Introduction
Ischemia/reperfusion injury (IRI) is commonly
observed in clinical settings following stroke, trauma surgery and
organ transplantation (1). Renal
IRI (RIRI), which is commonly observed following hypovolemic shock,
renal surgical procedures, renal transplantation and acute renal
arterial occlusion, is a major cause of acute renal failure, and is
a complex pathophysiological process leading to high rates of
morbidity and mortality (2–4).
However, no effective therapy is currently available, with the
exception of supportive treatment. Therefore, the identification of
effective therapeutic intervention strategies for RIRI is required.
The pathophysiological mechanism underlying RIRI is complex, and
involves the release of reactive oxygen species (ROS) (5), generation of pro–inflammatory
mediators (6), calcium overload
and activation of the apoptotic genes (7). A previous study demonstrated that ROS
are important in the pathophysiology of RIRI (8).
Erythropoietin (EPO) is a hematopoietic hormone
produced by the kidney and the fetal liver in response to hypoxia
(9). Previous studies have
revealed that EPO has numerous protective effects, including
anti–oxidative (10,11), anti–inflammatory (12) and anti apoptotic effects (13,14).
EPO can exert these protective effects against IRI in the brain
(15), liver, heart and intestine
(16,17). Furthermore, it has been reported
that renal expression levels of EPO are decreased following RIRI
(18). EPO exerts cytoprotective
effects by modulating a variety of signal transduction pathways,
which involve mitogen–activated protein kinase (MAPK), nuclear
factor–κB (NF–κB) and phosphatidylinositol 3–kinase (PI3K)/Akt
(19,20). It has been reported that PI3K/Akt
signaling pathway activation exerts protective effects on IRI, as
activated Akt elevates the expression of endothelial nitric oxide
synthase (eNOS) and the production of nitric oxide (NO) in
endothelial cells (21). However,
the protective mechanisms underlying the effects of EPO on RIRI
remain to be fully elucidated.
Selenium is an essential trace element, which
scavenges free radicals (22), and
its anti oxidative function is associated with selenoproteins,
including glutathione peroxidase (GSH–Px) and thioredoxin reductase
(23). Selenium deficiency has
been demonstrated to be closely associated with various diseases,
including cancer, coronary artery disease and osteoarthritis
(24–26). Previous studies have reported that
selenium supplementation has a number of beneficial effects in IRI
in different tissues, including the brain, heart, liver and kidney
(27–30).
To the best of our knowledge, no studies have been
performed on the effects of combined treatment with EPO and sodium
selenite in IRI. Therefore, the aim of the present study was to
investigate the synergistic effects of EPO and sodium selenite in
RIRI. In addition, the mechanisms underlying the action of EPO and
sodium selenite on RIRI were investigated.
Materials and methods
Determination of optimal treatment
components Animals, grouping and treatment
A total of 60 male Kun–Ming mice, weighing 18–22 g
(provided by the Experimental Animal Center of Xi'an Jiaotong
University, Xi'an, China) were used in the present study, in
accordance with the recommended guidelines on the Care and Use of
Laboratory Animals issued by the Chinese Council on Animal
Research. The present study was approved by the Ethics Committee at
Xian Jiaotong University (Xian, China). The mice were randomly
divided into six groups: In groups 1–6, drug dosage was determined
based on the Uniform Design's Table of U6 (66) (31). EPO was obtained from Shengyang
Sunshine Pharmaceutical Co., Ltd. (Shengyang, China). Sodium
selenite was purchased from Shanghai Tiancifu Bioengineering Co.,
Ltd. (Shanghai, China). Following treatment for 3 days with the
concentrations indicated in Table
I, an acute RIRI model was established in the mice. The dosage
ranges of EPO and sodium selenite were based on previous reports
(11–13,19–23).
Briefly, following anesthesia with 10% chloral hydrate, the
abdomens of the mice were opened through ventrimeson, and the renal
pedicle was exposed by incision. The right kidney was removed and
the left kidney pedicle was clamped for 1 h. For reperfusion, the
clamp was removed, and blood flow was allowed to return to the
renal tissues. Following reperfusion for 4 h, blood samples were
collected through the venous plexus behind the eyeball, and the
mice were sacrificed with 10% chloral hydrate. The samples were
placed into eppendorf tubes and centrifuged at 1,368 × g for 10 min
at room temperature, from which the plasma was collected and stored
at −80°C until further analysis.
 | Table IEffects of EPO and sodium selenite on
serum expression levels of BUN in mice with renal
ischemia/reperfusion injury (n=10 for each group). |
Table I
Effects of EPO and sodium selenite on
serum expression levels of BUN in mice with renal
ischemia/reperfusion injury (n=10 for each group).
| Group | EPO (IU/kg) | Se
(µg/kg) | BUN (mmol/l) | 1/BUN |
|---|
| 1 | 125 | 50 | 15.13±1.48 | 0.067±0.006 |
| 2 | 250 | 400 | 14.16±3.13 | 0.074±0.018 |
| 3 | 500 | 25 | 16.29±1.71 | 0.062±0.007 |
| 4 | 1,000 | 200 | 10.74±1.65 | 0.098±0.017 |
| 5 | 2,000 | 12.5 | 9.09±2.35 | 0.117±0.031 |
| 6 | 4,000 | 100 | 9.76±1.66 | 0.112±0.020 |
Treatment with EPO and sodium selenite in
RIPI Animals, grouping and treatment
Following treatment with the optimal dosage ratio of
EPO and sodium selenite, 70 male Sprague Dawley rats, weighing
180–220 g (obtained from the Experimental Animal Center of Xi'an
Jiaotong University) were used to undertake factorial analysis. The
rats were used in accordance with recommended guidelines on the
care and use of laboratory animals issued by the Chinese Council on
Animal Research. The study was approved by the Ethics Committee at
Xian Jiaotong University. The Kun–Ming mice and Sprague–Dawley rats
were fed with standard laboratory diets ad libitum, and were
maintained at a temperature of 20–25°C and a humidity of 45–55%
with illumination for 12 h in a specific pathogen–free
environment.
The rats were randomly divided into seven groups
(n=10/group): Sham group, intraperitoneal (i.p) injection with
normal saline; a model group, i.p injection with normal saline; EPO
group, i.p injection with 1,500 U/kg/day EPO; Se group (i.p)
injection with 150 µg/kg/day sodium selenite); EPO + Se (L)
group, i.p injection with 750 U/kg/day EPO and 75 µg/kg/day
sodium selenite); EPO + Se (M) group, i.p injection with 1,500
U/kg/day EPO and 150 µg/kg/day sodium selenite); EPO + Se
(H) group, intraperitoneal (i.p) injection with 3,000 U/kg/day EPO
and 300 µg/kg/day sodium selenite. Following three days of
treatment, an acute RIRI model was established. Briefly, following
anesthesia with 10% chloral hydrate, the renal pedicle of the rats
was exposed by incision. The right kidney was removed and the left
kidney pedicle was clamped for 1 h. The clamp was subsequently
removed and blood flow was permitted to return to the renal tissues
for reperfusion. Following 4 h reperfusion, blood samples were
collected through the abdominal aorta under anesthesia. The blood
samples were placed into eppendorf tubes and centrifuged at 1,368 ×
g for 10 min at room temperature, and the plasma was collected and
stored at –80°C until further analysis. The rats were sacrificed by
cervical dislocation, and the left kidney was separated into two
specimens, one of which was immediately stored in eppendorf tubes
and frozen in liquid nitrogen; whereas the other half was fixed in
10% formalin for hematoxylin and eosin (H&E) staining and
immunohistochemical examination.
Analysis of serum and renal biochemical
indices
The serum levels of BUN were measured via urease
methods using commercial kits (cat. no. C013–2; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). The levels of serum and
renal super oxide dismutase (SOD), malonic dialdehyde (MDA) and
renal glutathione peroxidase (GSH–Px) were measured using a Total
Superoxide Dismutase Assay kit (cat. no. A001–1; hydroxylamine
method), Malondialdehyde Assay kit (cat. no. A003–1; TBA method)
and Glutathione Peroxidase Assay kit (cat. no. A005; colorimetric
method), according to the manufacturer's protocol (Nanjing
Jiancheng Bioengineering Institute). Renal expression levels of
nitric oxide (NO) were determined indirectly as the concentration
of nitrite from nitrates, using an NO assay kit (cat. no. A012;
Nanjing Jiancheng Bioengineering Institute).
Histological changes in the kidney
Renal tissue samples were embedded in paraffin
(Xi'an Zaolutang Pharmaceutical Co, Ltd., Xi'an, China), sectioned
at 5 µm and stained with H&E (Xi'an Laibo Bio–technology
Co, Ltd., Xi'an, China). Renal tubule damage was scored by
calculating the percentage of tubules in the corticomedullary
junction, which exhibited cell necrosis, loss of the brush border,
cast formation and tubular dilatation. The scoring of renal damage
was performed in a double–blinded manner, as follows: Score 0, no
damage; score 1, 10%; score 2, 11–25%; score 3, 26–45%; score 4,
46–75%; score 5, >76%. Images of the representative fields were
captured using an Olympus DP71 digital camera (Olympus Corporation,
Tokyo, Japan).
Immunohistochemical detection in the
renal tissue samples
Immunohistochemistry was performed to examine the
expression levels of PI3K in the renal tissue samples. The tissue
sections were deparaffinized and rehydrated, followed by immersion
in citrate buffer (0.01 M at pH 6.0) and heating for 10 min (Gree
Co., Guangzhou, China) in a microwave oven for antigen retrieval.
Following cooling to room temperature, the tissue sections were
rinsed with distilled water twice and PBS twice, and then incubated
with 3% H2O2 solution to block endogenous
enzymes. The tissue sections were subsequently incubated with
normal goat serum to block non–specific binding following rinsing
with phosphate–buffered saline. Subsequently, the tissue sections
were incubated overnight at 4°C with monoclonal mouse anti human
PI3K primary antibody (cat. no. ab86714; Abcam, Cambridge, USA;
1:200), followed by incubation with the appropriate horseradish
peroxidase–conjugated secondary antibody at room temperature for 30
mins. Substrate–chromogen DAB reagent (Beijing Zhongshan Golden
Bridge Biotechnology Co. Ltd., Beijing, China) was added for
coloration, following which the tissue specimens were dehydrated,
counterstained with xylene, and mounted under glass cover slips.
Brown colored sites were observed at a final magnification of ×400
under a microscope by a pathologist, and images were captured using
an Olympus DP71 digital camera (Olympus Corporation, Tokyo,
Japan).
Statistical analysis
The data are presented as the mean ± standard error
of the mean. Multiple comparisons were performed using analysis of
variance with SPSS v13.0 software (SPSS, Inc., Chicago, IL, USA).
Weighted modification analyses were performed using DAS 1.0
software (Shanghai University of Traditional Chinese Medicine,
Shanghai, China). d represents the standardized dose and b
represents the coefficient of standardized dose; the value of b (d)
reflects the relative importance of the component. The higher the
value of b (d), the more significant the dose–dependency. The
component exhibiting the highest b value was deemed the predominant
drug. b (d1d2) represents the interaction between the two drugs,
with higher values representing increased synergy. Figures were
generated using Prism 5.0 software (GraphPad Software Inc, La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Determination of optimal treatment
components
Effects of EPO and sodium selenite on
serum expression levels of BUN in RIRI mice
To evaluate the protective effects of EPO and sodium
selenite on RIRI in mice, the expression levels of BUN were
quantified. The expression levels of BUN were significantly reduced
following injection with EPO and sodium selenite prior to RIRI. As
shown in Table I, the serum
expression levels of BUN decreased between 16.29±1.71 mmol/l (group
3) and 9.09±2.35 mmol/l (group 5).
Optimization formulation analysis
Further analysis demonstrated that the optimal
proportion of compounds in the preparation was 10:1 (10 IU EPO: 1
µg sodium selenite), which was calculated using DAS 1.0
software (24). The standardized
dose (d), the coefficient of the standardized dose (b), the value
of b (d) reflected the relative importance of the components of the
prescription (Table. II). The
higher the value of b (d), the higher the significance of the
dose–dependency. The component with the maximum b (d) value was the
predominant drug, whereas, b (d1d2) indicated the interaction
between the two drugs, of which a higher value indicated increased
synergy. EPO and sodium selenite exhibited synergistically
protective effects on RIRI. EPO was identified as the predominant
drug and sodium selenite served as the adjuvant. The theoretical
optimization formulation was determined as 4,000 IU/kg EPO and 400
µg/kg sodium selenite.
 | Table IIEffects of each component in
combination in its interaction with 1/BUN. |
Table II
Effects of each component in
combination in its interaction with 1/BUN.
| Component | Standardized
dose | b (d1) | b (d1d2) | P value | Optimized dose | Annotation |
|---|
| EPO | d1 | 2.085 | – | 0.013 | 4,000.0 IU/kg | Dmax of EPO |
| Se | d2 | 0.314 | – | 0.042 | 400.0
µg/kg | Dmax of Se |
| EPO+Se | d1d2 | – | 0.698 | 0.041 | 400.0 | Synergistic |
Effects of treatment with EPI and
sodium selenite in RIPI. EPO combined with sodium selenite
decreases serum expression levels of BUN in rats with RIRI
As shown in Fig. 1,
the serum expression levels of BUN in the RIRI model group were
significantly higher, compared with the sham group (P<0.05),
suggesting marked glomerular dysfunction. Compared with the model
group, intraperitoneal administration of medium (1,500 U/kg/day
EPO, 150 µg/kg/day Se); and high (3,000 U/kg/day EPO, 300
µg/kg/day Se) doses of EPO and sodium selenite combined
resulted in a significant reduction in serum expression levels of
BUN (P<0.01), which occurred in a dose–dependent manner.
However, no significant effects on the expression levels of BUN
were observed following treatment with EPO or sodium selenite
alone, compared with the model group.
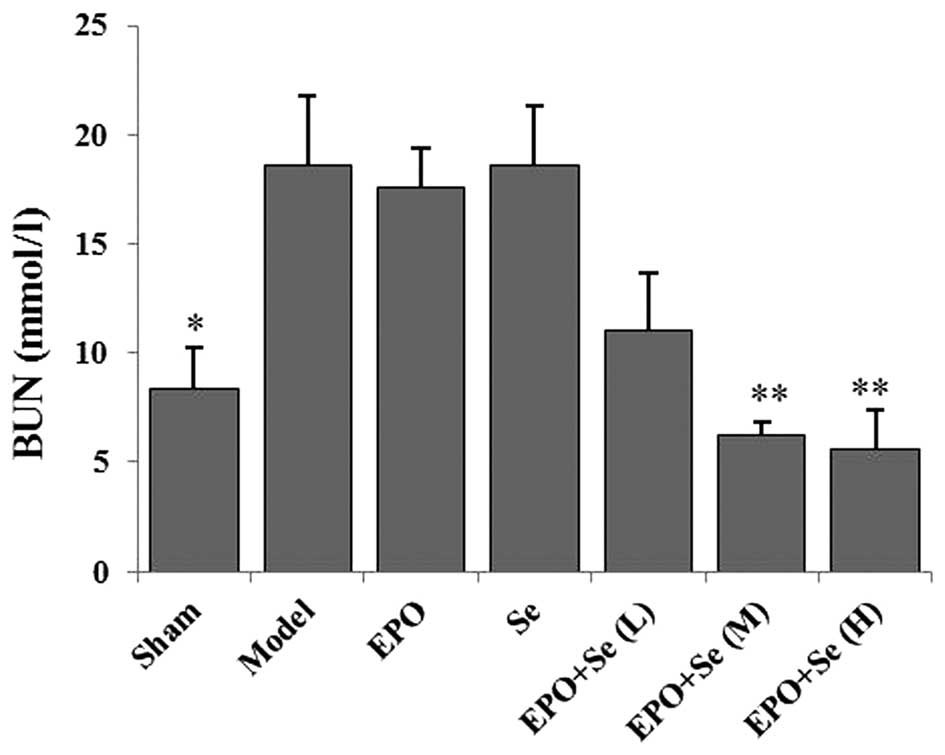 | Figure 1Effects of treatment with EPO
combined with sodium selenite on serum the expression levels of BUN
in rats with RIRI. Drugs were intraperitoneally injected prior to
RIRI. Sham, no RIRI, normal saline; model, normal saline; EPO,
1,500 U/kg/day EPO; Se, 150 µg/kg/day Se; EPO + Se (L), 750
U/kg/day EPO+75 µg/kg/day Se; EPO + Se (M), 1,500 U/kg/day
EPO+150 µg/kg/day Se; EPO + Se (H), 3,000 U/kg/day EPO+300
µg/kg/day Se. Data are presented as the mean ± standard
error of the mean. *P<0.05 and **P<0.01, vs. model
group. RIRI, renal ischemia/reperfusion injury; BUN, blood urea
nitrogen; EPO, erythropoietin; Se, sodium selenite. |
EPO combined with sodium selenite
improves serum biochemical parameters of rats with RIRI
As shown in Fig. 2A and
B, the serum and tissue expression levels of MDA in the model
group were significantly higher, compared with the sham group
(P<0.01). Pre–treatment with EPO and sodium selenite
significantly reduced the increased expression levels observed in
the model group to varying degrees. Furthermore, the expression
levels of MDA decreased in the sodium selenite–treated group,
compared with the model group (P<0.01). Antioxidant enzyme
activity levels were also measured, including those of SOD and
GSH–Px (Fig. 2C–E). Tissue
activities of SOD and GSH–Px in the model group were significantly
lower than those in the sham group (P<0.01). Pre–treatment with
EPO and sodium selenite significantly increased tissue activities
of GSH–Px. However, the serum expression levels of SOD in the
treated groups were not significantly different from those in the
model group (P>0.05). As shown in Fig. 2F the tissue levels of NO were
significantly lower in the model group, compared with the sham
group (P<0.05). However, pre–treatment with EPO and sodium
selenite increased tissue levels of NO, compared with the model
group (P<0.05).
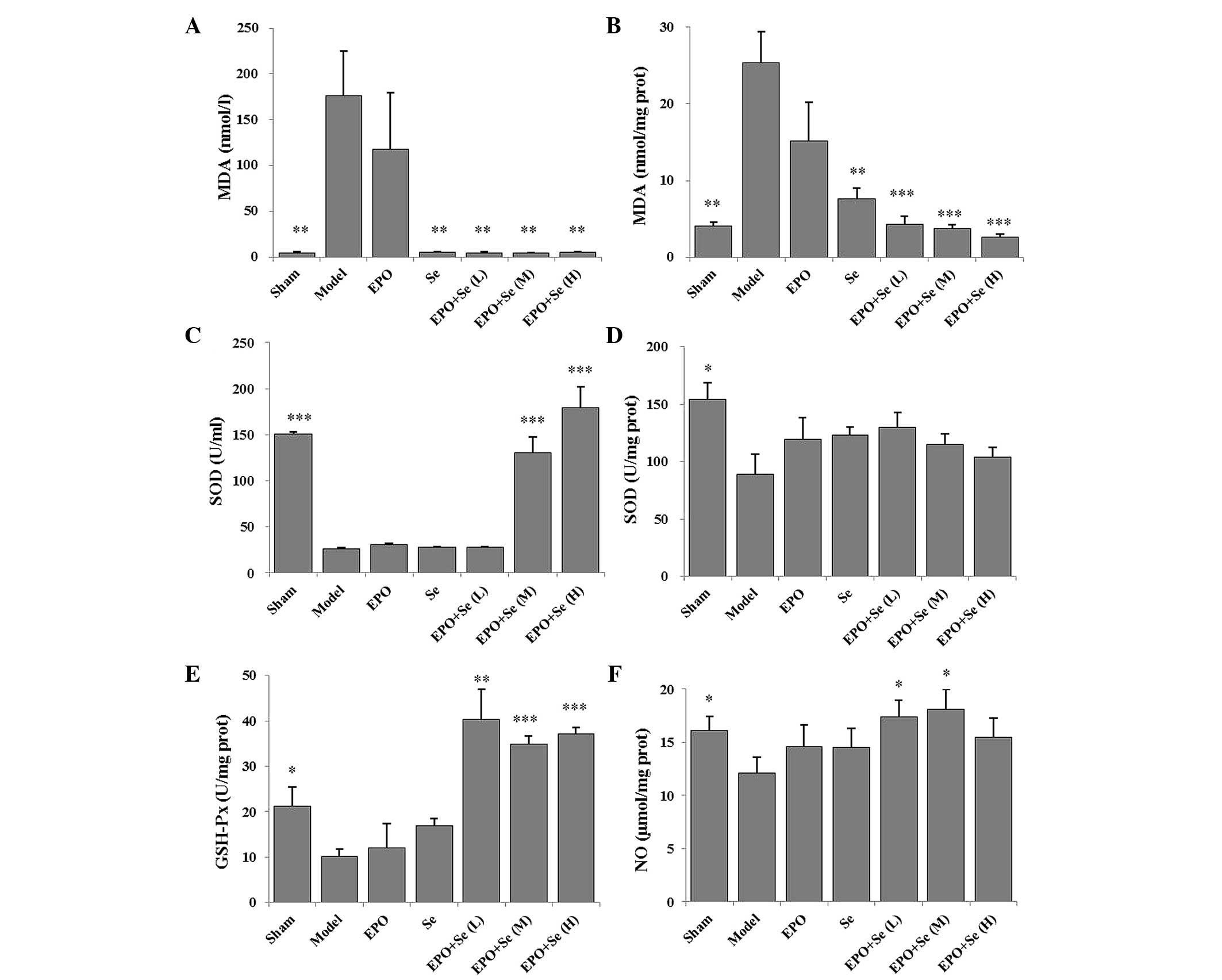 | Figure 2Effects of treatment with EPO and Se
on the expression levels of MDA, SOD, GSH–Px and NO in rats with
renal ischemia/reperfusion injury. (A) Serum expression levels of
MDA. (B) Tissue expression levels of MDA. (C) Serum SOD activity.
(D) Tissue SOD activity. (E) Tissue GSH–Px activity. (F) Tissue
expression levels of NO. Data are expressed as the mean ± standard
error of the mean. *P<0.05, **P<0.01
and ***P<0.001, vs. model group. EPO, erythropoietin;
Se, sodium selenite; MDA, malondialdehyde; SOD, superoxide
dismutase; GPH–Px, glutathione peroxidase; NO, nitric oxide. |
EPO and sodium selenite ameliorate
histological alterations in rats with RIRI
Histological alterations were observed in the renal
tissue samples of the rats in each group (Fig. 3). Histological analysis of the
H&E-stained renal sections in the sham group revealed an
integrated architectural structure of healthy glomerular and
tubular cells without necrosis (Fig.
3A). However, the renal sections of the model group showed
dilation of the Bowman's capsule, degeneration of the tubular
epithelium, necrosis of the tubular epithelium, tubular dilation,
protein cast, interstitial inflammatory cell infiltration and
congestion of the blood vessels (Fig.
3B). The degree of renal tissue damage was significantly
ameliorated in the rats pretreated with EPO and sodium selenite
(Fig. 3C–G). The tubule injury
scores (Fig. 3H) also showed that
EPO and sodium selenite reduced tubule injury in renal ischemia
reperfusion (P<0.01).
Immunohistochemical analysis of the
renal tissue samples and evaluation of the expression of PI3K
In order to further investigate the mechanisms
underlying the effects of EPO and sodium selenite on RIRI, the
protein expression levels of PI3K were quantified using
immunohistochemistry. Positive expression of PI3K was indicated by
brown staining following treatment with DAB (Fig. 4). Image–Pro Plus 6.0 was used to
analyze the immunohistochemical images, which revealed that
pre–treatment with EPO and sodium selenite significantly increased
the expression levels of PI3K in the renal tissue samples, compared
with the model group (P<0.01; Fig.
4). No differences in expression levels were observed following
treatment with EPO alone, sodium selenite alone or treatment with
EPO and sodium selenite combined (P>0.05). The results suggested
that EPO and sodium selenite upregulated the expression levels of
PI3K in the renal tissues of the RIRI rats.
Discussion
RIRI is often associated with rates of high
morbidity and mortality in acute kidney injury (32). However, the pathophysiology of RIRI
is complex and undefined, and there remains no effective treatment,
requiring future investigations into drug combinations.
Weighted modification design was first developed by
Zheng and Sun (31) using U6
(66), uniform design and 6×6 optimization latin square
design theory, and the laws of compound drug dose–effect
association to establish the multiple factors of multilevel data
analysis to develop an efficient, simple drug formula method. It
enables compound drug formula optimization, precise ratio
proportioning, and can reduce the number of experimental group
required (31). In the present
study, weighted modification design was used for combined EPO and
sodium selenite for the treatment of RIRI. In pre–renal injury, the
expression levels of BUN increase disproportionately to those of
creatinine due to the enhanced proximal tubular reabsorption, which
follows the enhanced transport of sodium and water. Acute RIRI is a
form of pre–renal injury. The results of the present study
demonstrated that co–treatment with EPO and sodium selenite induced
coordinated protection against RIRI, based on the expression levels
of BUN. EPO was the predominant effective drug, and sodium selenite
served as an adjuvant. The optimal ratio of treatment preparation
was 10:1 (10 IU EPO: 1 µg sodium selenite).
IRI leads to the generation of ROS, which are
critical in pathological processes and indirectly leads to lipid
peroxidation (33). The superoxide
radical is considered a 'primary' ROS, and is produced by the
xanthine oxidase enzyme in the early stages of ischemia. The
superoxide radical is depleted through a dismutation reaction,
which is catalyzed by SOD to produce the less reactive
H2O2, which is further converted to
H2O and O2 by catalase and GSH–Px enzymes,
preventing the formation of highly reactive hydroxyl radicals
(34,35). The results of the present study
demonstrated that pre–treatment with EPO and sodium selenite
significantly increased the expression levels of serum SOD and
renal SOD, as well as the activity of GSH–Px. MDA is a final
product of lipid peroxidation, and is generally accepted as a
sensitive marker of the rate of lipid peroxidation (34). In the present study, the serum and
renal expression levels of MDA were significantly higher in the
model group, compared with the groups pre–treated with EPO and
sodium selenite, which exhibited significantly reduced MDA content
and protected the tissue against further injury.
Concordant with these results, Hussein et al
(36) demonstrated that treatment
with EPO reduced the expression levels of renal MDA and increased
the activity of SOD and concentration of GSH, thereby improving
renal function in IRI. EPO may exert its antioxidative effects
directly by upregulating the activity of hemoglobin oxidase–1
(37). In addition, EPO may exert
beneficial effects on ischemic preconditioning indirectly by
inhibiting the activity of induced nitric oxide synthase (38), increasing the concentration of iron
and increasing the number of immature red blood cells in the
circulation to reduce cellular oxidative stress, as red blood cells
contain high levels of anti oxidative enzymes (39). Bozkurt et al (40) demonstrated that pre–treatment with
selenium significantly decreases tissue expression levels of MDA
and increases the activities of SOD and GSH–Px in tissues, which
prevents tissue oxidative damage induced IRI in rat ovaries. The
results of the present study suggested that treatment with EPO and
sodium selenite significantly reduced IRI induced oxidative stress
via antioxidative properties.
Evidence has indicated that EPO specifically
prevents the destruction of living cells surrounding areas of
injury by signaling through a non–hematopoietic receptor (41). The expression of EPO receptor is
also present in the kidney, liver, brain and heart (41–44).
Following the binding of EPO to its receptors, phosphorylation of
Janus kinase 2 occurs, which subsequently activated multiple
signaling cascades that recruit PI3K, MAPK and NF–κB (45). Activation of the PI3K/Akt signaling
pathway can regulate cell apoptosis, survival and the proliferation
of downstream factors. Akt phosphorylates and inhibits Bas,
resulting in B–cell lymphoma (Bcl) dissociation. Akt also activates
inhibitor of κB kinase α, which leads to NF–κB activation, and
induces the expression of anti–apoptotic genes, including Bcl–2,
resulting in inhibition of apoptosis (41,45).
Previous studies have reported that activation of the PI3K/Akt
signaling pathway is also essential for EPO–mediated organ
protection in renal and heart IRI (46,47).
A previous study also demonstrated that activation of the apoptosis
signal regulating kinase 1 (ASK1)/c–jun N–terminal kinase signaling
cascade in cerebral IRI, which is closely associated with oxidative
stress, can be suppressed by selenite through activation of the
PI3K/Akt signaling pathway in rat hippocampi (48). Yoon et al (49) demonstrated that selenite suppresses
H2O2 induced apoptosis, through inhibition of
ASK1 and stimulation of PI3K/Akt activities (49). The results of the present study
demonstrated that pre–treatment with EPO and sodium selenite
increased the protein expression levels of PI3K to varying degrees,
suggesting that activation of the PI3K/Akt signaling pathway during
reperfusion is important in the renal protective effects of EPO and
sodium selenite.
Further investigations demonstrated that activation
of the PI3K/Akt signaling pathway elevated the expression levels of
eNOS, thus increasing NO production. NO promotes vasorelaxation and
reduces apoptosis by decreasing oxidative stress via the inhibition
of NADPH oxidase (50). NO is also
reported to attenuate vasoconstriction, reduce apoptosis and
protect organs from IRI (51–53).
In a previous study, Teng et al (54) demonstrated that cardioprotection
effects induced by EPO were due to the stimulation of coronary
artery endothelial cells, upregulated eNOS activity and enhanced NO
production. In the present study, pre–treatment with EPO and sodium
selenite increased the tissue expression levels of NO.
In conclusion, the data of the present study
suggested that EPO and sodium selenite prevented much of the renal
damage from IRI. These beneficial effects were closely associated
with a decrease in oxidative stress and an increase in the
production of NO via stimulation of the PI3K/NO signaling pathway.
To the best of our knowledge, the present study is the first to
report that EPO and sodium selenite exhibit synergistic protection
on RIRI, and has important potential implications in the
development of novel strategies to prevent and treat renal
disease.
Abbreviations:
|
RIRI
|
renal ischemic/reperfusion injury
|
|
ROS
|
reactive oxygen species
|
|
EPO
|
erythropoietin
|
|
EPOR
|
erythropoietin receptor
|
|
MAPK
|
mitogen-activated protein kinase
|
|
NFκB
|
nuclear factorκB
|
|
PI3K
|
phosphatidylinositol-3 kinase
|
|
Se
|
sodium selenite
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
|
GSH-Px
|
glutathione peroxidase
|
|
BUN
|
blood urea nitrogen
|
|
Cr
|
creatinine
|
|
eNOS
|
endothelial nitric oxide synthase
|
|
NO
|
nitric oxide
|
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant. nos. 81471031
and 30901808), the Technology and Innovation Project of the Shaanxi
Province (grant. no. 2011KTCL03–20) and the National College
Students Innovation Training Project (grant. no. 201210698117).
References
|
1
|
Xia D, Shen K, Zhong W and Pan H:
Administration of minocycline ameliorates damage in a renal
ischemia/reperfusion injury model. Clin Invest Med. 34:E55–E63.
2011.PubMed/NCBI
|
|
2
|
Li YW, Zhang Y, Zhang L, Li X, Yu JB,
Zhang HT, Tan BB, Jiang LH, Wang YX, Liang Y, et al: Protective
effect of tea polyphenols on renal ischemia/reperfusion injury via
suppressing the activation of TLR4/NF–κB p65 signal pathway. Gene.
542:46–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salvadori M, Rosso G and Bertoni E: Update
on ischemia–reperfusion injury in kidney transplantation:
Pathogenesis and treatment. World J Transplant. 5:52–67.
2015.PubMed/NCBI
|
|
4
|
Kiliç K, Hanci V, Selek S, Sözmen M, Kiliç
N, Citil M, Yurtlu DA and Yurtlu BS: The effects of dexmedetomidine
on mesenteric arterial occlusion–associated gut ischemia and
reperfusion–induced gut and kidney injury in rabbits. J Surg Res.
178:223–232. 2012. View Article : Google Scholar
|
|
5
|
Cong G, Cui L, Zang M and Hao L:
Attenuation of renal ischemia/reperfusion injury by a
polysaccharide from the roots of Dipsacus asperoides. Int J Biol
Macromol. 56:14–19. 2013. View Article : Google Scholar
|
|
6
|
Wang F, Yu G, Liu SY, Li JB, Wang JF, Bo
LL, Qian LR, Sun XJ and Deng XM: Hydrogen–rich saline protects
against renal ischemia/reperfusion injury in rats. J Surg Res.
167:e339–e344. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He X, Xu X, Fan M, Chen X, Sun X, Luo G,
Chen L, Mu Q, Feng Y, Mao Q and Chao Z: Preconditioning with
hyperbaric oxygen induces tolerance against renal
ischemia/reperfusion injury via increased expression of heme
oxygenase–1. J Surg Res. 170:e271–e277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qiao X, Li RS, Li H, Zhu GZ, Huang XG,
Shao S and Bai B: Intermedin protects against renal
ischemia/reperfusion injury by inhibition of soxidative stress. Am
J Physiol Renal Physiol. 304:F112–F119. 2013. View Article : Google Scholar
|
|
9
|
Fisher JW: Erythropoietin: Physiology and
pharmacology update. Exp Biol Med (Maywood). 228:1–14. 2003.
|
|
10
|
Lippi G, Franchini M and Banfi G:
Biochemistry and physiology of anabolic androgenic steroids doping.
Mini Rev Med Chem. 11:362–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dimitrijevic ZM, Cvetkovic TP, Djordjevic
VM, Pavlovic DD, Stefanovic NZ, Stojanovic IR, Paunovic GJ and
Velickovic–Radovanovic RM: How the duration period of
erythropoietin treatment influences the oxidative status of
hemodialysis patients. Int J Med Sci. 9:808–815. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nairz M, Sonnweber T, Schroll A, Theurl I
and Weiss G: The pleiotropic effects of erythropoietin in infection
and inflammation. Microbes Infect. 14:238–246. 2012. View Article : Google Scholar :
|
|
13
|
Stoyanoff TR, Todaro JS, Aguirre MV,
Zimmermann MC and Brandan NC: Amelioration of
lipopolysaccharide–induced acute kidney injury by erythropoietin:
Involvement of mitochondria–regulated apoptosis. Toxicology.
318:13–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen S, Li J, Peng H, Zhou J and Fang H:
Administration of erythropoietin exerts protective effects against
glucocorticoid–induced osteonecrosis of the femoral head in rats.
Int J Mol Med. 33:840–848. 2014.PubMed/NCBI
|
|
15
|
Pellegrini L, Bennis Y, Velly L,
Grandvuillemin I, Pisano P, Bruder N and Guillet B: Erythropoietin
protects newborn rat against sevoflurane–induced neurotoxicity.
Paediatr Anaesth. 24:749–759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gul M, Cömert M, Çakmak GK, Kertis G,
Ugurbas E and Oner MO: Effect of erythropoietin on liver
regeneration in an experimental model of partial hepatectomy. Int J
Surg. 11:59–63. 2013. View Article : Google Scholar
|
|
17
|
Yu Y, Shiou SR, Guo Y, Lu L, Westerhoff M,
Sun J, Petrof EO and Claud EC: Erythropoietin protects epithelial
cells from excessive autophagy and apoptosis in experimental
neonatal necrotizing enterocolitis. PLoS One. 8:e696202013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Zou YR, Zhong X, Deng HD, Pu L,
Peng K and Wang L: Erythropoietin pretreatment ameliorates renal
ischaemia–reperfusion injury by activating PI3K/Akt signalling.
Nephrology (Carlton). 20:266–272. 2015. View Article : Google Scholar
|
|
19
|
Kwon MS, Kim MH, Kim SH, Park KD, Yoo SH,
Oh IU, Pak S and Seo YJ: Erythropoietin exerts cell protective
effect by activating PI3K/Akt and MAPK pathways in C6 Cells. Neurol
Res. 36:215–223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li XJ, Zhang GX, Sun N, Sun Y, Yang LZ and
Du YJ: Protective effects of erythropoietin on endotoxin–related
organ injury in rats. J Huazhong Univ Sci Technolog Med Sci.
33:680–686. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao L, Lu P, Li Y, Yang L, Feng H, Huang
Y, Zhang D, Chen J and Zhu D: Osthole relaxes pulmonary arteries
through endothelial phosphatidylinositol 3–kinase/Akt–eNOS–NO
signaling pathway in rats. Eur J Pharmacol. 699:23–32. 2013.
View Article : Google Scholar
|
|
22
|
Ošt'ádalová I: Biological effects of
selenium compounds with a particular attention to the ontogenetic
development. Physiol Res. 61(Suppl 1): S19–S34. 2012.
|
|
23
|
Guo F, Monsefi N, Moritz A and
Beiras–Fernandez A: Selenium and cardiovascular surgery: An
overview. Curr Drug Saf. 7:321–327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Turan B, Saini HK, Zhang M, Prajapati D,
Elimban V and Dhalla NS: Selenium improves cardiac function by
attenuating the activation of NF–kappaB due to ischemia/reperfusion
injury. Antioxid Redox Signal. 7:1388–1397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karaman S, Mansuroğlu B, Kizilbey K,
Derman S and Hazar AB: Selenium status in blood, urine, and hair
samples of newly diagnosed pediatric cancer patients. Turk J Med
Sci. 45:329–334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi XW, Guo X, Ren FL, Li J and Wu XM: The
effect of short tandem repeat loci and low selenium levels on
endemic osteoarthritis in China. J Bone Joint Surg Am. 92:72–80.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mehta SL, Kumari S, Mendelev N and Li PA:
Selenium preserves mitochondrial function, stimulates mitochondrial
biogenesis, and reduces infarct volume after focal cerebral
ischemia. BMC Neurosc. 13(79)2012. View Article : Google Scholar
|
|
28
|
Treska V, Kuntscher V, Hasman D, Neprasová
P, Kobr J, Racek J, Trefil L and Hes O: Importance of selenium for
the influence of ischemia–reperfusion syndrome after kidney
transplantation from a non–heart beating donor in a pig model.
Transplant Proc. 34:3057–3059. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin TT, Wang BM, Li XY, Pan Y, Wang W, Mu
Y, Liu JQ, Shen JC and Luo GM: An insight into the protection of
rat liver against ischemia/reperfusion injury by 2–selenium–bridged
beta–cyclodextrin. Hepatol Res. 39:1125–1136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ostadalova I, Vobecky M, Chvojkova Z,
Mikova D, Hampl V, Wilhelm J and Ostadal B: Selenium protects the
immature rat heart against ischemia/reperfusion injury. Mol Cell
Biochem. 300:259–267. 2007. View Article : Google Scholar
|
|
31
|
Zheng QS and Sun RY: Quantitative design
of drug compatibility by weighted modification method. Zhongguo Yao
Li Xue Bao. 20:1043–1051. 1999.
|
|
32
|
Shih YC, Lee PY, Cheng H, Tsai CH, Ma H
and Tarng DC: Adipose derived stem cells exhibit antioxidative and
anti–apoptotic properties to rescue ischemic acute kidney injury in
rats. Plast Reconstr Surg. 132:940e–951e. 2013. View Article : Google Scholar
|
|
33
|
Wang HB, Li YX, Hao YJ, Wang TF, Lei Z, Wu
Y, Zhao QP, Ang H, Ma L and Liu J: et al: Neuroprotective effects
of LBP on brain ischemic reperfusion neurodegeneration. Eur Rev Med
Pharmacol Sci. 17:2760–2765. 2013.PubMed/NCBI
|
|
34
|
Kim J, Jang HS and Park KM: Reactive
oxygen species generated by renal ischemia and reperfusion trigger
protection against subsequent renal ischemia and reperfusion injury
in mice. Am J Physiol Renal Physiol. 298:F158–F166. 2010.
View Article : Google Scholar
|
|
35
|
Guo C, Tong L, Xi M, Yang H, Dong H and
Wen A: Neuroprotective effect of calycosin on cerebral ischemia and
reperfusion injury in rats. J Ethnopharmacol. 144:768–774. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hussein A, Shokeir AA, Sarhan ME,
El–Menabawy FR, Abd Elmoneim HA, El–Nashar EM and Barakat NM:
Effects of combined erythropoietin and epidermal growth factor on
renal ischaemia/reperfusion injury: A randomized experimental
controlled study. BJU Int. 107:323–328. 2011. View Article : Google Scholar
|
|
37
|
Luo YH, Li ZD, Liu LX and Dong GH:
Pretreatment with erythropoietin reduces hepatic
ischemia/reperfusion injury. Hepatobiliary Pancreat Dis Int.
8:294–299. 2009.PubMed/NCBI
|
|
38
|
Yang FL, Subeq YM, Chiu YH, Lee RP, Lee CJ
and Hsu BG: Recombinant human erythropoietin reduces
rhabdomyolysis–induced acute renal failure in rats. Injury.
43:367–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu N, Tian J, Wang W, Cheng J, Hu D and
Zhang J: Effect and mechanism of erythropoietin on mesenchymal stem
cell proliferation in vitro under the acute kidney injury
microenvironment. Exp Biol Med (Maywood). 236:1093–1099. 2011.
View Article : Google Scholar
|
|
40
|
Bozkurt S, Arikan DC, Kurutas EB, Sayar H,
Okumus M, Coskun A and Bakan V: Selenium has a protective effect on
ischemia/reperfusion injury in a rat ovary model: Biochemical and
histopathologic evaluation. J Pediatr Surg. 47:1735–1741. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brines M and Cerami A: Emerging biological
roles for erythropoietin in the nervous system. Nat Rev Neurosci.
6:484–494. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lei DM, Piao SG, Jin YS, Jin H, Cui ZH,
Jin HF, Jin JZ, Zheng HL, Li JJ, Jiang YJ, Yang CW and Li C:
Expression of erythropoietin and its receptor in kidneys from
normal and cyclosporine–treated rats. Transplant Proc. 46:521–528.
2014. View Article : Google Scholar
|
|
43
|
Yang XF, He Y, Li HY, Liu X, Chen H, Liu
JB, Ji WJ, Wang B and Chen LN: Hepatoprotective effects of
erythropoietin on D galactosamine/lipopolysaccharide–induced
fulminant hepatic failure in mice. Mol Med Rep. 10:555–559.
2014.PubMed/NCBI
|
|
44
|
Sanchis–Gomar F, Garcia–Gimenez JL, Pareja
Galeano H, Romagnoli M, Perez–Quilis C and Lippi G: Erythropoietin
and the heart: physiological effects and the therapeutic
perspective. Int J Cardiol. 171:116–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rusai K, Prókai A, Szebeni B, Fekete A,
Treszl A, Vannay A, Müller V, Reusz G, Heemann U, Lutz J, et al:
Role of serum and glucocorticoid–regulated kinase–1 in the
protective effects of erythropoietin during renal
ischemia/reperfusion injury. Biochem Pharmacol. 79:1173–1181. 2010.
View Article : Google Scholar
|
|
46
|
Yang C, Zhao T, Lin M, Zhao Z, Hu L, Jia
Y, Xue Y, Xu M, Tang Q, Yang B, et al: Helix B surface peptide
administered after insult of ischemia reperfusion improved renal
function, structure and apoptosis through beta common
receptor/erythropoietin receptor and PI3K/Akt pathway in a murine
model. Exp Biol Med (Maywood). 238:111–119. 2013. View Article : Google Scholar
|
|
47
|
Xu X, Cao Z, Cao B, Li J, Guo L, Que L, Ha
T, Chen Q, Li C and Li Y: Carbamylated erythropoietin protects the
myocardium from acute ischemia/reperfusion injury through a
PI3K/Akt–dependent mechanism. Surgery. 146:506–514. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang Q, Zhang QG, Wu DN, Yin XH and Zhang
GY: Neuroprotection of selenite against ischemic brain injury
through negatively regulating early activation of ASK1/JNK cascade
via activation of PI3K/AKT pathway. Acta Pharmacol Sin. 28:19–27.
2007. View Article : Google Scholar
|
|
49
|
Yoon SO, Kim MM, Park SJ, Kim D, Chung J
and Chung AS: Selenite suppresses hydrogen peroxide induced cell
apoptosis through inhibition of ASK1/JNK and activation of
PI3–K/Akt pathways. FASEB J. 16:111–113. 2002.
|
|
50
|
Mastromarino V, Volpe M, Musumeci MB,
Autore C and Conti E: Erythropoietin and the heart: Facts and
perspectives. Clin Sci (Lond). 120:51–63. 2011. View Article : Google Scholar
|
|
51
|
Gliemann L, Nyberg M and Hellsten Y:
Nitric oxide and reactive oxygen species in limb vascular function:
What is the effect of physical activity? Free Radic Res. 8:71–83.
2014. View Article : Google Scholar
|
|
52
|
Dziodzio T, Biebl M and Pratschke J:
Impact of brain death on ischemia/reperfusion injury in liver
transplantation. Curr Opin Organ Transplant. 19:108–114. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang W, Han Y, Meng G, Bai W, Xie L, Lu
H, Shao Y, Wei L, Pan S, Zhou S, et al: Direct Renin inhibition
with aliskiren protects against myocardial ischemia/reperfusion
injury by activating nitric oxide synthase signaling in
spontaneously hypertensive rats. J Am Heart Assoc. 3:e0006062014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Teng R, Calvert JW, Sibmooh N, Piknova B,
Suzuki N, Sun J, Martinez K, Yamamoto M, Schechter AN, Lefer DJ and
Noguchi CT: Acute erythropoietin cardioprotection is mediated by
endothelial response. Basic Res Cardiol. 106:343–354. 2011.
View Article : Google Scholar : PubMed/NCBI
|


















