Introduction
Ischemic stroke is currently a leading cause of
cerebrovascular disease worldwide, and exhibits a high morbidity
and mortality among patients (1).
Ischemic stroke is induced by a transient or permanent occlusion in
the cerebral vessel, resulting in neuronal death and associated
behavioral deficits, including sensorimotor dysfunction, spatial
orientation disorder, and learning and memory impairment (2–4). In
addition, the mechanisms underlying stroke include oxidative
stress, blood-brain barrier dysfunction, neuronal apoptosis and
inflammation (5,6). Although tissue-type plasminogen
activator is used clinically and remains the only FDA-approved
treatment for ischemic stroke, it is not so effective for all
patients and only a small number of patients recover as a result of
the reperfusion injury and a narrow 3 h time-window for safe
administration (7). Therefore,
other effective therapeutic agents are required to assist the
patients with their diseases.
Lycopene, a member of the carotenoid family, is
found predominantly in tomatoes and other red colored fruits
(8). It has been previously
reported that lycopene has several biological functions in various
diseases. Lycopene protects the cell from lipid peroxidation and
oxidative DNA damage as a highly efficient antioxidant (8,9). In
addition, lycopene exhibits other properties, including
antiapoptosis (10),
anti-inflammation (11,12), antiamyloid (13), anti-ischemia (14), and antitumor properties (15). Since lycopene has a high
liposolubility, it can cross the blood-brain barrier (16). It has also been demonstrated that
lycopene is beneficial for certain neurological disorders,
including Alzheimer's disease (17,18).
Therefore, lycopene is potentially beneficial in other brain
diseases and the present study set out to investigate this.
Oxidative stress is important in ischemic stroke,
characterized by a dramatic increase in reactive oxygen species
(19). In normal cells, the
antioxidant system protects cells from various oxidative stresses.
Antioxidant/electrophile response element (ARE)-regulated phase II
detoxifying enzymes and antioxidants are one of the predominant
antioxidant pathways involved in attenuating increased oxidative
stress and maintaining the redox status in several tissues and
organs (20). Heme oxygenase-1
(HO-1) is an ARE-regulated enzyme and antioxidant, which is
regulated by the redox-sensitive transcription factor, nuclear
factor erythroid 2-related factor (Nrf2) (21). The function of HO-1 is to catalyze
heme to biliverdin, carbon monoxide and iron. It has been
previously reported that Nrf-2 activation protects the neurons from
ischemia (22). Under
physiological conditions, Nrf2 is located in the cytosol and binds
to Kelch-like ECH-associated protein 1 (Keap1). In response to
oxidative stress, Nrf2 dislocates from Keap1 and translocates to
the nucleus (23,24), where it forms a heterodimer with
its obligatory partner, Maf, and binds to the ARE sequence to
activate the transcription of numerous antioxidative and
electrophile detoxification genes, including HO-1, NAD(P)H:quinine
oxidoreductase 1 and glutamate-cysteine ligase (25).
The present study aimed to investigate whether
lycopene exerts a neuroprotective effect on the ischemic brain in a
bilateral common carotid artery occlusion (BCCAO) model. If so, the
present study aimed to determine whether it regulates Nrf2/HO-1
signaling in this ischemic model.
Materials and methods
Animals
A total of 60 C57BL/6 mice, aged 12 weeks and
weighing 20–24 g, were used in the experiments and were provided by
the Experimental Animal Center of the Tianjin Medical University
(Tianjin, China). The mice were maintained in cages under a
controlled-light environment (12 h light/dark cycles) and were
allowed free access to a rodent diet and tap water. The present
study was approved by the Ethics Committee of Tianjin Medical
University. All animals used in this study were cared for in
accordance with the Guidance for the Care and Use of Laboratory
Animals published by the United States National Institute of
Health.
Establishment of global cerebral
ischemia
BCCAO was used as a model of global cerebral
ischemia, as previously reported (26). Surgical operation was performed by
an individual in a blinded manner. The mice were anesthetized with
3% isoflurane (Baxter, Deerfield, IL, USA). Following induction,
the concentration of isoflurane was maintained at 1.5%. Isoflurane
was administered via a face mask, which was constructed to fit over
the animals' frontal area. A midline incision was made to the
region between the neck and sternum to expose the trachea. The
right and left common carotid arteries were located lateral to the
sternocleidomastoid and were carefully separated. Cerebral ischemia
was induced by clamping each of the arteries with two miniature
artery clips. Following 20 min of cerebral ischemia, the clips were
removed from each artery to allow for the reperfusion of blood
through the carotid arteries. Sham-operated mice underwent the
identical surgical procedure without artery occlusion. During the
surgical procedure, the pericranial temperature was monitored using
a temperature probe and maintained at 37.0–37.5°C using a heating
pad. Following surgery, the animals were placed in a warm
environment (30–33°C) to avoid biased results due to
hypothermia.
Drug administration
For the BCCAO + lycopene group, lycopene was
intraperitoneally administered at a dose of 20 mg/kg for seven
consecutive days prior to surgery. The mice in the sham group and
BCCAO group were injected solely with an equal concentration of
dimethyl sulfoxide (DMSO). Lycopene was dissolved in 2% DMSO.
Neurological tests
The treated mice were allowed to recover for 24 h
prior to subsequent tests. The mice were subjected to a modified
neurological examination designed to detect motor deficits.
Briefly, the mice were placed on a 10–20 cm screen (grid size
0.2×0.2 cm), which can be rotated from 0° (horizontal) to 90°
(vertical). The mice were placed on this screen, which was in a
horizontal position, and the screen was then rotated into the
vertical plane. The duration for which each mouse was able to hold
on to the vertical screen was recorded up to a maximum of 15 sec
(corresponding to a maximum of three points). Next, the mouse was
placed at the center of a horizontal wooden rod (diameter, 1.5 cm),
and the duration that the mouse was able to remain balanced on the
rod was recorded up to a maximum of 30 sec (corresponding to a
maximum of three points). Finally, a prehensile traction test was
performed. The duration that the mouse was able to cling to a
horizontal rope was recorded up to a maximum of 5 sec
(corresponding to a maximum of three points). From these tests, a
total motor score (TMS; nine possible points) was calculated. The
neurological assessments were performed at 24, 48 or 72 h
post-reperfusion by an observer in a blinded manner. The TMS has
been shown previously to be an accurate method for evaluating
global cerebral ischemic injury in mice (27).
Hematoxylin and eosin (HE) staining
Neuronal damage was assessed using HE staining
(Beyotime Institute of Biotechnology, Shanghai, China). On day 3
following the induction of ischemia, the animals were anesthetized
with sodium pentobarbital (50 mg/kg intraperitoneally;
Sigma-Aldrich, St. Louis, MO, USA) and transcardially perfused with
4% phosphate-buffered paraformaldehyde, following a flush with 0.1
M phosphate-buffered saline (PBS). The brains were removed,
post-fixed at 4°C in 4% paraformaldehyde overnight and then
sectioned on a freezing microtome. The brains were sectioned
backward from the optic chiasm into six consecutive sections (12
µm), which included the dorsal hippocampus, and were stained
with HE. The pyramidal neurons of the CA1 region were examined.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL)
The tissue sections were placed on slides and
incubated with TUNEL reaction mixture (Roche Diagnostics GmbH,
Mannheim, Germany), including enzyme solution (terminal
deoxynucleotidyl transferase) and tetramethylrhodamine-labeled
TUNEL-positive nucleotides, in a dark humidified chamber for 1 h at
37°C, followed by a final wash for 3×10 min with PBS and then
covered with water-based mounting medium (National Diagnotics,
Atlanta, GA, USA). The captured images were viewed and analyzed
using laser scanning confocal microscopy (FV1000; Olympus, Tokyo,
Japan).
Western blot analysis
Proteins were extracted following brain tissue
homogenization in radioimmunoprecipitation acid buffer (EMD
Millipore, Billerica, MA, USA). The total protein content was
determined using a bicinchoninic acid protein assay. The protein
samples (50 µg) were separated by electrophoresis on
SDS-PAGE gels (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) and were transferred onto polyvinylidene difluoride
membranes (EMD Millipore). The membranes were blocked with 5%
non-fat milk at room temperature for 2 h and were incubated
overnight with the appropriate primary antibodies. The antibodies
used were rabbit polyclonal anti-Nrf2 (1:200; cat. no. sc-13032;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), rabbit
polyclonal anti-HO-1 (1:200; cat. no. sc-10789; Santa Cruz
Biotechnology, Inc.), rabbit polyclonal anti-Histone1 (1:1,000;
cat. no. ab4270; Abcam, Cambridge, MA, USA), and mouse monoclonal
anti-β-actin (1:5,000; cat. no. sc-47778; Santa Cruz Biotechnology,
Inc.). Following extensive rinsing with Tris-buffered saline,
containing 0.1% Triton X-100 buffer, the membranes were incubated
with mouse anti-rabbit (cat. no. sc-2357) and goat anti-mouse (cat.
no. sc-2005) horseradish peroxidase-conjugated secondary antibodies
(1:2,000; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. The membranes were developed and a bar graph was
produced to depict the ratios of semi-quantitative results obtained
by scanning reactive bands and quantifying the optical density
using Image Lab version 4.0 software (Bio-Rad Laboratories,
Hercules, CA, USA).
Statistical analysis
All statistical analyses were performed using SPSS
11.0 for Windows software (SPSS, Inc., Chicago, IL, USA). All
values, with the exception of TMS, are presented as the mean ±
standard error of the mean, and were analyzed using a one-way
analysis of variance. Between-groups, differences were detected
based on post-hoc Student-Newman-Keuls tests. The TMS are expressed
as the medians and were analyzed using the Kruskal-Wallis test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Neurological score
As shown in Fig. 1,
the neurological score in the BCCAO group markedly decreased
compared with that in the sham group (P<0.05) at 24, 48 and 72 h
following the induction of ischemia. Lycopene treatment ameliorated
the injury and the neurological score was increased compared with
that in the BCCAO group (P<0.05).
HE staining
A total of 3 days following reperfusion, the number
of viable neurons in the CA1 region was markedly decreased in the
BCCAO group. Lycopene treatment significantly reduced the neuronal
degeneration in the CA1 region compared with that in the BCCAO
group (Fig. 2).
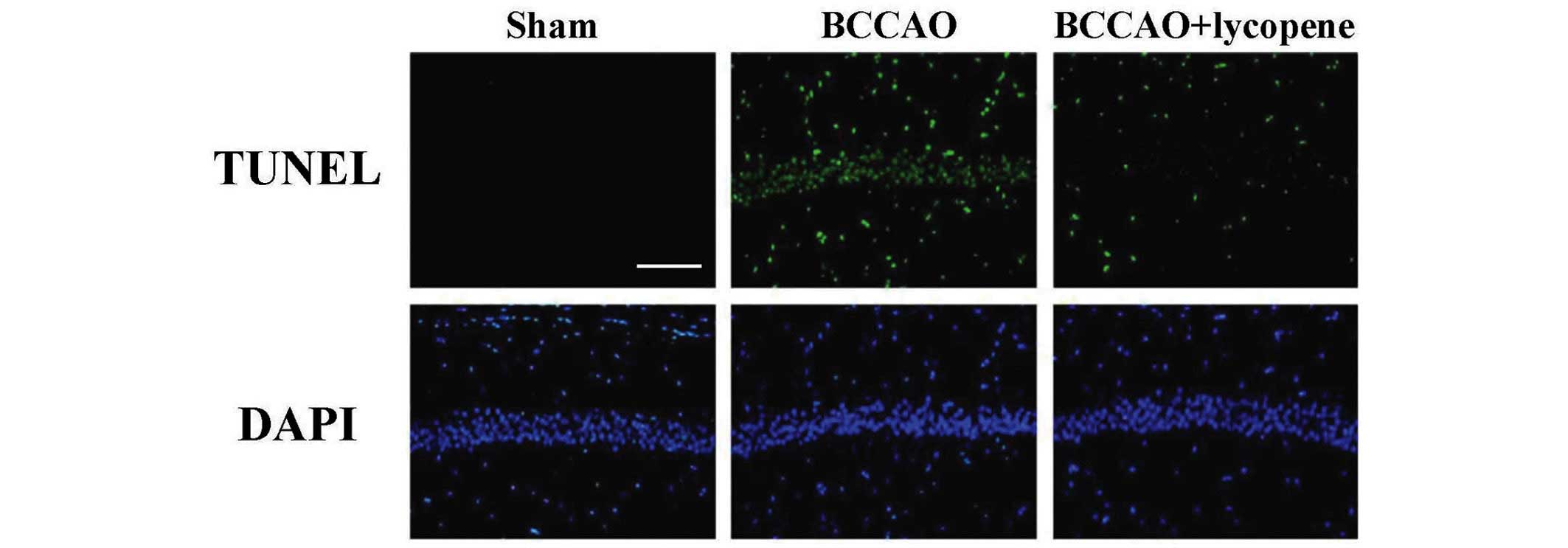
TUNEL
As shown in Fig. 3,
ischemia induced a marked neuronal apoptotic response compared with
the sham group. The survival of the neurons was markedly increased
when lycopene was administered, suggesting that lycopene treatment
attenuated the apoptosis of neurons, as indicated by the decrease
in the number of TUNEL-positive neurons in the CA1 region.
Oxidative stress
Global cerebral ischemia induced a dramatic decrease
in the production of GSH and a significant increase in the
production of reactive oxygen species (ROS). When lycopene was
administrated, the production of GSH was increased (P<0.05) and
the production of ROS was decreased (P<0.05), indicating that
lycopene protects the ischemic brain from oxidative stress
(Fig. 4).
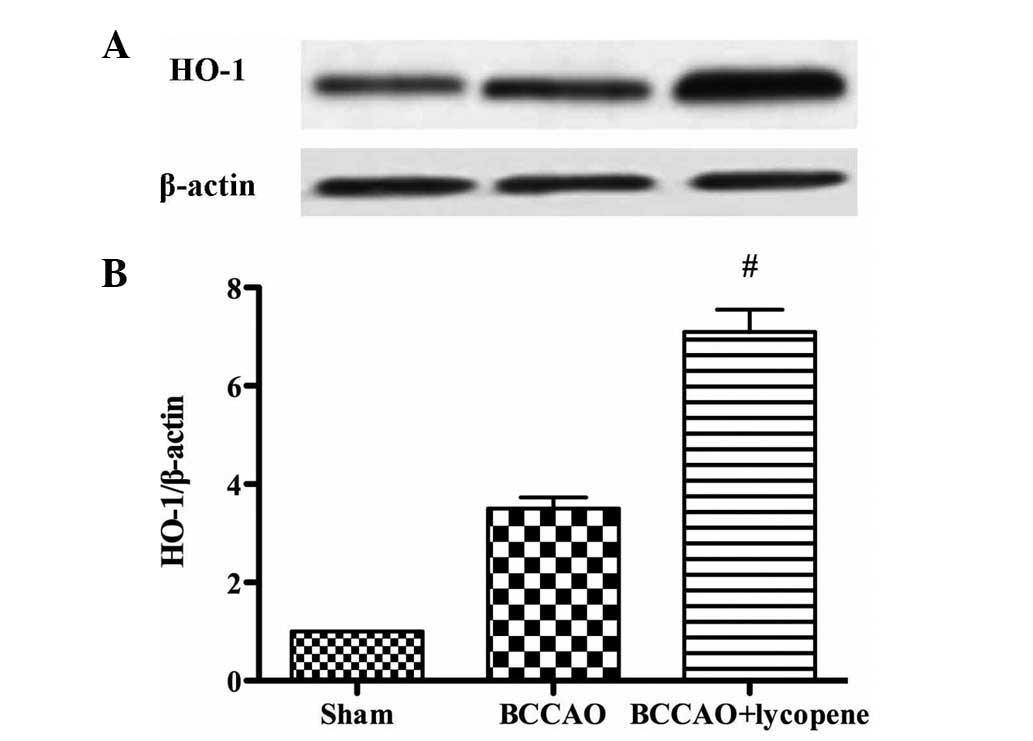
Effect of lycopene on the expression of
Nrf2 and HO-1
As shown in Figs. 5
and 6, the expression levels of
nuclear and total Nrf2 and HO-1 in the hippocampus were detected by
western blotting. The nuclear and total Nrf2 were markedly
upregulated in the lycopene treatment group. In addition, lycopene
significantly upregulated the expression of HO-1.
Discussion
The results of the present study demonstrated that
lycopene preconditioning has a neuroprotective effect in cerebral
ischemia-reperfusion in mice. Lycopene preconditioning
significantly improved the TMS and reduced neuronal death following
cerebral ischemia-reperfusion. It was revealed that lycopene
pretreatment induced an antiapoptotic effect and an antioxidative
stress effect, which is demonstrated by its ability to increase the
production of GSH and to decrease the production of ROS. In
addition, lycopene activated the expression of Nrf2 and HO-1 in
this global ischemic model.
Pyramidal neurons in the hippocampal CA1 region are
particularly vulnerable to ischemia. This region undergoes delayed
neuronal death, often reported as apoptosis in collaboration with
DNA fragmentation (28). In the
present study, a model of BCCAO was established and it was
subsequently determined that lycopene pretreatment protected the
brain from the ischemic injury, which is associated with its
anti-apoptotic effect and its antioxidative stress effect.
Neuronal apoptosis is an important pathological
process of ischemic stroke (29,30).
Rabuffetti et al (31)
demonstrated that the inhibition of apoptosis reduces ischemic
injury. Although two pathways of apoptosis, extrinsic and
intrinsic, have been recognized (32), the final phase of apoptosis
execution, which includes activation of executioner caspases (e.g.
caspase 3), is shared by each of these pathways (33). Lycopene, a member of the carotenoid
family, is found predominantly in tomatoes and other red colored
fruits (8). It has been reported
that lycopene protects against apoptosis in
hypoxia/reoxygenation-induced H9C2 myocardioblast cells (34). In addition, He et al
(35) reported that lycopene
attenuates inflammation and apoptosis in postmyocardial infarction
remodeling. The protective effect of lycopene in retinal
ischemia/reperfusion injury has been demonstrated. It has been
suggested that lycopene reduces the apoptosis of cells in the
ganglion cell layer (36). The
present study is in agreement with these studies and the results
suggested that lycopene attenuated neuronal apoptosis in global
ischemic brain.
Oxidative stress is also significant in ischemic
brain injury (37). In
ischemia/reperfusion injury, ROS is markedly produced and the
endogenous antioxidant system cannot eliminate many of them. As a
result, ROS leads to lipid peroxidation and DNA damage. It has been
suggested that lycopene protects pancreatic acinar cells against
severe acute pancreatitis (38).
Additionally, lycopene prevents experimental priapism against
oxidative damage, as reported previously (39), and attenuates oxidative stress in
fructose-drinking insulin resistant rats (40). Consistent with these studies, the
present study suggested that lycopene attenuates oxidative stress
induced by global ischemia in the brain.
HO-1 is a rate-limiting enzyme, catalyzing the
degradation of heme into carbon monoxide, biliverdin and ferritin
(41). HO-1 is regulated by the
transcription factor Nrf2 at the transcriptional level (42). Under physiological conditions, Nrf2
is located in the cytosol by binding to Keap1 (43). In the presence of ROS, Nrf2 is
released from Keap1 and translocates into the nucleus, activating
the transcription of HO-1. In the present study, brain
ischemia/reperfusion injury leads a dramatic increase in the
generation of ROS. Consequently, nuclear Nrf2 was increased and
HO-1 was upregulated following ischemia-reperfusion injury. In
addition, lycopene pretreatment significantly induced an increase
in the expression levels of Nrf2 and HO-1.
In conclusion, these findings suggested that
lycopene provided significant neuroprotection in mice subjected to
global cerebral ischemia by inhibiting neuronal apoptosis and
attenuating oxidative stress, which is associated with the
activation of Nrf2/HO-1 signaling.
References
|
1
|
Li M, Qu YZ, Zhao ZW, Wu SX, Liu YY, Wei
XY, Gao L and Gao GD: Astragaloside IV protects against focal
cerebral ischemia/reperfusion injury correlating to suppression of
neutrophils adhesion-related molecules. Neurochem Int. 60:458–465.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amano M, Hasegawa M, Hasegawa T and
Nabeshima T: Characteristics of transient cerebral ischemia-induced
deficits on various learning and memory tasks in male Mongolian
gerbils. Jpn J Pharmacol. 63:469–477. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Csiszar A: Anti-inflammatory effects of
resveratrol: Possible role in prevention of age-related
cardiovascular disease. Ann N Y Acad Sci. 1215:117–122. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jung JE, Kim GS, Chen H, Maier CM,
Narasimhan P, Song YS, Niizuma K, Katsu M, Okami N, Yoshioka H, et
al: Reperfusion and neurovascular dysfunction in stroke: from basic
mechanisms to potential strategies for neuroprotection. Mol
Neurobiol. 41:172–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
del Zoppo GJ and Mabuchi T: Cerebral
microvessel responses to focal ischemia. J Cereb Blood Flow Metab.
23:879–894. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Diaz-Ruiz A, Zavala C, Montes S,
Ortiz-Plata A, Salgado-Ceballos H, Orozco-Suarez S, Nava-Ruiz C,
Pérez-Neri I, Perez-Severiano F and Ríos C: Antioxidant,
antiinflammatory and antiapoptotic effects of dapsone in a model of
brain ischemia/reperfusion in rats. J Neurosci Res. 86:3410–3419.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Armstead WM, Ganguly K, Kiessling JW,
Riley J, Chen XH, Smith DH, Stein SC, Higazi AA, Cines DB, Bdeir K,
et al: Signaling, delivery and age as emerging issues in the
benefit/risk ratio outcome of tPA For treatment of CNS ischemic
disorders. J Neurochem. 113:303–312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuhad A, Sethi R and Chopra K: Lycopene
attenuates diabetes-associated cognitive decline in rats. Life Sci.
83:128–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sandhir R, Mehrotra A and Kamboj SS:
Lycopene prevents 3-nitropropionic acid-induced mitochondrial
oxidative stress and dysfunctions in nervous system. Neurochem Int.
57:579–587. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujita K, Yoshimoto N, Kato T, Imada H,
Matsumoto G, Inakuma T, Nagata Y and Miyachi E: Lycopene inhibits
ischemia/reperfusion-induced neuronal apoptosis in gerbil
hippocampal tissue. Neurochem Res. 38:461–469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ip BC, Hu KQ, Liu C, Smith DE, Obin MS,
Ausman LM and Wang XD: Lycopene metabolite, apo-10′-lycopenoic
acid, inhibits diethylnitrosamine-initiated, high fat diet-promoted
hepatic inflammation and tumorigenesis in mice. Cancer Prev Res
(Phila). 6:1304–1316. 2013. View Article : Google Scholar
|
|
12
|
Palozza P, Simone R, Catalano A, Monego G,
Barini A, Mele MC, Parrone N, Trombino S, Picci N and Ranelletti
FO: Lycopene prevention of oxysterol-induced proinflammatory
cytokine cascade in human macrophages: Inhibition of NF-κB nuclear
binding and increase in PPARγ expression. J Nutr Biochem.
22:259–268. 2011. View Article : Google Scholar
|
|
13
|
Qu M, Li L, Chen C, Li M, Pei L, Chu F,
Yang J, Yu Z, Wang D and Zhou Z: Protective effects of lycopene
against amyloid β-induced neurotoxicity in cultured rat cortical
neurons. Neurosci Lett. 505:286–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yue R, Xia X, Jiang J, Yang D, Han Y, Chen
X, Cai Y, Li L, Wang WE and Zeng C: Mitochondrial DNA oxidative
damage contributes to cardiomyocyte ischemia/reperfusion-injury in
rats: Cardioprotective role of lycopene. J Cell Physiol.
230:2128–2141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ip BC, Liu C, Ausman LM, von Lintig J and
Wang XD: Lycopene attenuated hepatic tumorigenesis via differential
mechanisms depending on carotenoid cleavage enzyme in mice. Cancer
Prev Res (Phila). 7:1219–1227. 2014. View Article : Google Scholar
|
|
16
|
Khachik F, Carvalho L, Bernstein PS, Muir
GJ, Zhao DY and Katz NB: Chemistry, distribution, and metabolism of
tomato carotenoids and their impact on human health. Exp Biol Med
(Maywood). 227:845–851. 2002.
|
|
17
|
Bun S, Ikejima C, Kida J, Yoshimura A,
Lebowitz AJ, Kakuma T and Asada T: A combination of supplements may
reduce the risk of Alzheimer's disease in elderly Japanese with
normal cognition. J Alzheimers Dis. 45:15–25. 2015.
|
|
18
|
Prakash A and Kumar A: Implicating the
role of lycopene in restoration of mitochondrial enzymes and BDNF
levels in β-amyloid induced Alzheimers disease. Eur J Pharmacol.
741:104–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen H, Yoshioka H, Kim GS, Jung JE, Okami
N, Sakata H, Maier CM, Narasimhan P and Goeders CE: mechanisms of
cell death and potential molecular targets for neuroprotection.
Antioxid Redox Signal. 14:1505–1517. 2011. View Article : Google Scholar :
|
|
20
|
He M, Siow RC, Sugden D, Gao L, Cheng X
and Mann GE: Induction of HO-1 and redox signaling in endothelial
cells by advanced glycation end products: A role for Nrf2 in
vascular protection in diabetes. Nutr Metab Cardiovasc Dis.
21:277–285. 2011.
|
|
21
|
Itoh K, Chiba T, Takahashi S, Ishii T,
Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et
al: An Nrf2/small Maf heterodimer mediates the induction of phase
II detoxifying enzyme genes through antioxidant response elements.
Biochen Biophys Res Commun. 236:313–322. 1997. View Article : Google Scholar
|
|
22
|
Alfieri A, Srivastava S, Siow RC, Modo M,
Fraser PA and Mann GE: Targeting the Nrf2-Keap1 antioxidant defence
pathway for neurovascular protection in stroke. J Physiol.
589:4125–4136. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dinkova-Kostova AT, Holtzclaw WD, Cole RN,
Itoh K, Wakabayashi N, Katoh Y and Yamamoto Mand Talalay P: Direct
evidence that sulfhydryl groups of Keap1 are the sensors regulating
induction of phase 2 enzymes that protect against carcinogens and
oxidants. Proc Natl Acad Sci USA. 99:11908–11913. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang DD: Mechanistic studies of the
Nrf2-Keap1 signaling pathway. Drug Metab Rev. 38:769–789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wakabayashi N, Dinkova-Kostova AT,
Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW and
Talalay P: Protection against electrophile and oxidant stress by
induction of the phase 2 response: fate of cysteines of the Keap1
sensor modified by inducers. Proc Natl Acad Sci USA. 101:2040–2045.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang HP, Sun YY, Chen XM, Yuan LB, Su BX,
Ma R, Zhao RN, Dong HL and Xiong L: The neuroprotective effects of
isoflurane preconditioning in a murine transient global cerebral
ischemia-reperfusion model: The role of the Notch signaling
pathway. Neuromolecular Med. 16:191–204. 2014. View Article : Google Scholar
|
|
27
|
Homi HM, Mixco JM, Sheng H, Grocott HP,
Pearlstein RD and Warner DS: Severe hypotension is not essential
for isoflurane neuroprotection against forebrain ischemia in mice.
Anesthesiology. 99:1145–1151. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miyamoto O, Tamae K, Kasai H, Hirakawa H,
Hayashida Y, Konishi R and Itano T: Suppression of hyperemia and
DNA oxidation by indomethacin in cerebral ischemia. Eur J
Pharmacol. 459:179–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brouns R and De Deyn PP: The complexity of
neurobiological processes in acute ischemic stroke. Clin Neurol
Neurosurg. 111:483–495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Doyle KP, Simon RP and Stenzel-Poore MP:
Mechanisms of ischemic brain damage. Neuropharmacology. 55:310–318.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rabuffetti M, Sciorati C, Tarozzo G,
Clementi E, Manfredi AA and Beltramo M: Inhibition of
caspase-1-like activity by Ac-Tyr-Val-Ala-Asp-chloromethyl ketone
induces long-lasting neuroprotection in cerebral ischemia through
apoptosis reduction and decrease of proinflammatory cytokines. J
Neurosci. 20:4398–4404. 2000.PubMed/NCBI
|
|
32
|
Chen Mand Wang J: Initiator caspases in
apoptosis signaling pathways. Apoptosis. 7:313–319. 2002.
View Article : Google Scholar
|
|
33
|
Thornberry NA and Lazebnik Y: Caspases:
Enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen F, Sun ZW, Ye LF, Fu GS, Mou Y and Hu
SJ: Lycopene protects against apoptosis in hypoxia/reoxygenation
induced H9C2 myocardioblast cells through increased autophagy. Mol
Med Rep. 11:1358–1365. 2015.
|
|
35
|
He Q, Zhou W, Xiong C, Tan G and Chen M:
Lycopene attenuates inflammation and apoptosis in post-myocardial
infarction remodeling by inhibiting the nuclear factor-κB signaling
pathway. Mol Med Rep. 11:374–378. 2015.
|
|
36
|
He M, Pan H, Chang RC, So KF, Brecha NC
and Pu M: Activation of the Nrf2/HO-1 antioxidant pathway
contributes to the protective effects of Lycium barbarum
polysaccharides in the rodent retina after
ischemia-reperfusion-induced damage. PloS One. 9:e848002014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Y, Zhang L and Liang J: Activation of
the Nrf2 defense pathway contributes to neuroprotective effects of
phloretin on oxidative stress injury after cerebral
ischemia/reperfusion in rats. J Neurol Sci. 351:88–92. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lv JC, Wang G, Pan SH, Bai XW and Sun B:
Lycopene protects pancreatic acinar cells against severe acute
pancreatitis by abating the oxidative stress through JNK pathway.
Free Radic Res. 49:151–163. 2015. View Article : Google Scholar
|
|
39
|
Ciftci O, Oguz F, Beytur A, Polat F,
Altıntas R and Oguzturk H: Lycopene prevents experimental priapism
against oxidative and nitrosative damage. Eur Rev Med Pharmacol
Sci. 18:3320–3325. 2014.PubMed/NCBI
|
|
40
|
Yin Q, Ma Y, Hong Y, Hou X, Chen J, Shen
C, Sun M, Shang Y, Dong S, Zeng Z, et al: Lycopene attenuates
insulin signaling deficits, oxidative stress, neuroinflammation,
and cognitive impairment in fructose-drinking insulin resistant
rats. Neuropharmacology. 86:389–396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Siow RC, Sato H and Mann GE: Heme
oxygenase-carbon monoxide signalling pathway in atherosclerosis:
Anti-atherogenic actions of bilirubin and carbon monoxide?
Cardiovasc Res. 41:385–394. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wei Y, Gong J, Yoshida T, Eberhart CG, Xu
Z, Kombairaju P, Sporn MB, Handa JT and Duh EJ: Nrf2 has a
protective role against neuronal and capillary degeneration in
retinal ischemia-reperfusion injury. Free Radic Biol Med.
51:216–224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheng X, Siow RC and Mann GE: Impaired
redox signaling and antioxidant gene expression in endothelial
cells in diabetes: a role for mitochondria and the nuclear
factor-E2-related factor 2-Kelch-like ECH-associated protein 1
defense pathway. Antioxid Redox Signal. 14:469–487. 2011.
View Article : Google Scholar
|