis, via the activation of
the PI3K/Akt signaling pathway. Therefore luteolin may have
protective effects, and further study is required to fully
elucidate the protective mechanisms.
References
|
1
|
Fahn S and Cohen G: The oxidant stress
hypothesis in Parkinson's disease: Evidence supporting it. Ann
Neurol. 32:804–812. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith MA, Perry G, Richey PL, Sayre LM,
Anderson VE, Beal MF and Kowall N: Oxidative damage in Alzheimer's.
Nature. 382:120–121. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Halliwell B: Oxidative stress and
neurodegeneration: Where are we now? J Neurochem. 97:1634–1658.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li MH, Jang JH, Sun B and Surh YJ:
Protective effects of oligomers of grape seed polyphenols against
beta-amyloid-induced oxidative cell death. Ann N Y Acad Sci.
1030:317–329. 2004. View Article : Google Scholar
|
|
5
|
Fukui K, Takatsu H, Shinkai T, Suzuki S,
Abe K and Urano S: Appearance of amyloid beta-like substances and
delayed-type apoptosis in rat hippocampus CA1 region through aging
and oxidative stress. J Alzheimers Dis. 8:299–309. 2005.PubMed/NCBI
|
|
6
|
Kadowaki H, Nishitoh H, Urano F, Sadamitsu
C, Matsuzawa A, Takeda K, Masutani H, Yodoi J, Urano Y, Nagano T
and Ichijo H: Amyloid beta induces neuronal cell death through
ROS-mediated ASK1 activation. Cell Death Differ. 12:19–24. 2005.
View Article : Google Scholar
|
|
7
|
Heo SR, Han AM and Kwon YK: p62 protects
SH-SY5Y neuroblastoma cells against H2O2-induced injury through the
PDK1/Akt pathway. Neurosci Lett. 23;450(1): 45–50. 2009. View Article : Google Scholar
|
|
8
|
Satoh T, Sakai N, Enokido Y, Uchiyama Y
and Hatanaka H: Free radical-independent protection by nerve growth
factor and Bcl-2 of PC12 cells from hydrogen peroxide-triggered
apoptosis. J Biochem. 120:540–546. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu BP and Yang R: Critical evaluation of
the free radical theory of aging. A proposal for the oxidative
stress hypothesis. Ann N Y Acad Sci. 786(1 Near-Earth Ob): 1–11.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Floyd RA: Antioxidants, oxidative stress,
and degenerative neurological disorders. Proc Soc Exp Biol Med.
222:236–245. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sultana R, Newman S, Mohmmad-Abdul H,
Keller JN and Butterfield DA: Protective effect of the xanthate,
D609, on Alzheimer's amyloid betapeptide (1–42)-induced oxidative
stress in primary neuronal cells. Free Radic Res. 38:449–458. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koh SH, Kwon H, Park KH, Ko JK, Kim JH,
Hwang MS, Yum YN, Kim OH, Kim J, Kim HT, et al: Protective effect
of diallyl disulfide on oxidative stress-injured neuronally
differentiated PC12 cells. Brain Res Mol Brain Res. 133:176–186.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi SJ, Kim MJ, Heo HJ, Hong B, Cho HY,
Kim YJ, Kim HK, Lim ST, Jun WJ, Kim EK and Shin DH: Ameliorating
effect of Gardenia jasminoides extract on amyloid beta
peptide-induced neuronal cell deficit. Mol Cells. 24:113–118.
2007.PubMed/NCBI
|
|
14
|
Zhang HY, Liu YH, Wang HQ, Xu JH and Hu
HT: Puerarin protects PC12 cells against beta-amyloid-induced cell
injury. Cell Biol Int. 32:1230–1237. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soubra L, Sarkis D, Hilan C and Verger P:
Dietary exposure of children and teenagers to benzoates, sulphites,
butylhydroxyanisol (BHA) and butylhydroxytoluen (BHT) in Beirut
(Lebanon). Regul Toxicol Pharmacol. 47:68–77. 2007. View Article : Google Scholar
|
|
16
|
Pandurangan AK, Dharmalingam P, Ananda
Sadagopan SK and Ganapasam S: Effect of luteolin on the levels of
glycoproteins during azoxymethane-induced colon carcinogenesis in
mice. Asian Pac J Cancer Prev. 13:1569–1573. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung HA, Jin SE, Min BS, Kim BW and Choi
JS: Anti-inflammatory activity of Korean thistle Cirsium maackii
and its major flavonoid, luteolin 5-O-glucoside. Food Chem Toxicol.
50:2171–2179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Manju V and Nalini N: Protective role of
luteolin in 1,2-dimeth-ylhydrazine induced experimental colon
carcinogenesis. Cell Biochem Funct. 25:189–194. 2007. View Article : Google Scholar
|
|
19
|
Sun GB, Sun X, Wang M, Ye JX, Si JY, Xu
HB, Meng XB, Qin M, Sun J, Wang HW and Sun XB: Oxidative stress
suppression by luteolin-induced heme oxygenase-1 expression.
Toxicol Appl Pharmacol. 265:229–240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Casagrande F and Darbon JM: Effects of
structurally related flavonoids on cell cycle progression of human
melanoma cells: Regulation of cyclin-dependent kinases CDK2 and
CDK1. Biochem Pharmacol. 61:1205–1215. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsui T, Kobayshi M, Hayashida S and
Matsumoto K: Luteolin, a flavone, does not suppress post prandial
blood glucose absorption through the inhalation of
alpha-glucosidase action. Biosci, Biotechnol and Biochem.
66:689–692. 2002. View Article : Google Scholar
|
|
22
|
Ji BS and Gao Y: Protective effect of
trihexyphenidyl on hydrogen peroxide-induced oxidative damage in
PC12 cells. Neurosci Lett. 437:50–54. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee YM, Park SH, Shin DI, Hwang JY, Park
B, Park YJ, Lee TH, Chae HZ, Jin BK, Oh TH and Oh YJ: Oxidative
modification of peroxiredoxin is associated with drug-induced
apoptotic signaling in experimental models of Parkinson disease. J
Biol Chem. 283:9986–9998. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Markesbery WR: Oxidative stress hypothesis
in Alzheimer's disease. Free Radic Biol Med. 23:134–147. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jenner P: Oxidative stress in Parkinson's
disease. Ann Neurol. 53(Suppl 3): S26–S36; discussion S36–S38.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Emerit J, Edeas M and Bricaire F:
Neurodegenerative diseases and oxidative stress. Biomed
Pharmacother. 58:39–46. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gozal E, Sachleben LR Jr, Rane MJ, Vega C
and Gozal D: Mild sustained and intermittent hypoxia induce
apoptosis in PC-12 cells via different mechanisms. Am J Physiol
Cell Physiol. 288:C535–C542. 2005. View Article : Google Scholar
|
|
28
|
Hirose M, Takatori M, Kuroda Y, Abe M,
Murata E, Isada T, Ueda K, Shigemi K, Shibazaki M, Shimizu F, et
al: Effect of synthetic cell-penetrating peptides on TrkA activity
in PC12 cells. J Pharmacol Sci. 106:107–113. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Zhang QB, Zhang ZS, Zhang JJ and
Li PC: Synthesized phosphorylated and aminated derivatives of
fucoidan and their potential antioxidant activity in vitro. Int J
Biol Macromol. 44:170–174. 2009. View Article : Google Scholar
|
|
30
|
Erten SF, Kocak A, Ozdemir I, Aydemir S,
Colak A and Reeder BS: Protective effect of melatonin on
experimental spinal cord ischemia. Spinal Cord. 41:533–538. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qian H and Liu D: The time course of
malondialdehyde production following impact injury to rat spinal
cord as measured by microdialysis and high pressure liquid
chromatography. Neurochem Res. 22:1231–1236. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Misao J, Hayakawa Y, Ohno M, Kato S,
Fujiwara T and Fujiwara H: Expression of bcl-2 protein, an
inhibitor of apoptosis, and Bax, an accelerator of apoptosis, in
ventricular myocytes of human hearts with myocardial infarction.
Circulation. 94:1506–1512. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang R, Zhang HY and Tang XC: Huperzine A
attenuates cognitive dysfunction and neuronal degeneration caused
by beta-amyloid protein-(1–40) in rat. Eur J Pharmacol.
421:149–156. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Datta SR, Dudek H, Tao X, Masters S, Fu H,
Gotoh Y and Greenberg ME: Akt phosphorylation of BAD couples
survival signals to the cell-intrinsic death machinery. Cell.
91:231–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Martin D, Salinas M, Fujita N, Tsuruo T
and Cuadrado A: Ceramide and reactive oxygen species generated by
H2O2 induce caspase-3-independent degradation
of Akt/protein kinase B. J Biol Chem. 277:42943–42952. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang X, McCullough KD, Franke TF and
Holbrook NJ: Epidermal growth factor receptor-dependent Akt
activation by oxidative stress enhances cell survival. J Biol Chem.
275:14624–14631. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma R, Xiong N, Huang C, Tang Q, Hu B,
Xiang J and Li G: Erythropoietin protects PC12 cells from
beta-amyloid(25–35)-induced apoptosis via PI3K/Akt signaling
pathway. Neuropharmacology. 56:1027–1034. 2009. View Article : Google Scholar : PubMed/NCBI
|